Abstract
Urinary neutrophil gelatinase-associated lipocalin (uNGAL) is known to predict the prolonged delayed graft function after kidney transplantation. We examined the relation of uNGAL with histological findings of acute tubular injury (ATI). Analyses were made in biopsies taken at 6 weeks, 3 months, and 6 months after kidney transplantation. uNGAL was measured in the spot urines, normalized to urinary creatinine excretion, and correlated to biopsy findings and clinical, laboratory, and demographic variables. Controls included healthy individuals, individuals after kidney donation and ICU patients with acute kidney failure. Renal transplant recipients without ATI did not display elevated uNGAL levels compared to the healthy controls. Transplant patients with ATI had a higher uNGAL excretion at 6 weeks than patients without ATI (27,435 versus 13,605 ng/g; P = 0.031). This increase in uNGAL was minor compared to ICU patients with acute renal failure (2.05 × 106 ng/g). Patients with repeated findings of ATI or severe ATI did not have higher urinary NGAL levels compared to those with only one ATI finding or moderate ATI. Female recipient gender and urinary tract infection were identified as potential confounders. uNGAL has a relation with histological signs of acute tubular injury. The usability of this biomarker in renal allograft recipients is limited because of the low sensitivity.
1. Introduction
Acute kidney injury early after transplantation may arise from the donor's condition, prolonged cold ischemia time, and early posttransplant injuries like rejection and drug toxicity. Immediate clinical correlates of this injury may be delayed graft function or a less-than-optimal glomerular filtration rate. However, acute kidney injury carries adverse long-term consequences as patients with acute tubular injury early posttransplantation have an inferior long-term allograft function [1]. Therefore, markers, which facilitate early diagnosis of acute kidney injury or even provide prognostic information, would be helpful in the posttransplant care of these patients.
Traditionally, serum parameters as creatinine and urine output are used to monitor kidney function in transplanted patients. However, a rise in serum creatinine already implies a significant amount of kidney damage, which limits its ability to detect impaired graft function at early stages. Recently, novel biomarkers such as Kim-1, IL-18, and neutrophil gelatinase-associated lipocalin (NGAL) have been proven useful in detecting nontransplant acute kidney damage [2]. NGAL, a 25-kDa protein, is a member of the lipocalin family, which is expressed in many human tissues, including the kidney. In children and adults with cardiopulmonary surgery, NGAL was specifically and early elevated in those patients that developed acute renal failure a few days after surgery [3]. Parikh et al. reported that urinary NGAL could be an early predictor of DGF after kidney transplantation [4]. In a previous study by Mishra et al. with immunochemical staining of renal allografts, a correlation was established between an increased expression of NGAL and prolonged cold ischemia time, elevated serum creatinine levels, and dialysis requirement [5]. In another study, urinary NGAL was more accurate in predicting dialysis within one week of transplantation than serum creatinine [6].
Acute kidney injury primarily affects renal tubules and NGAL is produced in injured tubular epithelial cells. Therefore, we addressed the question whether urinary NGAL correlates with histologically confirmed acute tubular injury using protocol biopsies taken within the first six months after transplantation.
