Abstract
Morbidity and mortality are greater among socially disadvantaged racial/ethnic groups and those of lower socioeconomic status (SES). Greater chronic stress exposure in disadvantaged groups may contribute to this by accelerating cellular aging, indexed by shorter age-adjusted telomere length. While studies consistently relate shorter leukocyte telomere length (LTL) to stress, the few studies, mostly from the UK, examining associations of LTL with SES have been mixed. The current study examined associations between educational attainment and LTL among 2,599 high-functioning black and white adults age 70-79 from the Health, Aging and Body Composition Study. Multiple regression analyses tested associations of race/ethnicity, educational attainment and income with LTL, adjusting for potential confounders. Those with only a high school education had significantly shorter mean LTL (4806 basepairs) than those with post-high school education (4926 basepairs; B=125, SE= 47.6, p = .009). A significant interaction of race and education (B = 207.8, SE = 98.7, p = .035) revealed more beneficial effects of post-high school education for blacks than for whites. Smokers had shorter LTL than non-smokers, but the association of education and LTL remained significant when smoking was covaried (B = 119.7, SE = 47.6, p = .012). While higher income was associated with longer LTL, the effect was not significant (p > .10). This study provides the first demonstration of an association between educational attainment and LTL in a US population where higher education appears to have a protective effect against telomere shortening, particularly in blacks.
Keywords: Socioeconomic status, education, telomere length, race, health disparities
INTRODUCTION
All-cause mortality and rates of cardiovascular disease, stroke, arthritis, diabetes, and some forms of cancer are higher among socially disadvantaged populations (Braveman et al., 2010; Centers for Disease Control and Prevention (CDC), 2011; Singh and Hiatt, 2006). Despite differences in contributing factors and mechanisms of specific diseases, individuals with lower socioeconomic status and/or who belong to some racial or ethnic groups are at higher risk for all of these adverse outcomes (Adler and Stewart, 2010). Disparities in morbidity and mortality are seen at all stages of the lifespan, but their extent differs by age. Disparities are marked at birth, narrow during childhood and adolescence, and increase again during late adulthood up to age 65. After age 65, disparities associated with income persist but narrow, and differences between blacks and whites not only diminish but, in some cases, actually reverse, with lower mortality rates among older blacks than older whites (House et al., 1994; Kim and Miech, 2009).
The association of both race/ethnicity and socioeconomic status with morbidity and mortality from a wide range of diseases suggests the existence of a common biological process related to social disadvantage that increases vulnerability to multiple health problems. Cellular aging, as reflected by LTL, is potentially such a process. Telomeres are DNA-protein complexes that cap the ends of chromosomes and protect against damage; when telomeres become too short, cells become more susceptible to neoplastic transformation, functionally impaired and unable to divide, reaching senescence (Blackburn, 2000, 2001). Senescent cells, in turn, tend to secrete pro-inflammatory cytokines and contribute to disease processes (Freund et al., 2010; O’Donovan et al., 2011a). Such processes underlie many of the chronic diseases that are overrepresented in socially disadvantaged groups and prospective studies have established that individuals with shorter telomeres have higher risk for such diseases and for early mortality (Cawthon et al., 2003; Epel et al., 2009; Njajou et al., 2009; Willeit et al., 2010).
Social disadvantage may increase morbidity and mortality through greater exposure to social conditions that promote leukocyte telomere shortening. For example, individuals with less education and lower income are exposed to greater chronic stress, which may affect LTL by elevating inflammation and promoting oxidative stress (Kiecolt-Glaser et al., 2011; O’Donovan et al., 2011a). People also may cope with stress exposures by engaging in health-damaging behaviors such as use of tobacco and other substances, poor nutrition, and lack of exercise (Hatch and Dohrenwend, 2007; Matthews et al., 2010), all of which have been linked to shorter LTL (Puterman et al., 2010; Sun et al., 2012; Valdes et al., 2005).
