Abstract
Background
Roux-en-Y gastric bypass (RYGB) surgery is very effective in reducing excess body weight and improving glucose homeostasis in obese subjects. Changes in the pattern of gut hormone secretion are thought to play a major role, but the mechanisms leading to both changed hormone secretion and beneficial effects remain unclear. Specifically, it is not clear whether changes in the number of hormone-secreting enteroendocrine cells, or changes in the releasing stimuli, or both, are important.
Methods
We estimated numbers of enteroendocrine cells after immunohistochemical staining in fixed tissue samples from rats at 10–11 months after RYGB.
Key Results
Numbers of glucagon-like peptide-1 (GLP-1) (L-cells, co-expressing peptide YY (PYY)), cholecystokinin (CCK), neurotensin, and 5-HT-immunoreactive cells were significantly increased in the Roux and common limbs, but not the biliopancreatic limb in RYGB rats compared with sham-operated, obese rats fed high-fat diet, and chow-fed controls. This increase was mostly accounted for by general hyperplasia of all intestinal wall layers of the nutrient-perfused Roux and common limbs, and less to increased density of expression. The number of ghrelin cells in the bypassed stomach was not different among the three groups.
Conclusions & Inferences
The findings suggest that the number of enteroendocrine cells increases passively as the gut adapts, and that the increased total number of L- and I-cells is likely to contribute to the higher circulating levels of GLP-1, PYY, and CCK, potentially leading to suppression of food intake and stimulation of insulin secretion. Whether changes in releasing stimuli also contribute to altered circulating levels will have to be determined in future studies.
Keywords: bariatric surgery, ghrelin, high-fat diet, hyperplasia, intestinal wall, obesity, stomach
INTRODUCTION
Bariatric surgery has become one of the most effective treatment options for obesity and metabolic diseases.1–3 Roux-en-Y gastric bypass surgery (RYGB), in particular, leads to sustained body weight loss in morbidly obese patients and is increasingly considered in less obese and even in normal weight patients to cure diabetes and other obesity-associated diseases.4 Although several mechanisms have been proposed, it is not known how RYGB surgery produces these beneficial effects.5 Thus, investigation of such potential mechanisms in clinical and preclinical studies is gaining momentum, as it may lead to the identification of novel drug targets, more efficient surgical procedures, and improved long-term behavioral compliance after surgery.
One of the leading candidate mechanisms is a change in the pattern of gut hormone secretion favoring a catabolic profile of processes involved in the control of energy balance and an improved glucose homeostasis. Increased secretion of the lower gut hormones GLP-1 and PYY has been consistently found after RYGB in both human subjects6–9 and rat models.9–11 The role of other gut hormones and factors with known appetiteinducing effects such as ghrelin12,13 and appetite-suppressing effects such as CCK 14,15 and apolipoprotein A-IV (Apo-AIV),16 is less clear and controversial.
It will be important to identify the mechanisms leading to both changes in gut hormone secretion and their actions on processes of energy balance regulation in the brain and other organs. GLP-1, GLP-2, and PYY are secreted from L-cells which are enriched in the more distal parts of the gut, and it is thought that increased exposure to undigested nutrients of the Roux and common limbs after RYGB surgery causes their increased secretion. GLP-2 is thereby acting as a growth factor leading to hyperplasia and subsequent hypertrophy of the gut wall and in turn, an increased number of L-cells.17–21
The first studies on enteroendocrine cell proliferation have been carried out in obese human subjects22 and obese Zucker rats23 after jejuno-ileal bypass surgery. Increased densities of CCK and somatostatin-immunoreactive mucosal enteroendocrine cells in the duodenum proximal to the bypass were observed in humans22 and increased numbers of CCK-immunoreactive cells in both the bypassed and functional loops of the jejunum in rats.23 Also, after biliopancreatic diversion in rats, a procedure similar to RYGB, but with a preserved pyloric sphincter region, there is significant hypertrophy of the mucosa and external muscle layers in the common limb,24 and an increased number of cells co-expressing gastric inhibitory peptide (GIP) and GLP-1 in the jejunum now receiving nutrients directly from the stomach.25 Finally after true RYGB in rats there was increased villus height and crypt depth, as well as an increased number of villus and crypt goblet cells.26 In another study in rats and humans, GLP-2 levels were significantly increased and there was evidence for increased mitotic rate and crypt cell proliferation.27
We have developed a rat model for RYGB that exhibits many of the salient features of such surgery in obese human subjects, namely a sustained weight and fat mass loss, increased plasma levels of GLP-1, PYY, and amylin,11 improved glucose tolerance,11 and a shift in food preference away from fatty and calorie dense foods.28 The aim of the present study was to assess effects on gut hyperplasia and hypertrophy and numbers of enteroendocrine cells expressing CCK, GLP-1, 5-HT, neurotensin, and ghrelin in our rat model of RYGB. A preliminary account of this analysis has been published as an abstract.29
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats weighing ~200 g (Harlan Industries, Indianapolis, IN, USA) were housed individually in wire-mesh cages at a constant temperature of 21–23 °C with a 12 h light-dark cycle (lights on at 07:00, off at 19:00). Food and water were provided ad libitum unless otherwise indicated. Animals were made obese by putting them on a two-choice diet for 14–16 weeks consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001; Purina LabDiet, Richmond IN, USA) and high-fat diet (sweet HF diet; Kcal%: Carb, 35; Fat, 45; Prot, 20, D12451; Research Diets, New Brunswick, NJ, USA), with each of the diets containing sufficient minerals and vitamins. Small amounts of liquid Ensure diet (Kcal%: Carb, 64; Fat, 21.6; Prot, 14.4; Abbott Laboratories, Columbus, OH, USA) was also provided before surgery. They were then randomly assigned to either RYGB or sham surgery. After surgery, only liquid Ensure was provided as a source of food for the first 10 days, before giving back increasing amounts of regular chow and HF diet. A lean control group without surgery was placed on a regular chow diet throughout the experiment. All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Roux-en-Y gastric bypass and sham surgery
Details of the RYGB surgical procedure have been reported earlier.11,28 Briefly, the procedure resulted in a gastric pouch of about 20% of the total gastric volume, connected to a 15-cm-long Roux limb, a 25-cm-long common limb, and a roughly 40-cm-long biliopancreatic limb.
Sham-surgery consisted of the same procedure as for RYGB, except that the transected jejunum was re-anastomosed, one small incision in the jejunum 25 cm from ileocecal valve and one in the gastric fundus were sutured closed, and the cutting stapler was laid over the stomach without firing. Thus, a similar amount of surgical trauma was inflicted, but the normal flow of nutrients was preserved in sham-operated rats. To overcome potential deficits in iron absorption and development of anemia, rats were administered a macromolecular dextran–iron complex (Iron Dextran injectable, catalog # 93963, 5 mg, sc; Town and Country, Ashland, OH, USA) once a week for the first 2 weeks after RYGB surgery. Additional doses were administered to individual anemic animals if indicated.