2. Materials and Methods
2.1. Study Population and Sample Collection
The study population consisted of 140 adult kidney transplant recipients randomly chosen from our protocol biopsy archive (n = 35 patients who never had any signs of ATI and n = 105 patients with at least one finding of ATI during the protocol biopsy program). Controls included 9 healthy individuals (2-kidney controls), 13 individuals after kidney donation (1-kidney control), and 5 ICU patients with acute kidney failure. Renal protocol biopsies are regularly performed 6 weeks and 3 and 6 months after kidney transplantation in the transplant center of the Medical School of Hannover since 2001. All patients are eligible for the protocol biopsies unless certain medical conditions like bleeding disorders and other comorbidities advice against a biopsy. In addition to these regular protocol biopsies, biopsies for cause are performed in case of unclear allograft impairment. Only data of patients undergoing regular protocol biopsies were included in this study. Demographic and clinical data of patients participating in this program were collected prior to and at the time of transplantation. The demographics for patients with and without ATI are depicted in Table 1. For patients without ATI n = 23 samples were taken at 6 weeks, n = 17 at 3 months, and n = 16 at 6 months after transplantation. For patients with signs of ATI n = 44 samples were taken at 6 weeks, n = 3 samples at 3 months, and n = 55 samples at 6 months. After transplantation, clinical data and routine laboratory results were collected corresponding to the time points of the three protocol biopsies. Spot urine was frozen in −80°C at time of the biopsy. Biopsies were evaluated according to the updated Banff classification [7]. Data and sample collections were performed with an informed consent of the patients and with the approval of the ethics board of the Medical School Hannover.
Table 1.
Demographic and clinical data of patients with and without ATI.
| ATI | ATI+ | All patients | P value | |
|---|---|---|---|---|
| N = 35 | N = 105 | N = 140 | ||
| Recipient | ||||
| Age (years) | 49 ± 12 (49,20–69) | 51 ± 12 (52,25–73) | 51 ± 12 (50,20–73) | |
| Gender (% m/f) | 65,7/34,3 | 61,9/38,1 | 62,9/37,1 | |
| Retransplanted pat. (%) | 8,6 | 10,5 | 10,0 | |
| Preformed antibodies neg. (%) | 94,3 | 93,3 | 93,5 | |
| Lowest s-creatinine within the first 6 weeks of tx | 160 ± 83 | 182 ± 87 | 176 ± 86 | |
| Hypercholesteroemia before tx (%) | 31,4 | 37,1 | 35,7 | |
| Arterial hypertension before tx (%) | 100 | 96,2 | 97,1 | |
| Donor | ||||
| Age (years) | 47 ± 15 (50,7−70) | 51 ± 13 (52,16–77) | 50 ± 14 (51,7–77) | |
| Gender (% m/f) | 60/40 | 53,3/46,7 | 55/45 | |
| Type | ||||
| Deceased (%) | 82,9 | 95,2 | 92,1 | 0.029 |
| Living (%) | 17,1 | 4,8 | 7,9 | |
| CMV IgG positive (%) | 60 | 53,8 | 55,4 | |
| s-creatinine (μmol/L) | 95 ± 36 | 93 ± 44 | 93 ± 42 | |
| Graft factors at tx | ||||
| Cold ischemia time (h) | 14 ± 7 | 17 ± 7 | 17 ± 7 | 0.032 |
| Mean number of HLA mismatches | 2,26 ± 1,6 | 2,4 ± 1,72 | 2,36 ± 1,68 | |
| Initial function of the graft (%) | 68,6 | 28,6 | 38,6 | 0.000 |
| Need of dialysis following tx (%) | 31,4 | 71,4 | 62,1 | 0.000 |
| Immunosuppressive regimen (%) | ||||
| CyA-MMF-Steroids-IL2 | 37,1 | 31 | 32,6 | |
| CyA-MMF-Steroids-ATG | 0 | 1 | 0,7 | |
| CyA-MMF-Steroids-no induction | 0 | 3 | 2,2 | |
| CyA-MMF-IL2 | 14,3 | 31 | 26,7 | |
| CyA-MMF-ATG | 0 | 1 | 0,7 | |
| Other, IL2 | 28,6 | 14 | 17,8 | |
| Other, ATG | 11,4 | 7 | 8,1 | |
| Other, no induction | 8,6 | 12 | 11,1 |
ATI−: patients without ATI; ATI+: patients with ATI; tx: transplantation; median and range of values in brackets; CyA: cyclosporine A; MMF: mycophenolate mofetil; ATG: antithymocyte globulin; IL2: interleukin 2 receptor antagonist.