Based on the above, we would expect that both SES and race/ethnicity would be linked to shorter LTL. However, different patterns of association of income, education and occupation with LTL have been found across studies. In a British sample of 1552 female twins, LTL was unrelated to the women’s income or education, but was shorter among those in manual versus non-manual occupational classes (Cherkas et al., 2006). Among Scottish men age 45-64 years, LTL was shorter among the 489 men who developed coronary heart disease (CHD) than among the 1058 controls; in the overall sample those who were employed had longer LTL than did those lacking employment, but LTL was not related to the men’s educational attainment or neighborhood deprivation (Batty et al., 2009). Among 382 Glasgow residents age 35-64 years, LTL was shorter in those with lower income (Shiels et al., 2011). However, while lower educational attainment was associated with shorter LTL among 448 male and female participants in the Whitehall study of British civil servants, neither current income nor current or pre-retirement occupational grade was associated with LTL (Steptoe et al., 2011). Similarly, among 4,441 British women aged 41-80 years, education was significantly related to LTL while social class was not (Surtees et al., 2011).
The above studies come from the UK, where health disparities are generally understood in relation to the occupational status of oneself and one’s family members. In the US, health dispa rities researchers have focused relatively more on differences in health status associated with race and ethnicity. Among racial and ethnic groups, African-Americans are at the highest risk for chronic stress, social disadvantage and discrimination as well as for chronic diseases and early mortality. Accordingly, blacks in the US might be expected to have shorter LTL than whites, but the opposite has been found in some studies. Zhu et al. (2011) found that black adolescents had longer LTL than whites, and among participants up to age 80 in the Bogalusa Heart Study and the Family Heart Study, Hunt et al. (2008) reported longer LTL among blacks compared with whites, adjusting for age and BMI. While Diez Roux et al. (2009) found no significant black-white differences in LTL in the MESA Study, the relatively small sample of 279 blacks and 182 whites with a wide age range (age 45-84) may have been underpowered to detect an effect.
Apart from racial/ethnic differences in LTL, it is also important to see if effects of SES differ by race/ethnicity. Given the history of discrimination and segregation in the US, race/ethnicity may moderate the impact of education on health. On the one hand, higher education has served as an important element in social mobility and blacks may show greater benefit of higher educational attainment. However, since discriminatory policies and practices may not only affect quality and amount of education, but may diminish the relative value that blacks obtain from a given level of education compared to whites, blacks may show fewer health benefits of higher education.
To address these novel issues, the current study tested associations of educational attainment and income with LTL among a large sample of black and white men and women age 70-79 years of age. We examined whether LTL is shorter among those with less educational attainment and with less income, whether these effects differ among blacks and whites, and lastly, if they are explained in part by differences in health behaviors.
METHODS
Data Source
The Health Aging and Body Composition (Health ABC) Study recruited black and white men and women age 70-79 in Pittsburgh, PA and Memphis, TN. Participants were eligible if they reported no difficulty walking ¼ mile or going up 10 steps without resting or performing basic activities of daily living. Participants were excluded if they reported active treatment for cancer in the prior three years, plans to move out of the study area in the next three years, or current participation in a randomized trial of a lifestyle intervention. Of the initial sample of 3075, stratified by gender and race, 2,740 (85%) had complete data and were included in the current analysis. The group excluded due to incomplete data did not vary significantly from the final sample in terms of age, sex, exercise, obesity, smoking, or history of heart disease, diabetes, lung disease, or arthritis, but did include a significantly higher proportion of blacks (47.9% vs. 40.5%, p < 0.01) and a lower proportion of people with a history of cancer (15.1% vs. 19.5 %, p < 0.02).