Tissue preparation and immunohistochemistry
Ten to eleven months after surgery, rats were euthanized after overnight food deprivation, and the gastrointestinal tract was harvested. This time-point after surgery was chosen because the animals were involved in behavioral tests and hormone measurements, the results of which were reported previously.11,28 Furthermore, this extended postsurgical period allowed us to assure the long-term effectiveness of the procedure on body weight and adiposity, and most likely captured the final state of adaptive changes. Half the tissue samples from each location were immersion-fixed in 10% buffered formalin for later immunohistochemical processing, and the other half was rapidly frozen in liquid nitrogen for later quantitative PCR or Western immunoblotting. After washing in saline, the fixed samples were soaked in 18% sucrose, 0.05% sodium azide in 0.1 mol L−1 phosphate buffered saline (PBS) solution for cryoprotection. Tissue samples were then frozen and 30-µm-thick sections were cut in a cryostat and separated into five series. For immediate processing, sections were held in PBS (+4 °C), whereas for long-term storage (−20 °C), a cryoprotectant solution (50% PBS, 30% ethylene glycol, 20% glycerol) was used. Free-floating sections were pretreated with 0.5% sodium borohydride in PBS to minimize aldehyde cross-linking of the fixative and treated with a blocking solution containing 5% normal goat or donkey serum in PBS containing 0.5% Triton X-100 (PBST). Appropriate washing in PBS followed all incubations. The sections were incubated in primary antibody (CCK-antibody 1 : 400, raised in rabbit against synthetic CCK-39; Peninsula, Belmont, CA, USA; GLP-1 mouse monoclonal antibody 1 : 5000, ImmunDiagnostik, Bensheim, Germany, distributed by ALPCO, Windham, NH, USA; rabbit anti-neurotensin 1 : 5000, ImmunoStar, Hudson, WI, USA; 5-HT antibody 1 : 50 000, rabbit anti-serotonin, ImmunoStar; goat anti-ghrelin, 1 : 1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA.) diluted in PBST containing 0.1% gelatin and 0.05% sodium azide, overnight at room temperature, or at 4 °C for 20 h, under gentle agitation by an orbital shaker. After thoroughly rinsing in PBS, they were reacted with Cy-3-conjugated antirabbit, antigoat, or antimouse IgG secondary antibody (Jackson Immuno Research, West Grove, PA, USA) before mounting and cover slipping in an aqueous medium. Staining was completely absent in control experiments with omission of the primary antibody or, for CCK-39, with incubation in primary antibody that had been preabsorbed by adding 10 µmol sulfated CCK-8 for 4 h at room temperature. Further, manufacturer-supplied data indicate that this antibody is more selective for CCK than antibodies raised against CCK-8, as it is not inhibited by pre-absorption in 10 nmol gastrin I (G17), Tetrin (G4), and Somatostatin-14.
Immunohistochemical analysis
Whole sections were mounted onto Superfrost glass slides using Fluoromount G (Southern Biotechnology, Birmingham, AL, USA) as the mounting medium. Sections were viewed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss Microscopy, Thornwood, NY, USA). Counts were performed visually. Cells that were immunoreactive but did not have clear enteroendocrine cell morphology, such as 5-HT-positive mast cells, were not counted. Counts were based on averages from two to four intestinal cross-sections from each limb for each animal. For ghrelin cell counts, the average of seven sections from corpus of the stomach was used.