2.2. NGAL Detection
Urinary NGAL was measured in the spot urine using the Quantikine human lipocalin-2/NGAL Immunoassay (Cat.no. DLCN 20 by R&D Systems), according to the manufacturer's protocol. Urinary NGAL excretion was normalized to urinary creatinine excretion to correct for differences in NGAL due to urine dilution. Urinary NGAL excretion is presented as the amount of urinary NGAL in ng per g of urine creatinine.
2.3. Statistical Analyses
Descriptive data were reported as medians with ranges. Comparisons of categorical data between groups were performed with Fisher's exact test and the chi-square test for two or more samples. Numerical data were compared with the Kruskal-Wallis test and the Mann-Whitney test. Linear regression analyses were performed with log transformed NGAL values as the dependent variable and the demographical, clinical, and laboratory factors that had been significantly different in univariate analyses. Variables from the univariate analyses were separately examined for the samples at 6 weeks and 6 months using the backward selection. SPSS statistical software package version 18.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Statistical significance was assumed for P < 0.05. Note that for better visualisation the logarithm to base 10 was used in all figures with NGAL results, because these values were highly variable and not normally distributed.
3. Results
3.1. uNGAL Excretion in Patients after Kidney Transplantation Remains Constant over Time and Is Comparable to Healthy Controls
First we compared the uNGAL excretion levels of renal allograft recipients without ATI to different control groups. There was no significant difference in uNGAL excretion in transplanted patients versus healthy individuals with 1 kidney (i.e., kidney donors) and healthy individuals with 2 kidneys (Figure 1). In nontransplanted patients with acute renal failure, the NGAL/creatinine ratio was significantly higher 2.0490e + 006 ng/g (382498 to 1.0470e + 007) compared to healthy individuals with one or two kidneys and to transplanted patients without signs of ATI in the biopsies up to 6 months after transplantation (P < 0.001). Subsequent analyses represent a cross-sectional study of samples where not all patients provided urine samples at every time point, showing no time-associated differences in samples taken at 6 weeks, 3 months, and 6 months after transplantation (Figure 2(a)). Figure 2(b) depicts the dynamic change of uNGAL excretion at 6 weeks, 3 months and 6 months after transplantation in individual patients without ATI.
Figure 1.
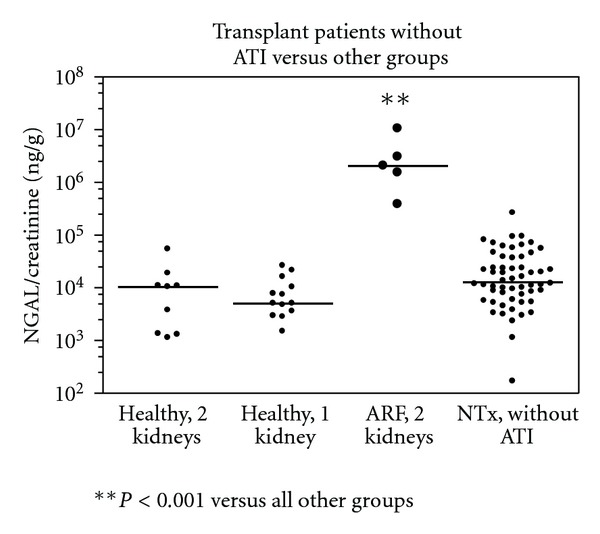
Urinary NGAL/creatinine ratios in transplanted patients versus other groups. Shown are the urinary NGAL/creatinine levels of the two control groups (healthy individuals with 2 (n = 9) or 1 kidney (n = 13)) versus ICU patients with acute renal failure (ARF, 2 kidney (n = 5)) and transplanted patients without ATI (n = 56). Patients with acute renal failure had significantly higher urinary NGAL levels compared to the other groups (P < 0.001).
Figure 2.