Measures
Outcome variable
Leukocyte telomere length (LTL)
LTL was assessed with quantitative polymerase chain reaction (Q-PCR). The ratio of telomere-repeat copy number to single-gene copy number (T/S ratio) was assessed in study samples by comparing them to a reference sample. The telomere length of the reference sample was obtained with the use of 64 DNA samples with known mean telomere restriction fragment (TRF) lengths. Then, each participant’s T/S ratio was calculated as the mean T/S ratio of three samples. This mean T/S ratio was then converted to LTL in base pairs by multiplying T/S ratio by the known telomere length of the reference DNA, 4,270bp. The slope of the linear regression line through a plot of T/S ratio (x axis) versus mean TRF length (y axis) is the number of base pairs of telomeric DNA corresponding to a single T/S unit. See Cawthon (2002) for more information on these methods. LTL was missing for 480 participants. Because there was some variability between assays, we controlled for telomere batch in all LTL analyses, constructing a dummy variable for each batch to enter into regression models, as done in previous analyses of LTL in the Health ABC sample (Njajou et al., 2009).
Predictor variables
Education
Participants reported the highest grade of education they had completed: less than high school, high school graduate, some post-high school education, and college graduate. A little over half (52.7%) had a high school degree or less.
Income
Participants reported their income within five categories (<10k, 10-25k, >25k and < 50k - <100k, 100k or more). 312 participants did not provide information on income and were excluded, leaving 2,287 participants (74.4% of original sample) for the income analysis. Income was dichotomized at $25k per year, with 52.1% reporting incomes of $25k or less per year.
Stratifying variables
Race and sex
These demographics were self-reported by subjects, 40.5% of the current sample was black and 51.6% was female.
Covariates
Smoking status
Participants indicated whether they currently smoked, previously smoked, or never smoked. The small number of participants who indicated that they currently smoked (10.3%) were combined with previous smokers, leading to groupings of ever smoked (56.5%) or never smoked (43.5%).
Body mass index
Obesity was derived from a body mass index (BMI) recorded during in-person visits. 25.6% of the sample were categorized as obese with a BMI (weight in kilograms divided by the squared value of height in meters) greater than 30.
Exercise
Exercise was measured as the number of kilocalories expended per kilogram of weight per week. Kilocalories (kcal) expended per week was assessed by asking subjects how many minutes per week they spent doing certain activities (e.g. walking, stair climbing, light housework), and assigning each activity a kcal/kg/hour based on previous literature (Ainsworth et al., 1993). Those kcal/kg/hour values for each activity were then summed to create an estimate of total amount of kcal/kg spent per week.
Health conditions
Participants reported presence or history of a range of diseases. This information was adjudicated using standardized algorithms considering various sources of information: self-report of a medical diagnosis, medication use, and results of screening tests from a clinical examination where appropriate. Five health conditions were used as control variables: arthritis, cancer, diabetes, heart disease (myocardial infarction, angina pectoris, congestive heart failure, leg claudication, transient ischemic attack, stroke, rheumatic heart disease, or hypertension), and lung disease (chronic bronchitis, emphysema, chronic obstructive pulmonary disease, and asthma).
Statistical Analysis
T-tests and chi-square tests were used to compare differences between those included and excluded from the final sample and to compare sample characteristics by race. Linear regressions were used to test the relationship between predictor variables and LTL. All models controlled for telomere assay batch (in order to adjust for inter-batch variability), and study city (Pittsburgh vs. Memphis), as well as presence or absence of each of the health conditions.
Using linear regression, we first regressed LTL on age, gender, and race (Model A), followed by education dichotomized as high school graduate or less versus some college or college graduate (Model B). Subsequent models tested moderation effects by adding interaction terms between education and the sociodemographic variables (Model C) and explored potential mediation by adding health behaviors as additional predictors (Model D). Following this, Models A and B were then repeated using income dichotomized at $25K per year in place of education. Telomere assay batch, site and chronic conditions were used as control variables for all models. All analyses were performed using Stata version 9.2.