Morphological measurements
Tissue measurements were performed visually using a Zeiss Axioplan fluorescence microscope in conjunction with a 10 × 10 ocular grid at 20× magnification. A micro slide ruler was used to determine that the 10 × 10 grid had a 50 µm resolution. For sampling purposes, two sections were chosen from each limb of each animal. Measurements of tissue thickness were taken at four points on each section for the longitudinal muscle, circular muscle, sub-mucosa, and mucosa (combined thickness of crypts and villi). If four measurements could not be taken on each of the two sections selected, two additional sections were chosen for that particular limb and counted to provide additional measurements. The average thickness of the tissue was calculated for each limb of each animal, and these averages were used for statistical analysis.
For measurements of total and mucosal cross-sectional areas, dark-field images were generated in a Leitz microscope with a 1× macro lens and areas determined using the paper weighing method. Average cross-sectional areas were calculated from at least three representative sections per rat and limb. Enteroendocrine cell densities were calculated as number of cells per mm2.
Statistical analysis
Morphological measurements and enteroendocrine cell counts were analyzed by two-way anovawith gut location as within-subject factor and surgical group as between-subject factor, followed by Bonferroni-adjusted multiple comparisons. Ghrelin cell counts were analyzed by one-way analysis of variance. All data were expressed as mean ± SEM.
RESULTS
RYGB-induces hypertrophy of the Roux and common limbs
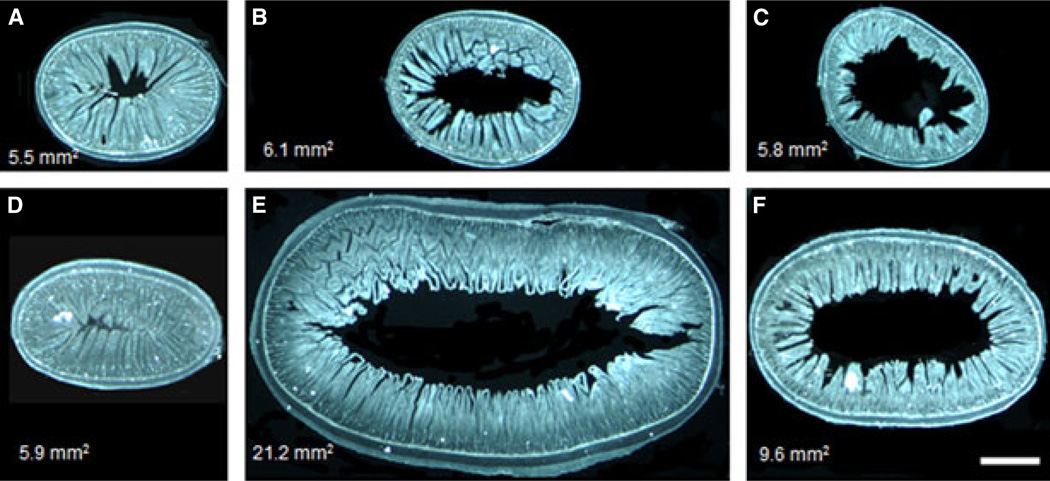
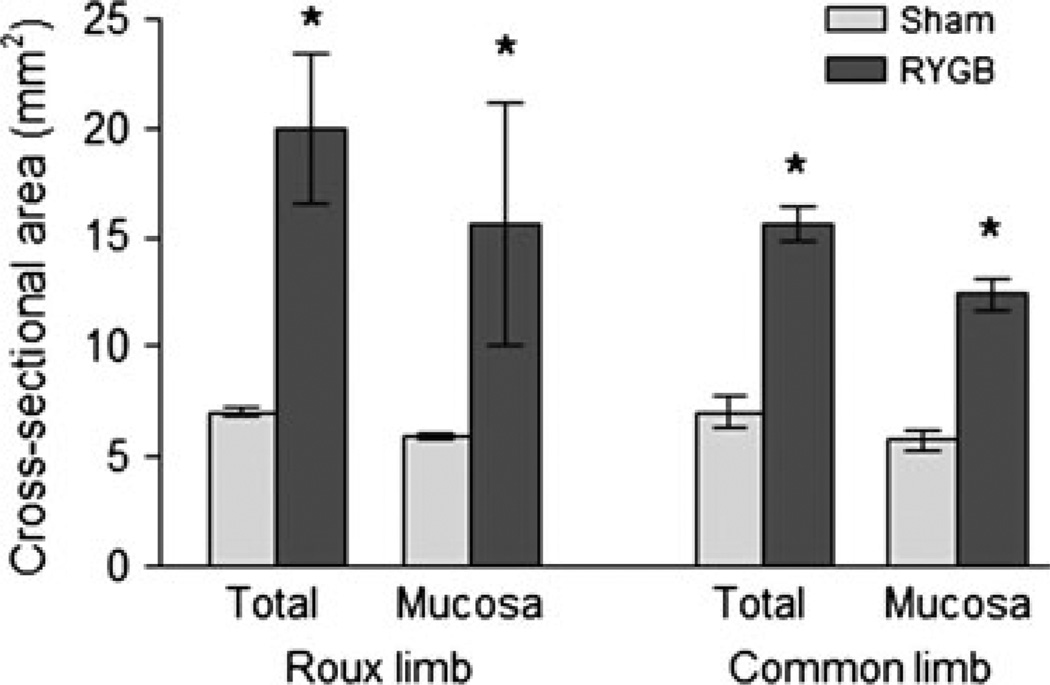
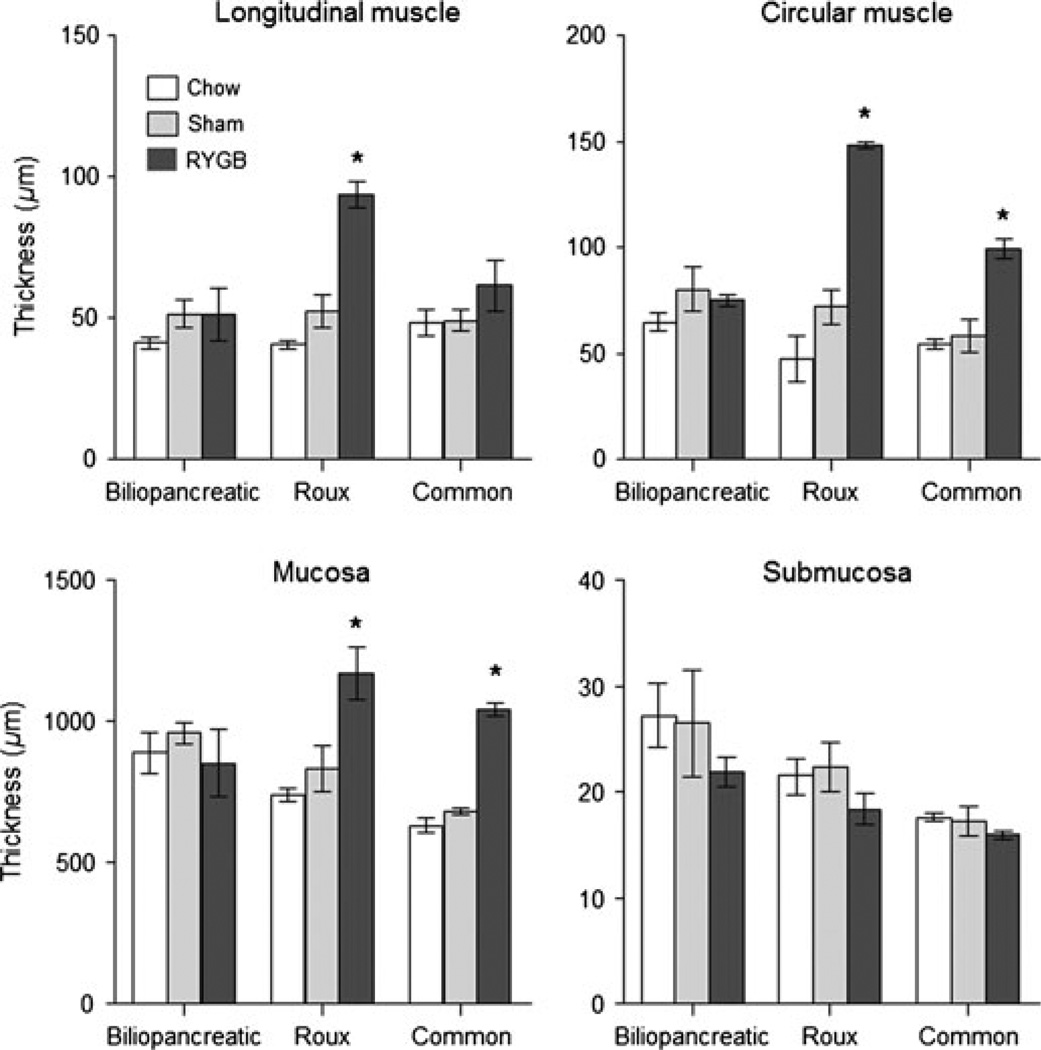
The diameter of the Roux and common limbs but not the biliopancreatic limb was greatly increased compared with the corresponding gut segments of sham-operated rats (Fig. 1). Similarly, the total and mucosal surface areas of the cross-sectioned Roux and common limbs were significantly increased (Fig. 2). Both the longitudinal and circular muscle layers of the Roux limb and the circular muscle layer in the common limb were about two-fold thicker in RYGB rats compared with both other groups (Fig. 3). No differences in the thickness of the external muscle layers were observed in the biliopancreatic limb. The total mucosa (crypt plus villous height) in the Roux and common limbs was also significantly thicker in RYGB rats compared with both control groups. No significant differences were observed for the submucosa thickness.
Figure 1.
Representative dark field images of cross-sections of the biliopancreatic (A, D), Roux (B, E), and common (C, F) limbs or their corresponding gut segments of rats at 10–11 months after sham (A–C) or RYGB surgery (D–F), showing hypertrophy of the Roux and common limbs but not the biliopancreatic limb. Surface areas in mm2 of the representative cross-sectioned segments are shown in the left bottom corner of each panel. Scale bar in F, 1.0 mm.
Figure 2.
Average total and mucosal cross-sectional areas of the Roux and common limbs from rats at 10–11 months after Roux-en-Y gastric bypass surgery (n = 4) or sham-operation (n = 4). *P < 0.05 compared with sham-operated rats, based on t-tests.