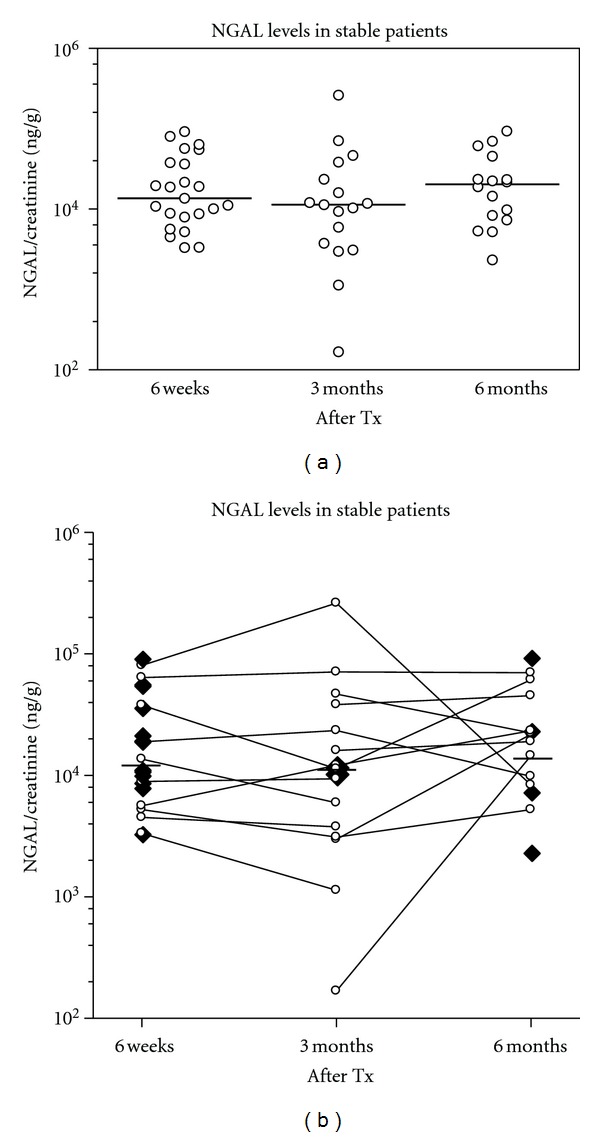
(a) NGAL excretion in stable patients without ATI at 6 weeks, 3 months, and 6 months after transplantation. Shown is the urinary NGAL/creatinine ratio at 6 weeks (n = 23), 3 months (n = 17), and 6 months (n = 16) in patients after kidney transplantation. (b) Individual course of NGAL excretion of the stable patients at 6 weeks, 3 months, and 6 months after transplantation. Depicted are single measurements (n = 13 at 6 weeks, n = 2 at 3 months, n = 4 at 6 months) as single black squares and serial measurements (6 weeks to 3 months: n = 4 patients, 3 months to 6 months: n = 5 patients, and 6 weeks to 3 months and 6 months: n = 6 patients) as open circles connected by lines. Total measurements at 6 weeks: n = 23, at 3 months: n = 17, and 6 months: n = 16.
3.2. uNGAL Is Not Sensitive to Detect ATI in Transplanted Patients
Next, we tested the sensitivity of urinary NGAL as a biomarker to indicate ATI in transplanted patients. At six weeks, patients with ATI had urinary NGAL values that were two-fold higher than the values from patients without ATI in the biopsies, yet there was a considerable overlap of values between the two groups. At 6 months, no differences between patients with and without ATI were apparent (Figure 3(a)). Figure 3(b) depicts the dynamic change of uNGAL excretion in patients with ATI. Multiple findings of ATI did not correlate with higher uNGAL levels (Figure 4(a)). Moreover, the extent of ATI, whether diffuse or focal, did not cause a difference of the NGAL ratios (Figure 4(b)).
Figure 3.