RESULTS
The sample is described in Table l. Blacks were younger than whites, and more likely to be female, to be obese and to have arthritis, diabetes, heart disease and lung disease, though they were less likely to have had cancer. In addition, blacks were less likely than whites to have obtained post-high school education and to have incomes greater than $25,000/year. LTL was longer among blacks than whites.
TABLE 1.
Descriptive Statistics, by Race
| Characteristic | Black | White | p-value |
|---|---|---|---|
| N=2,599 | N=1,053 | N=1,546 | |
| Age, years, mean(SD) | 73.4 (2.9) | 73.7 (2.84) | 0.001 ** |
| Gender, % | |||
| Female | 57.8 | 47.4 | <0.001 ** |
| Male | 42.2 | 52.6 | |
| Hx of Cancer, % | 11.8 | 24.8 | <0.001 ** |
| Diabetes, % | 21.1 | 10.7 | <0.001 ** |
| Arthritis, % | 59.5 | 54.7 | 0.030 * |
| Lung Disease, % | 21.8 | 17.1 | 0.001 ** |
| Heart Disease, % | 71.7 | 55.9 | <0.001 ** |
| Education, % | |||
| <= High school | 69.8 | 40.6 | <0.001 ** |
| >= Some college | 30.2 | 59.4 | |
| Smoking, % | |||
| Ever smoked | 55.3 | 57.3 | 0.382 |
| Never smoked | 44.7 | 42.7 | |
| Obesity, % | 35.9 | 18.6 | <0.001 ** |
| Exercise, kcal/kg/week, mean (SD) |
80.1 (75.0) | 84.0 (63.8) | 0.127 |
| Income Greater than 25K, % | 25.2 | 63.5 | <0.001 ** |
| Telomere length, bp, mean(SD) | 4933 (1332) | 4815 (1374) | 0.044 * |
Notes: All continuous variables tested using t-tests. All dichotomous variables testing using chi-squared tests.
p<.05
p<.01
Education
Across the sample, individuals who had some education beyond high school had longer mean LTL than those with a high school education or less (4926 versus 4806 base pairs, respectively). Table 2 presents results of the multivariate models of LTL regressed on age, gender, race, education and the interaction of education and sociodemographic characteristics. Model B represents the test of our primary hypothesis that LTL will be longer among those with more education adjusting for age, gender, and race/ethnicity. This was confirmed; independent of age, gender, race, chronic conditions, study site, and LTL assay batch. LTL was significantly associated with education (B = 125, SE = 47.6, p = .009). Comparing Models A and B reveals that the association of race/ethnicity and LTL became stronger once education was entered into the model; the regression coefficient for race increased from 64 (SE = 49, p = .193) in the initial model to 98 (SE = 50.6, p = .053) in the model including education.
TABLE 2.
Linear Regressions Predicting Telomere Length (Interaction Term Analysis), Full Sample
| N=2,599 | Model A |
Model B |
Model C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef | SE | p | Coef | SE | p | Coef | SE | p |
| Age | −26.6 | 7.7 | 0.001 ** | −26.3 | 7.9 | 0.001 ** | −18.2 | 10.8 | 0.090 |
| Female | 270.5 | 46.3 | <0.001 ** | 277.3 | 46.3 | <0.001 ** | 259.5 | 63.2 | <0.001 ** |
| Black | 64.0 | 49.0 | 0.192 | 98.0 | 50.6 | 0.053 | 10.7 | 64.6 | 0.869 |
| Education > HS | - | - | - | 125.0 | 47.6 | 0.009 ** | 1193.3 | 1167.2 | 0.307 |
| Age × Education | - | - | - | - | - | - | −15.8 | 15.8 | 0.318 |
| Female × Education | - | - | - | - | - | - | 24.2 | 91.2 | 0.791 |
| Black × Education | - | - | - | - | - | - | 207.8 | 98.7 | 0.035 * |
Notes: All models adjusted for chronic conditions, study site, and telomere batch.