Figure 3.
Thickness of intestinal wall layers in the biliopancreatic, Roux, and common limbs (or their equivalent) in non-operated, chow-fed lean rats (n = 4), sham-operated, high-fat fed obese rats (n = 4), and Roux-en-Y gastric bypass rats (n = 4), 10–11 months after surgery. * P < 0.05 compared with both chow-fed controls and sham-operated rats, based on anovafollowed by Bonferroni-adjusted multiple comparisons.
Increased number but not density of enteroendocrine cells in the Roux and common limbs
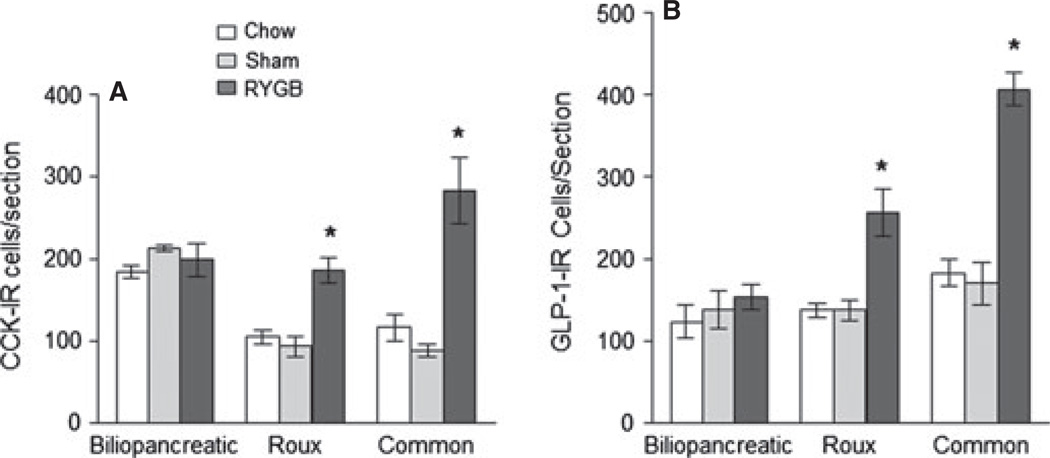
CCK-IR cells anova yielded significant effects of surgical treatment (F[2,24] = 20.7, P < 0.001) and gut location (F[2,24] = 9.9, P < 0.001), as well as a significant interaction (F[4,24] = 7.6, P < 0.001). As expected, in chow fed lean control rats, almost twice as many CCK-IR cells were expressed in the upper small intestine (the area corresponding to the biliopancreatic limb), compared with the lower small intestine (the area corresponding to the Roux and common limbs). (Figs 4 and 5). These numbers were not significantly different in high fat diet-induced obese rats with sham operation. However, in RYGB rats, the numbers of CCK-IR cells were significantly higher in the Roux limb (t = 3.5, P < 0.005) and common limbs (t = 7.4, P < 0.001), but not in the biliopancreatic limb. Compared with sham-operated rats, the increase was about two-fold in the Roux limb and about three-fold in the common limb.
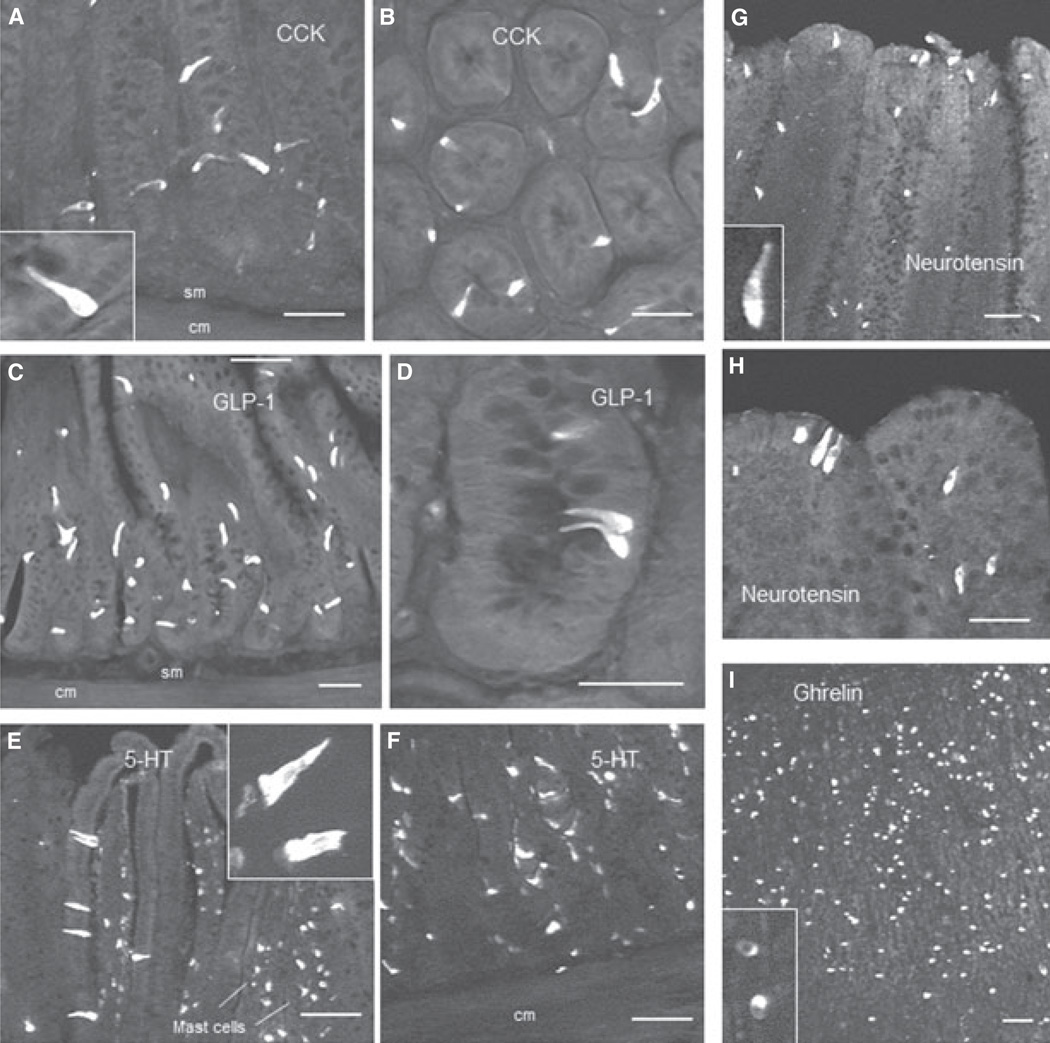
Figure 4.