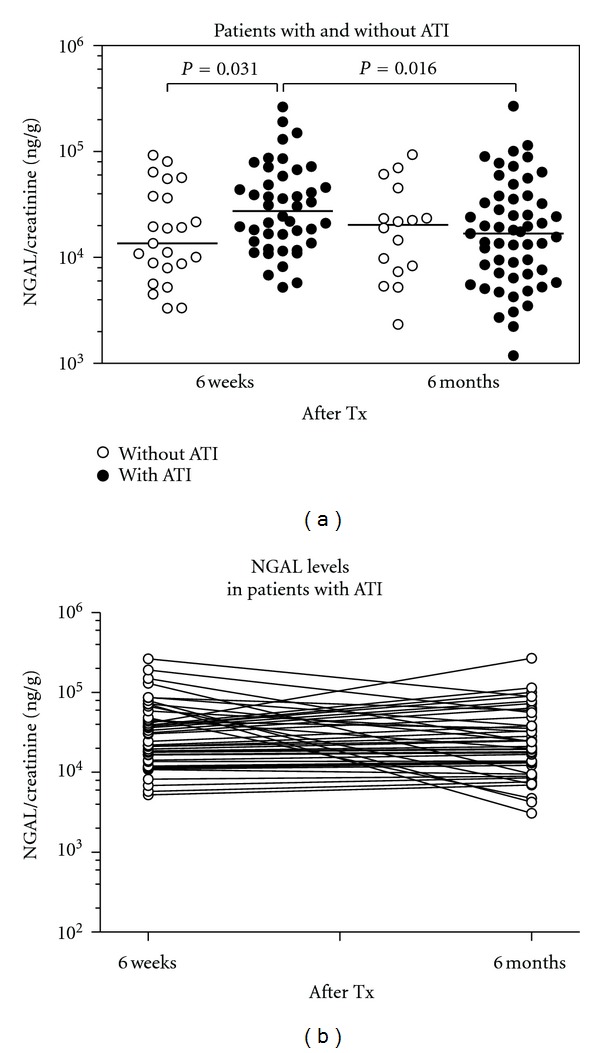
(a) Comparison of urinary NGAL/creatinine ratios in patients with and without ATI after kidney transplantation. Patients with ATI 6 weeks (n = 44) after transplantation have significantly higher urinary NGAL levels than patients without ATI (n = 23). In patients with ATI, the urinary NGAL excretion at 6 months (n = 55) is significantly lower than at 6 weeks. No difference in uNGAL ratios was seen between patients with and without ATI at 6 months. (b) Individual course of NGAL levels in patients with ATI at 6 weeks and 6 months after transplantation. Patients with singular measurement are presented by single values only (dots; n = 11 patients for the 6-month measurements). Serial measurements: n = 44 patients for 6 weeks and 6 months (connected by lines).
Figure 4.
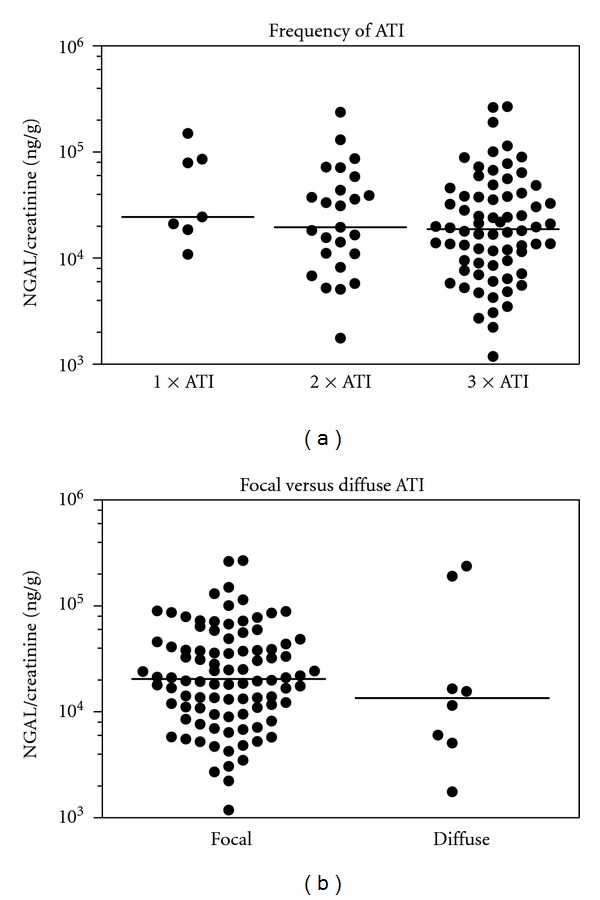
(a) Urinary NGAL/creatinine ratio depending on the frequency of ATI. Shown is the urinary NGAL/creatinine ratio depending on whether patients had 1 time (n = 7), 2 times (n = 25), or 3 times (n = 70) findings of ATI in biopsies taken while being followed in the biopsy program. (b) Urinary NGAL/creatinine ratio in Tx patients with focal versus diffuse ATI. No significant differences were found in the urinary NGAL excretion of patients with focal (n = 94) versus diffuse (n = 8) ATI findings after Tx.