p<.05
p<.01
Race/ethnicity and education
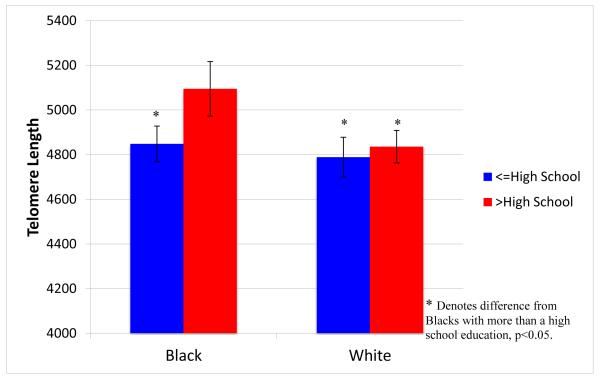
Model C in Table 2 revealed that while neither the association of age nor gender with LTL differed by race/ethnicity, the interaction of education and race/ethnicity was significant (B = 207.8, SE = 98.7, p = .035). As seen in Figure 1, this interaction arises from the stronger association of education with LTL among blacks than among whites. Blacks with more than a high school education had substantially longer LTL (mean 5107 basepairs, 95% confidence interval [CI] 4980-5324) than blacks with a high school education or less (mean 4859 basepairs, 95% CI 4776-4942). Although LTL was somewhat longer among whites who had post-high school education (mean 4829, 95% CI 4754-4904) than those who did not (mean 4795, 95% CI 4704-4887), the difference was not significant. This clarifies the finding from Models A and B that controlling for educational attainment increased the significance of the racial/ethnic differences in LTL. Proportionately fewer blacks than whites had obtained education beyond high school; it was only when there was an adjustment for the difference in educational levels that the longer LTL among blacks, especially those with more education, could be clearly seen.
FIGURE 1.
Age- and Gender-adjusted TL, by Race and Education*
*adjusted for site, batch, and chronic conditions
Graded association
In secondary analyses, we explored whether there was a graded association between LTL and four levels of education within each racial/ethnic group (see Supplemental Table 1). Among whites the mean LTL increased across each higher level of education, but there were no significant differences between educational levels. Blacks showed a different pattern, with increasing LTL with high school graduation and with some post high school education, but a small, insignificant dip in LTL among college graduates. Blacks with some post high school education had significantly longer LTL than did blacks who did not graduate from high school, as well as longer LTL than whites of all educational levels.
Health behaviors
We examined smoking, exercise and obesity as potential confounders and/or mediators of the relationship between education and LTL. As shown in Model C in Table 3, past and current smokers had shorter LTL than those who never smoked (B = −131.5, SE = 47.5, p = .006), while the associations of exercise and obesity with LTL were not significant. However, adjusting for smoking as well as for exercise and obesity did not substantially weaken the association between education and LTL (B = 119.7, SE = 47.6, p = .012).
TABLE 3.
Linear Regressions Predicting Telomere Length (Health Behavior Analysis), Full Sample
| N=2,599 | Model A |
Model B |
Model D |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef | SE | p | Coef | SE | p | Coef | SE | p |
| Age | −26.9 | 7.9 | 0.001 ** | −26.3 | 7.9 | 0.001 ** | −28.1 | 8.0 | <0.001 ** |
| Female | 270.5 | 46.3 | <0.001 ** | 277.3 | 46.3 | <0.001 ** | 244.1 | 48.2 | <0.001 ** |
| Black | 64.0 | 49.0 | 0.192 | 98.0 | 50.6 | 0.053 | 107.4 | 51.0 | 0.035 * |
| Education > HS | - | - | - | 125.0 | 47.6 | 0.009 ** | 119.7 | 47.6 | 0.012 * |
| Exercise | - | - | - | - | - | - | −0.1 | 0.3 | 0.717 |
| Obesity | - | - | - | - | - | - | −85.5 | 54.2 | 0.114 |
| Smoking | - | - | - | - | - | - | −131.5 | 47.5 | 0.006 ** |
Notes: All models adjusted for chronic conditions, study site, and telomere batch.
p<.05
p<.01
Income
Finally, we tested whether income was related to LTL in this sample by substituting income for education in the linear regression models. Controlling for age, gender and race/ethnicity, income was not significantly related to LTL (see Table 4; Model B). Since there was no main effect for income, interactions were not examined.