Examples of CCK (A,B), GLP-1 (C,D), and 5-HT (E,F), immunoreactive enteroendocrine cells in the Roux limb, neurotensin immunoreactive cells in the common limb (G,H), and ghrelin immunoreactive cells in the gastric antrum of the remnant stomach (I), 10–11 months after Roux-en-Y gastric bypass surgery. Note that different magnifications are used to emphasize shape and distribution of enteroendocrine cells in villi and crypts. Abbreviations: cm, circular muscle; sm, submucosa. Scale bars, 100 µm for all panels.
Figure 5.
Number of CCK (A) and GLP-1 (B) immunoreactive cells in the biliopancreatic, Roux, and common limbs (or their equivalent) in nonoperated, chow-fed lean rats (n = 3), sham-operated, high-fat fed obese rats (n = 4), and Roux-en-Y gastric bypass rats (n = 4), 10–11 months after surgery. * P < 0.05 compared with both chow-fed controls and sham-operated rats, based on anova followed by Bonferroni-adjusted multiple comparisons.
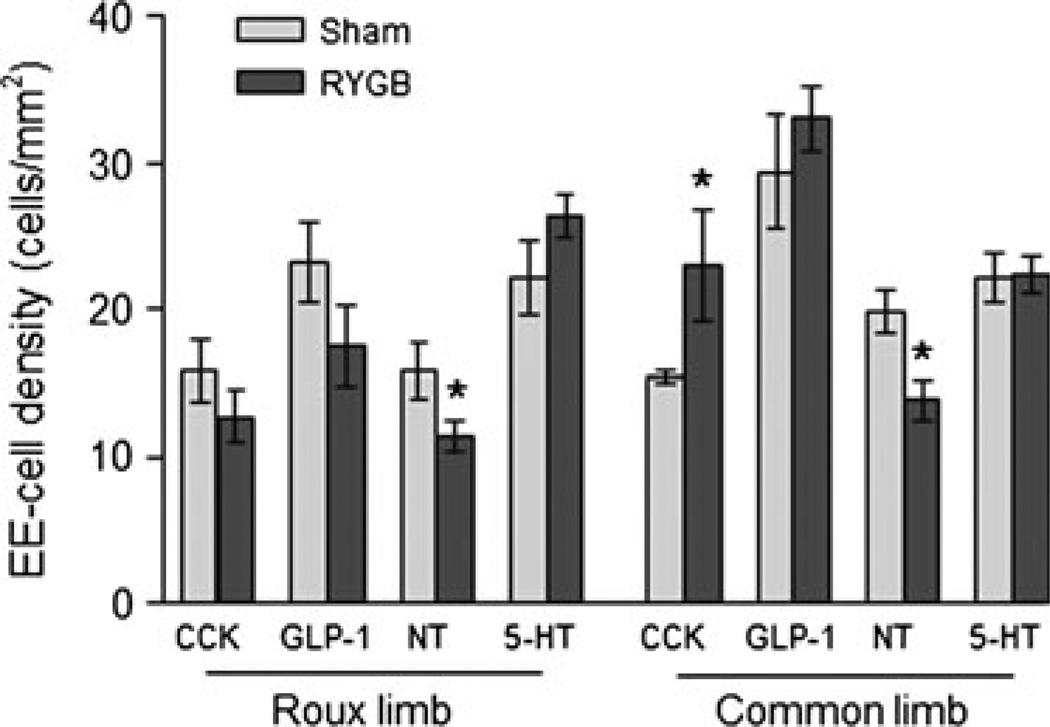
In the Roux limb, the density of CCK-cells was not significantly different after RYGB, compared with sham-operated rats (Fig. 8), because the cross-sectional area of the mucosa was also increased about three-fold (Fig. 2). In the common limb, the density of CCK-cells was significantly higher after RYGB compared with sham-operated rats (Fig. 8), as the increased cell number was not fully compensated by increased mucosal area. GLP-1-IR cells As for CCK, anova yielded significant effects of surgical treatment (F[2,24] = 34.4, P < 0.001) and gut location (F[2,24] = 23.2, P < 0.001), as well as a significant interaction (F[4,24] = 8.2, P < 0.001). There was only a slight and non-significant gradient in the expression of GLP-1-IR cells across the small intestine in chow-fed, lean control rats, with the proximal part (biliopancreatic and Roux limbs) expressing about 150 cells per section and the distal part (common limb) expressing about 200 cells per section (Figs 4 and 5). There were no significant differences between chow-fed lean and sham-operated, high fat-fed obese rats. In contrast, after Roux-en-Y gastric bypass surgery, there was a significant (t = 4.2, P < 0.001) two-fold increase of GLP-1-IR cell expression in the Roux limb, and a significant (t = 8.4, P < 0.001) 2.5-fold increase in the common limb, compared with sham-operated rats. Most of the CCK-IR and GLP-1-IR cells were found in the crypt area, and there were no obvious differences in this distribution pattern among the three groups. The density of GLP-1 cells was, however, not significantly different after RYGB, compared with sham-operated rats (Fig. 8).
Figure 8.
Mucosal cross-sectional area (A) and enteroendocrine cell densities (B) in the Roux and common limbs of rats at 10–11 months after Roux-en-Y gastric bypass (n = 4) or sham surgery (n = 4). * P < 0.05 compared with sham-operated rats, based on anova followed by Bonferroni-adjusted multiple comparisons.
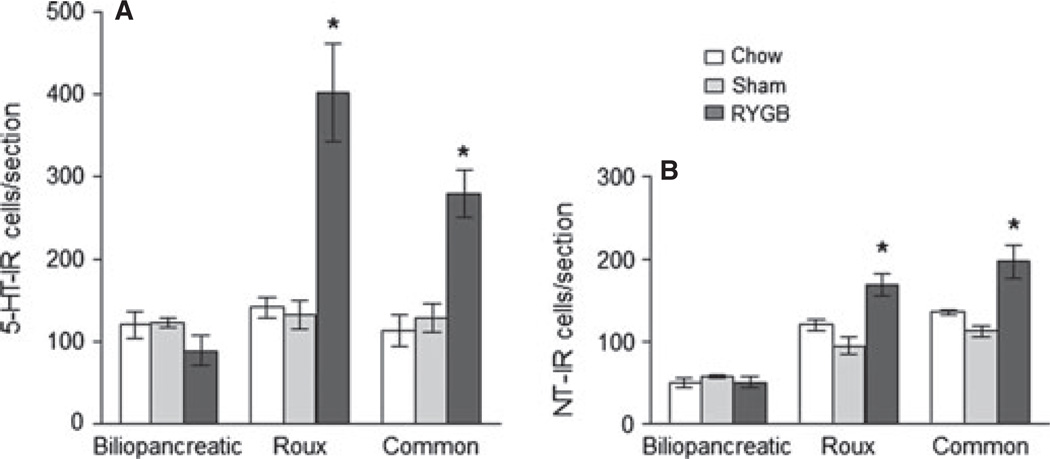
5-HT-IR cells The 5-HT antibody stained both enteroendocrine and mast cells, but only enteroendocrine cells were counted based on their distinctive location in the epithelium and elongated shape (Figs 4 and 6). anova yielded significant effects of surgical treatment (F[2,26] = 23.5, P < 0.001) and gut location (F[2,26] = 13.7, P < 0.001), as well as a significant interaction (F[4,26] = 10.5, P < 0.001). In chow-fed control rats we found similar numbers of 5-HT-IR enteroendocrine cells per cross-section at the locations corresponding to the three surgical limbs, and sham surgery did not result in significant changes. After RYGB, however, there was a significant increase in 5-HT-IR enteroendocrine cells in the Roux (t = 7.26, P < 0.001) and common limb (t = 4.0, P < 0.01), but not in the bypassed biliopancreatic limb. The density of 5-HT-cells was, however, not significantly different after RYGB, compared with sham-operated rats (Fig. 7).