3.3. Association of NGAL Levels with Clinical Variables and Exploration of Potential Confounders
In an explorative analysis, we correlated NGAL levels to allograft damage (e.g., acute tubular injury, delayed graft function, donor age) and to additional clinical, laboratory, and demographic variables (for a complete list see Supplementary Materials available online at doi:10.1155/2012/563404). The main findings of the univariate analysis are depicted in Table 2. Female recipient gender was associated with significant higher NGAL ratios at 6 weeks and 6 months after transplantation. Regarding donor gender, a numerical trend towards higher NGAL values for allografts from female donors became apparent at 6 months after transplantation. A lower GFR at the time of sampling was weakly associated with higher NGAL ratios at 6 months. Furthermore, we analyzed whether acute impairment of the allograft function at the time of biopsy had any relation to NGAL levels. Serum creatinine levels at the day of biopsy showed changes relative to the baseline values before the biopsy from −29 to 129% in patients without acute tubular injury and from −40 to 60% in patients with acute tubular injury. However, a correlation of the change in creatinine with uNGAL excretion could not be established for these patients (r 2 ≤ 0.1; data not shown).
Table 2.
Urinary NGAL levels (NGAL/creatinine; (ng/g)), and associations with various clinical, laboratory and demographic variables. Results are separately reported for the 6-week and 6-month samples. The remaining variables which were tested without significant differences are reported in the Supplementary Materials.
| 6 weeks | P value | 6 months | P value | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Donor gender | 18,875 (n = 47) |
23,910 (n = 37) |
0.31 | 12,264 (n = 35) |
21,785 (n = 37) |
0.071 |
| Recipient gender | 18,242 (n = 54) |
37,855 (n = 30) |
0.011 | 11,491 (n = 44) |
32,613 (n = 28) |
0.000 |
| GFRa | R = −0.204 | 0.066 | R = −0.321 | 0.006 | ||
|
| ||||||
| 6 weeks | P value | 6 months | P value | |||
| Not present | Present | Not present | Present | |||
|
| ||||||
| Delayed graft function | 18,875 (n = 13) | 21,315 (n = 71) |
0.218 | 17,554 (n = 45) |
19,354 (n = 27) |
0.866 |
| Isometric vacuolizationb | 19,187 (n = 61) | 31,224 (n = 23) |
0.031 | 22,436 (n = 47) |
9,480 (n = 25) |
0.008 |
| cGrade >0b | 19,031 (n = 70) | 39,575 (n = 14) |
0.033 | 14,218 (n = 44) |
21,115 (n = 28) |
0.583 |
| Urinary tract infection at the time of sampling | 17,901 (n = 63) | 50,548 (n = 14) |
0.000 | 13,760 (n = 56) |
64,889 (n = 6) |
0.014 |
| Panel reactive antibodies at transplantation > 0% | 22,952 (n = 76) |
11,696 (n = 7) |
0.019 | 17,194 (n = 66) |
33,929 (n = 6) |
0.342 |
| Blood transfusion before transplantation | 19,530 (n = 57) |
25,876 (n = 18) |
0.449 | 13,286 (n = 47) |
30,322 (n = 18) |
0.022 |
| MMF in the initial immunosuppression | 27,842 (n = 31) |
19,187 (n = 53) |
0.245 | 24,208 (n = 32) |
17,875 (n = 40) |
0.019 |
| Coronary heart disease before transplantation | 16,502 (n = 70) |
21,822 (n = 14) |
0.116 | 19,354 (n = 61) |
8,969 (n = 11) |
0.043 |
aGFR calculated according to the Cockroft and Gault formula at the time of sampling, baccording to the BANFF classification, at the time of sampling.