TABLE 4.
Linear Regressions Predicting Telomere Length (Interaction Term Analysis), Full Sample
| N=2,599 | Model A |
Model B |
Model C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef | SE | p | Coef | SE | p | Coef | SE | p |
| Age | −25.6 | 8.5 | 0.003 ** | −24.5 | 8.5 | 0.004 ** | −21.5 | 11.5 | 0.062 |
| Female | 296.5 | 49.7 | <0.001 ** | 309.3 | 50.3 | <0.001 ** | 301.5 | 69.2 | <0.001 ** |
| Black | 60.8 | 52.8 | 0.250 | 91.8 | 56.3 | 0.103 | 47.1 | 70.6 | 0.505 |
| Income > 25k | - | - | - | 85.8 | 53.9 | 0.111 | 453.0 | 1258.4 | 0.719 |
| Age × Income | - | - | - | - | - | - | −5.7 | 17.0 | 0.860 |
| Female × Income | - | - | - | - | - | - | 17.5 | 99.3 | 0.860 |
| Black × Income | - | - | - | - | - | - | 114.3 | 110.1 | 0.299 |
Notes: All models adjusted for chronic conditions, study site, and telomere batch.
p<.05
p<.01
DISCUSSION
Aristotle observed that “education is the best provision for old age.” The data reported here provide empirical evidence of life-long health benefits of educational attainment even in a long-lived population. More than forty years after their education was completed, men and women who obtained at least some education after high school had significantly longer LTL than those who had only a high school education or less. In contrast, the association of income with LTL was not significant.
The current findings are largely in line with the UK studies that found associations of LTL with education but not with income (Steptoe et al., 2011; Surtees et al., 2011). However, although, education was significantly associated with LTL controlling for age, gender, and race across the whole sample, results differed within racial/ethnic groups. Education was significantly related to LTL among blacks while whites showed only small, non-significant increases in LTL at each higher level of education. Cross-national differences and cohort effects could contribute to differences in findings between whites in Health ABC and British civil servants in the Whitehall studies for whom education was linked to LTL. Education has played an increasingly powerful role in societal stratification for all groups in the US over the past few decades, and may have had less impact on life circumstances among the older white participants in Health ABC compared to the relatively younger British civil servants. In contrast, for reasons discussed below these dynamics may have been somewhat different for blacks.
The finding that, for the sample as a whole, education but not income was related to LTL, is consistent with the view that educational attainment is the most fundamental indicator of SES. Education provides opportunities for social mobility and is a major determinant of future occupation and earnings and overall social status across a person’s lifetime. Education also provides access to social networks and to resources that may reduce exposure to chronic stress and/or increase one’s ability to deal effectively with potential threats or stressors. Numerous studies have now linked chronic stress exposure in humans with accelerated cellular aging as measured by LTL (Epel et al., 2004; Damjanovic et al., 2007; Kotrschal et al., 2007; Puterman et al., 2010). In addition, LTL has been linked to appraisals of exaggerated threat in response to a standardized stressor (O’Donovan et al., 2012). Psychosocial resources available to individuals with more education may reduce the severity of such appraisals (Pavlova and Silbereisen, 2012). Reduced threat appraisals may have particular benefit in individuals who encounter more life stress and this may be especially helpful to blacks who are relatively more likely than whites to experience chronic stress as the result of discrimination and barriers to achieving higher social standing in the US (Geronimous et al., 2006).