Figure 6.
Number of 5-HT (A) and neurotensin (B) immunoreactive cells in the biliopancreatic, Roux, and common limbs (or their equivalent) in non-operated, chow-fed lean rats (n = 3), sham-operated, high-fat fed obese rats (n = 4), and Roux-en-Y gastric bypass rats (n = 4), 10–11 months after surgery. *P < 0.05 compared with both chow-fed controls and sham-operated rats, based on anova followed by Bonferroni-adjusted multiple comparisons.
Figure 7.
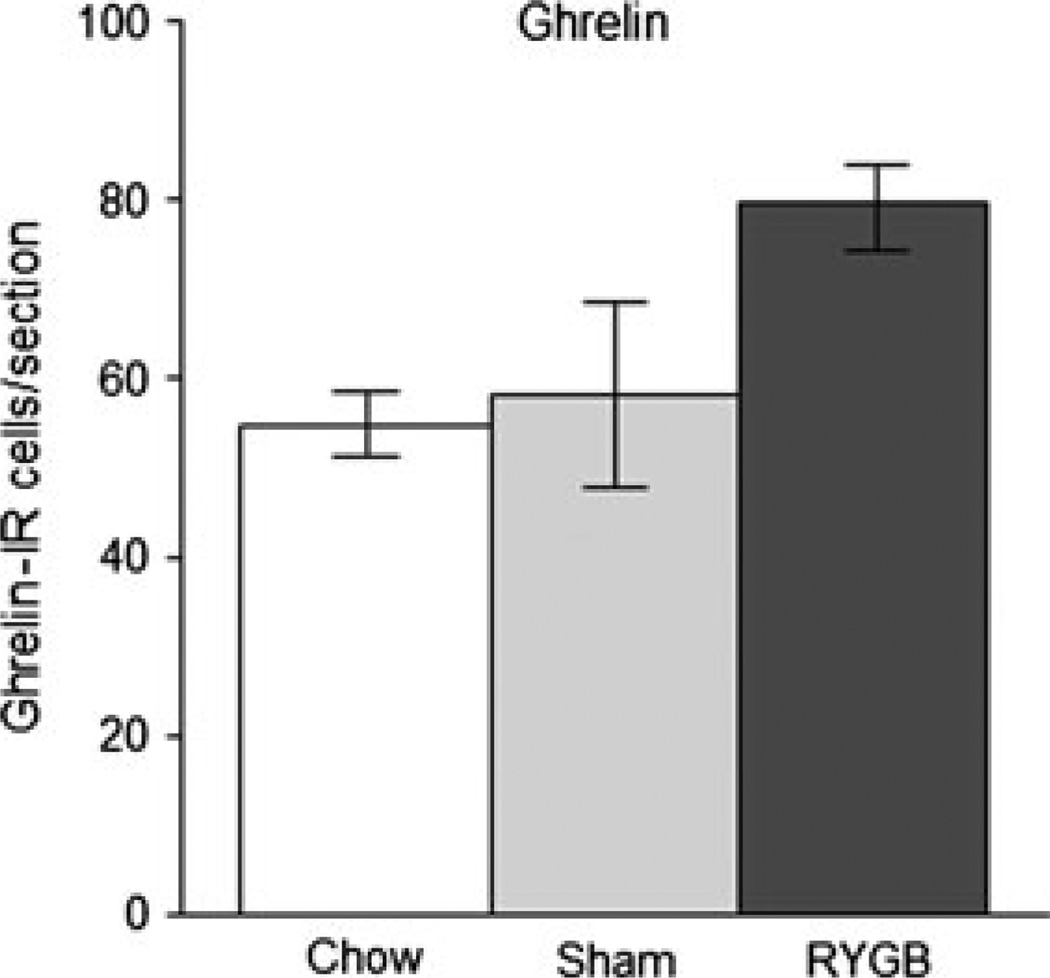
Number of ghrelin-immunoreactive cells in the gastric mucosa of non-operated, chow-fed lean rats (n = 4), sham-operated, high-fat fed obese rats (n = 4), and Roux-en-Y gastric bypass rats (n = 4), 10–11 months after surgery.
Neurotensin-IR cells anova yielded significant effects of surgical treatment (F[2,23] = 21.7, P < 0.001) and gut location (F[2,23] = 81.4, P < 0.001), as well as a significant interaction (F[4,23] = 6.8, P < 0.001). In chow-fed control rats, there was a proximal-to-distal gradient in the number of neurotensin-IR enteroendocrine cells, with fewer cells at the location corresponding to the biliopancreatic limb and more in the mid-jejunum (corresponding to the Roux limb) and the distal jejunum (corresponding to the common limb) (Figs 4 and 6). Furthermore, as determined for the biliopancreatic limb and unlike the other types of cells, the majority of neurotensin-IR cells were located in the villi (29 ± 6 cells) and a minority in the crypts (13 ± 1 cells; P < 0.05). These numbers of cells were not much different in sham-operated rats, but in RYGB rats, there were significantly more neurotensin-IR cells in the Roux-limb (t = 5.8, P < 0.001) and in the common limb (t = 6.1, P < 0.001), but not in the biliopancreatic limb. However, because the cross-sectional areas of the Roux and common limb mucosa was increased about two–three-fold (Fig. 2), the density of neurotensin cells was not increased, but significantly decreased after RYGB, compared with sham-operated rats (Fig. 8).
No change in ghrelin-IR cells There were very few ghrelin-IR cells in the small intestines and no obvious differences between the treatment groups (data not shown). In the corpus of the bypassed stomach, we found slightly more ghrelin-IR cells in RYGB rats compared with sham-operated rats and chow-fed control rats, but the difference was not statistically significant (P = 0.096) (Figs 3 and 7).
DISCUSSION
The gut is increasingly recognized as a key player in the development of metabolic disease,30 and because gut surgery currently is the most effective long-term treatment for obesity and other indices of metabolic syndrome, it is now also referred to as ‘metabolic surgery’ (e.g., 31). A changed pattern in gut hormone secretion appears to be an important ingredient in the beneficial effects of at least Roux-en-Y gastric bypass and perhaps other types of bariatric surgery.6,7,9 The factors leading to increased plasma levels of anorexigenic gut hormones such as GLP-1, PYY, and CCK after RYGB are not well understood. It has not been clear whether these increases in circulating hormones are due to overstimulation or proliferation of the enteroendocrine cells or to reduced degradation of the secreted hormones, or a combination of some of these factors. Here, we confirm earlier findings by others showing hypertrophy and hyperplasia of the Roux and common limbs after RYGB in both humans and rats,24,27 and we show that the increased numbers of GLP-1, CCK, 5-HT, and neurotensin-immunoreactive enteroendocrine cells is mainly due to the increased mucosal area, with only a minor contribution of increased cell density. The increased circulating levels of these hormones may thus be due to the increased total number of cells. However, our data do not rule out additional mechanisms such as increased releasing stimuli and increased secretory efficiency of the cells.
Our study confirms and extends reports of gut hypertrophy after various types of gut surgery. The approximately 2.5-fold increase in the area of both external muscle layers and the mucosa we observed 10–11 months after RYGB was very similar to the increase reported at 7 weeks after biliopancreatic diversion by Nadreau et al.,24 suggesting that the hypertrophic adaptation happens relatively early after surgery and then remains unchanged. This is consistent with other studies showing significant increases of plasma GLP-2 levels and the growth factors IGF-1 and bFGF already 2 weeks after RYGB in Sprague–Dawley rats32 and increased GLP-2 levels and crypt cell proliferation 3 weeks after RYGB in Wistar rats.27
Increased circulating levels of GLP-1 and PYY secreted from intestinal L-cells have been most strongly associated with the beneficial effects on body weight and glucose homeostasis after Roux-en-Y gastric bypass surgery. It is conceivable that increased levels of both of these hormones suppress food intake33–35 and that increased GLP-1 through its actions on insulin secretion helps normalize glucose tolerance.36,37 In one study using PYY-deficient mice, it has been claimed that this gut hormone is required for bypass surgery to significantly lower body weight.38 However, because it was a modified bypass surgery with the upper duodenum anastomosed to the greater curvature of the stomach and the observation period including only the first 10 days after surgery,39 the study can only be regarded as preliminary. Using the same entero-gastric anastomosis mouse model, it was earlier shown that blockade of GLP-1 receptor signaling by systemic infusion of Exendin-4 (9–39) during days 5 and 10 after surgery was able to partially prevent the profound anorexia, but not the body weight loss.39 Clearly, additional experiments in rodents with more standard gastric bypass surgery and longer observation periods will be necessary to demonstrate a requirement of elevated GLP-1 and/or PYY-signaling for the powerful beneficial effects of RYGB. Our immunohistochemical study suggests that at least one factor in the increased circulating GLP-1 and PYY levels is an increased number of L-cells. The fact that the number of L-cells only increased in the limbs that were exposed to luminal nutrients (Roux and common limbs), but not the biliopancreatic limb, suggests that the stimulus depends on a direct consequence of the abnormal perfusion with undigested nutrients and cannot only depend on circulating levels of the trophic hormone GLP-2. It will be important to demonstrate whether paracrine actions of GLP-2 are limited to the perfused limbs or if an additional signal is responsible for hyperplasia.
CCK was the first gut hormone implicated in satiation.40 Until recently, it has not received much attention as a possible mediator of RYGB-induced anorexia, probably because no changes in the postprandial CCK-response to a mixed protein-fat meal was reported 6 months after Roux-en-y gastric bypass or vertical banded gastroplasty in an early study.14 Similarly, no changes in plasma CCK levels were found 1 month after RYGB in an early rat model.41 However, in more recent clinical studies, postprandial CCK responses were significantly increased 2 weeks after RYGB in one study,15 but not 3 weeks after surgery in another study.42 Differences in the postprandial CCK response were also found between patients with RYGB or sleeve gastrectomy.43,44 More consistent findings were reported in patients after jejuno-ileal bypass surgery, with postprandial CCK levels significantly increased 3 months45 and even 20 years after surgery.46 In one of the rare studies using immunohistochemistry to identify specific types of enteroendocrine cells, Buchan et al. found increased numbers of CCK-immunoreactive cells, but it was not clear whether the increased number was due to increased density or general hyperplasia of the mucosa. Increased density of CCK cells was claimed in a study with intestinal biopsies taken at the duodeno-jejunal flexure from patients 1 to 30 years after jejuno-ileal bypass surgery.22 Thus, together with our present observations, it is likely that increased numbers of CCK cells are partly responsible for increased postprandial CCK levels and that this may contribute to early satiety after RYGB and jejuno-ileal bypass surgeries.
Neurotensin is another gut hormone that has received relatively little attention regarding its potential role in the effects of bariatric surgeries, even though its plasma levels were consistently found increased after various surgeries. Early studies in patients after jejuno-ileal bypass surgery showed elevated neurotensin levels47,48 and elevated neurotensin levels were associated with signs of the dumping syndrome 3 months after RYGB.49 Elevated postprandial levels of pro-neurotensin levels were also shown in more recent clinical studies after RYGB.50,51 Together with our findings of increased numbers of neurotensin-immunoreactive enteroendocrine cells after RYGB in rats, these clinical observations suggest that neurotensin is an interesting candidate for mediating some of the beneficial effects of RYGB and jejuno-ileal surgeries.
In contrast to the other gut hormones, ghrelin is strongly orexigenic, and reduced levels of this hormone could plausibly explain the reduced hunger drive after RYGB.13,52,53 This interpretation is supported by the observation that postoperative weight loss was correlated with the magnitude of the decrease in circulating ghrelin levels in a rat model for RYGB.12 However, several other clinical studies did not find significantly decreased fasting ghrelin levels after RYGB compared with untreated obese subjects,54–57 but in some of these studies, meal-induced suppression of ghrelin was enhanced.56,57 In a recent prospective study, fasting and postprandial ghrelin did show small decreases at 26 and 52 weeks after RYGB, but these changes were not statistically significant.7 Thus, although the effects of RYGB on ghrelin are highly variable, a relative ghrelin deficiency compared with the expected rise due to hypophagia and weight loss appears to be a common observation, keeping ghrelin as a potential candidate for RYGB’s effects on food intake. As the great majority of ghrelin producing cells are located in the gastric mucosa, the much smaller population of ghrelin cells in the small intestine seems irrelevant to explain decreases in circulating ghrelin levels. Our findings in the stomach suggest that there is no decrease in the total number of ghrelin-immunoreactive cells. However, elimination of some ghrelin cells by the surgical procedure of dividing the stomach and/or decreased stimulation of its secretion could account for lower circulating levels. Alternatively, reduced ghrelin levels could be the result of reduced stimulatory and/or increased suppressive humoral and neural signals.