The finding that education but not income was related to LTL could also reflect greater error in the measurement of income. Education can be measured with greater precision since people are more willing and able to report years of schooling and highest degree than they are to report their income. In addition, concerns about reporting income results in more missing data on income than on education, as was the case in the present study. Such concerns also lead to imprecision in the measurement of income since it is asked using ranges instead of specific amounts. Moreover, income can vary across the lifespan, as can the number of people in a household who are dependent on it which affects the “value” of any particular income. In contrast, educational attainment is relatively stable across adult life, affording it greater potential to influence factors linked to the cumulative process of cellular aging.
The finding that black-white differences became significant only after adjusting for educational attainment may help explain conflicting findings on racial/ethnic differences in LTL in the literature. If blacks on average have longer LTL than whites and have lower educational attainment, the former could be masked unless the latter is taken into account. Thus, findings on racial differences in LTL will depend partly on the range of SES of the sample, and whether it is adjusted for in analyses. Studies that aim to understand race-related health disparities need to include assessment of education. More generally, assessment of multiple indicators of SES, in interaction with race, can better identify the complex social factors that shape disparities in aging (Adler, 2011).
In this cohort, blacks did not simply benefit to the same extent as did whites from high school graduation, but had significantly longer LTL in late life than similarly educated whites. This finding is consistent with research showing a greater difference in life expectancy among blacks than among whites between those with low and high education (Crimmins and Saito, 2001). It is also consistent with findings of a reversal in survival of blacks and whites in older adults. Even though this reversal has not emerged in mortality rates among Health ABC participants to date, other studies have found that mortality rates among African-Americans are lower than those of European-Americans in old age (Kim and Miech, 2009; Johnson, 2000; Wing et al., 1985). The reasons for this “mortality cross-over” are not well understood. Since telomere shortening occurs in advance of disease and mortality, identifying protective factors that are associated with longer LTL in blacks, may shed light on the dynamics of the mortality cross-over. These findings from the Health ABC cohort suggest education as a key factor in the US and point to the importance of gaining a better understanding of how education may act to buffer aging at the basic level of the cell.
Theories of relative status may help explain the finding that blacks benefitted more from post high-school education than did whites (Adler and Snibbe, 2003; Marmot, 2006). Since relatively fewer blacks than whites obtain higher education (Leventhal-Weiner and Wallace, 2011), and this was even more the case in the era in which Health ABC participants were educated, blacks who had post high school education would likely have enjoyed higher status within the black community than would comparably-educated whites within their communities. Relative status within one’s own community may translate into better health, including longer LTL. This differential benefit for more educated blacks could account for the finding that they had longer LTL than any other group.
Strengths and limitations
This study is the first to examine the relationship of both SES and race/ethnicity with telomere length in a large US sample. The Health ABC study has a number of strengths, including a well-characterized representative sample of healthy older adults from two communities. However, the sample and the inclusion criteria may introduce some bias. Starting with an older sample introduces selection bias since those with less education and income are more likely to die before age 70, the lowest age of inclusion in the sample. Eliminating those in poorer health may have further skewed the sample towards those with longer LTL as well as more education and higher income. This could have truncated the variation in both predictor and outcome variables and made it more difficult to find a significant association between LTL and education and income and may explain, in part, why whites did not show a significant benefit from post high school education.
As with all studies linking education and LTL, differential selection associated with educational attainment at younger ages could be an alternative explanation for this association. Individuals who attained higher education or failed to do so may have had attributes or experiences that also affected their health in later life. Although the effect of education remained significant when adjusted for risk behaviors, such as smoking, which are known also to be associated with both LTL and educational attainment, other factors such as early neglect or trauma exposure, which could affect academic performance and shorten LTL (O’Donovan et al., 2011b; Tyrka et al., 2010) were not assessed.
CONCLUSION
In this sample of healthy older adults, higher levels of educational attainment but not income were associated with longer LTL, which confers reduced risk for multiple diseases of aging. While LTL was somewhat longer among whites with more education, the effects were not significant. In contrast, blacks who obtained some education beyond high school had significantly longer LTL than any other group. These findings may shed light on the mortality cross-over between blacks and whites in later life and point to the importance of assessing both education and race in studies of telomere length and diseases of aging.