Finally, 5-HT secreted from enterochromaffin cells is an important signaling molecule linking luminal stimuli with local enteric reflex activity and the extrinsic sensory innervation by vagal and dorsal root afferents.58,59 Altered mucosal 5-HT signaling has been implicated in inflammatory bowel disease,60 and it could play an important role in bringing about the drastic changes in motility and secretion of the Roux and common limbs that are suddenly faced with large quantities of undigested nutrients. Increased intestinal 5-HT signaling could also contribute to the feelings of nausea, discomfort, and early satiety with the ingestion of larger meals. Our findings suggest that, although not increased disproportionately to the increased mucosa, the role of 5-HT enterochromaffin cells and their downstream signaling would be worth studying in future experiments.
ACKNOWLEDGMENTS
We thank Andrew C. Shin with help in tissue harvesting and Katie Bailey for editorial help.
FUNDING
This research was supported by National Institutes of Health Grant DK047348.
Footnotes
DISCLOSURE
None of the authors declares a conflict of interest.
AUTHOR CONTRIBUTIONS
MBM carried out experiments, analyzed the data and helped with writing the manuscript. HZ did the surgeries. LMP developed the immunohistochemistry protocols. HRB designed the study and wrote the paper.
REFERENCES
- 1.Richards NG, Beekley AC, Tichansky DS. The economic costs of obesity and the impact of bariatric surgery. Surg Clin North Am. 2011;91:1173–1180. doi: 10.1016/j.suc.2011.08.010. vii–viii. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(Suppl 7):S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 3.Mechanick JI, Kushner RF, Sugerman HJ, et al. Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318–336. doi: 10.4158/EP.14.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2-an integrative review of early studies. Obes Surg. 2010;20:776–790. doi: 10.1007/s11695-010-0113-3. [DOI] [PubMed] [Google Scholar]
- 5.Tam CS, Berthoud HR, Bueter M, et al. Could the mechanisms of bariatric surgery hold the key for novel therapies? report from a Pennington Scientific Symposium. Obes Rev. 2011;12:984–994. doi: 10.1111/j.1467-789X.2011.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33(Suppl 1):S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 7.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Rouxen-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc. 2005;19:942–946. doi: 10.1007/s00464-004-8825-x. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JM, Kuemmerle JF, O’Dorisio TM, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770. doi: 10.1097/00000658-199006000-00016. discussion 770-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 16.Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009;17:46–52. doi: 10.1038/oby.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigalet DL, Bawazir O, Martin GR, et al. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557–1566. doi: 10.1007/s10620-006-9077-5. [DOI] [PubMed] [Google Scholar]
- 18.Rowland KJ, Brubaker PL. Life in the crypt: a role for glucagon-like peptide-2? Mol Cell Endocrinol. 2008;288:63–70. doi: 10.1016/j.mce.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- 20.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- 22.Ockander L, Hedenbro JL, Rehfeld JF, Sjolund K. Jejunoileal bypass changes the duodenal cholecystokinin and somatostatin cell density. Obes Surg. 2003;13:584–590. doi: 10.1381/096089203322190781. [DOI] [PubMed] [Google Scholar]
- 23.Chan CB, Buchan AM, Green KA, Pederson RA. The effect of jejunoileal bypass (JIB) in the obese Zucker rat on a sub-group of enteroendocrine cells. Int J Obes. 1987;11:285–293. [PubMed] [Google Scholar]
- 24.Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30:419–429. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 25.Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923–E932. doi: 10.1152/ajpendo.00422.2010. [DOI] [PubMed] [Google Scholar]
- 26.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Rouxen-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297:G950–G957. doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50–56. doi: 10.1097/SLA.0b013e3181d3d21f. [DOI] [PubMed] [Google Scholar]
- 28.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthoud HR, Mumphrey MB, Patterson LM, Zheng H. Roux-en-Y gastric bypass surgery-induced changes in enteroendocrine cell abundance and cytokine gene expression. Neurogastroenterol Motil. 2012;24:132. doi: 10.1111/nmo.12034. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inabnet WB, 3rd, Winegar DA, Sherif B, Sarr MG. Early outcomes of bariatric surgery in patients with metabolic syndrome: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2012;214:550–556. doi: 10.1016/j.jamcollsurg.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45:987–995. doi: 10.1016/j.jpedsurg.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 33.le Roux CW, Batterham RL, Aylwin SJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 34.Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 35.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang RM, Owji AA, Smith DM, Ghatei MA, Bloom SR. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest. 1995;95:417–421. doi: 10.1172/JCI117671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandarana K, Gelegen C, Karra E, et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60:810–818. doi: 10.2337/db10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Smith GP, Gibbs J, Jerome C, Pi-Sunyer FX, Kissileff HR, Thornton J. The satiety effect of cholecystokinin: a progress report. Peptides. 1981;2:57–59. doi: 10.1016/0196-9781(81)90011-5. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683–690. doi: 10.1016/j.soard.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y Gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchan AM, Pederson RA, Koop I, Gourlay RH, Cleator IG. Morphological and functional alterations to a sub-group of regulatory peptides in human pancreas and intestine after jejuno-ileal bypass. Int J Obes Relat Metab Disord. 1993;17:109–113. [PubMed] [Google Scholar]
- 46.Naslund E, Gryback P, Hellstrom PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–392. doi: 10.1038/sj.ijo.0800418. [DOI] [PubMed] [Google Scholar]
- 47.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471–480. [PubMed] [Google Scholar]
- 48.Naslund E, Gryback P, Backman L, et al. Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Dig Dis Sci. 1998;43:945–952. doi: 10.1023/a:1018806129102. [DOI] [PubMed] [Google Scholar]
- 49.Sirinek KR, O’Dorisio TM, Howe B, McFee AS. Neurotensin, vasoactive intestinal peptide, and Roux-en-Y gastrojejunostomy. Their role in the dumping syndrome. Arch Surg. 1985;120:605–609. doi: 10.1001/archsurg.1985.01390290083014. [DOI] [PubMed] [Google Scholar]
- 50.Christ-Crain M, Stoeckli R, Ernst A, et al. Effect of gastric bypass and gastric banding on proneurotensin levels in morbidly obese patients. J Clin Endocrinol Metab. 2006;91:3544–3547. doi: 10.1210/jc.2006-0256. [DOI] [PubMed] [Google Scholar]
- 51.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 52.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 53.Fruhbeck G, Diez-Caballero A, Gil MJ, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 54.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 55.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 56.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 57.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 58.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci. 2006;127:250–257. doi: 10.1016/j.autneu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Grundy D. 5-HT system in the gut: roles in the regulation of visceral sensitivity and motor functions. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):63–67. [PubMed] [Google Scholar]
- 60.Crowell MD, Wessinger SB. 5-HT and the brain-gut axis: opportunities for pharmacologic intervention. Expert Opin Investig Drugs. 2007;16:761–765. doi: 10.1517/13543784.16.6.761. [DOI] [PubMed] [Google Scholar]