Supplementary Material
Highlights.
This study demonstrates the positive impact of educational attainment on leukocyte telomere length many years later, especially for African-Americans.
ACKNOWLEDGEMENTS
The authors thank the Health ABC Study staff at the Pittsburgh and Memphis study sites and at the UCSF Coordinating Center, as well as the Health ABC Study participants for their time and efforts. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. The study was conducted on behalf of the Health, Aging and Body Composition (Health ABC) Study and was funded National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Current Directions in Psychological Science. 2003;12:119–123. [Google Scholar]
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann. N. Y. Acad. Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Adler NE. Cell aging and social disadvantage: perspectives on mechanisms underlying health disparities from “across the pond”. Brain Behav. Immun. 2011;25:1290–1291. doi: 10.1016/j.bbi.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports. Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, Samani NJ, Ford I. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. J. Epidemiol. Community Health. 2009;63:839–41. doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am. J. Public. Health. 2010;100(Suppl 1):S186–196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention CDC health disparities and inequalities report - United States, 2011. Morbidity and Mortality Weekly Report. 2011;60:1–116. [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970-1990: gender, racial, and educational differences. Soc. Sci. Med. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J. Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8:251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am. J. Community. Psychol. 2007;40:313–332. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J. Health Soc. Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NE. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37:267–283. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom. Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Miech R. The Black-White difference in age trajectories of functional health over the life course. Soc. Sci. Med. 2009;68:717–725. doi: 10.1016/j.socscimed.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biol. Lett. 2007;3:128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal-Weiner R, Wallace M. Racial differences in high school dropout rates: an analysis of US metropolitan areas. Research in Social Stratification and Mobility. 2011;29:393–413. [Google Scholar]
- Marmot M. Status syndrome: a challenge to medicine. JAMA. 2006;295:1304–1307. doi: 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann. N. Y. Acad. Sci. 2010;1186:146–173. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM, Health ABC Study Association between telomere length, specific causes of death, and years of healthy life in Health, Aging, and Body Composition, a population-based cohort study. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS one. 2011a;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel ES, Lin J, Wolkowitz O, Cohen BE, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma is associated with short leukocyte telomere length in posttraumatic stress disorder. Biol. Psychiatry. 2011b;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama J, Lin J, Puterman E, Adler N, Kemeny M, Wolkowitz O, Blackburn E, Epel E. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behavior and Immunity. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova MK, Silbereisen RK. Perceived level and appraisal of the growing expectations for active ageing among young-old in Germany. Research on Aging. 2012;34:80–99. [Google Scholar]
- Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PloS ONE. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, MacIntyre A, Johnson PCD, Batty D, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, McGinty A, McLean JS, Millar K, Sattar N, Tannahill C, Velupillai YN, Packard CJ. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid Cohort. PLoS ONE. 2011;6:e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979-2003. Int. J. Epidemiol. 2006;35:903–919. doi: 10.1093/ije/dyl089. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimäki M, Marmot M, Blackburn E, Erusalimsky JD. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav. Immun. 2011;25:1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Stampfer MJ, Franks PW, Manson JE, Rexrode KM. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS One. 2012;7:e38374. doi: 10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Educational attainment and mean leukocyte telomere length in women in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Brain Behav. Immun. 2011;26:414–418. doi: 10.1016/j.bbi.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao H, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol. Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wing S, Manton KG, Stallard E, Hames CG, Tryoler HA. The black/white mortality crossover: investigation in a community-based study. J. Gerontol. 1985;40:78–84. doi: 10.1093/geronj/40.1.78. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, Stallmann-Jorgensen I, Mooken G, Bundy V, Snieder H, van der Harst P, Dong Y. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. The Journal of Pediatrics. 2011;158:215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.