Isometric vacuolization and prevalence of chronic graft changes (cGrade>0) according to the current BANFF classification at 6 weeks after transplantation were associated with significantly higher uNGAL levels. However, after 6 months, uNGAL ratios were lower in patients with isometric vacuolization. In addition, patients with urinary tract infection at the time of sampling had significantly higher uNGAL ratios at 6 weeks as well as 6 months (Table 2).
Next, linear regression analyses were performed with log transformed NGAL values to determine the contribution of the identified variables of the explorative univariate analysis to the observed NGAL levels. Significant variables from the univariate analyses were separately examined for the samples at 6 weeks and 6 months using the backward selection. For the 6-week samples (Table 3(a)), presence of urinary tract infection and isometric vacuolization of tubular epithelial cells in the concomitant biopsy were associated with higher NGAL levels (r 2 = 0.296; unadjusted R Square of model fit). Recipient gender was not a significant factor in the regression model at 6 weeks (r 2 = 0.138; P = 0.131). In the 6-month samples (Table 3(b)), urinary tract infection, female recipient gender, and absence of isometric vacuolization were associated with higher NGAL levels (r 2 = 0.357).
Table 3.
Linear regression analysis of significant correlates to uNGAL excretion.
(a) Samples from 6 weeks.
| Model component | Regression coefficient | SE | P value | Tolerance |
|---|---|---|---|---|
| Constant | 5.029 | 0.224 | <0.001 | |
| Urinary tract infection | 0.519 | 0.110 | <0.001 | 0.980 |
| Isometric vacuolization | 0.272 | 0.091 | 0.004 | 1.0 |
(b) Samples from 6 months.
| Model component | Regression coefficient | SE | P value | Tolerance |
|---|---|---|---|---|
| Constant | 4.364 | 0.338 | <0.001 | |
| Urinary tract infection | 0.335 | 0.151 | 0.03 | 0.967 |
| Female recipient | 0.427 | 0.097 | <0.001 | 0.970 |
| Isometric vacuolization | −0.293 | 0.099 | 0.004 | 0.994 |
SE: standard error of the regression coefficient; tolerance indicates collinearity between the variables of mode I (1.0 = no collinearity). Calculated GFR's are based on the serum creatinine and Cockroft and Gault formula and square roots of GFR values are used for the analysis.
Since the univariate analysis indicated a relation between donor and recipient gender and NGAL levels, the possible gender combinations are additionally presented (Table 4). Significant differences were observed between the male donor/male recipient group compared to all other possible combinations, except the female/male combination at six weeks (P = 0.20).
Table 4.
Correlation of uNGAL ratios to the different donor/recipient gender combinations.
| Gender combinations donor/recipient |
NGAL in 6-week samplesa (ng/g) | NGAL in 6-month samplesb (ng/g) |
|---|---|---|
| Male/male | 14,259 | 5,670 |
| n = 28 | n = 20 | |
| Male/female | 37,717c | 32,893e |
| n = 19 | n = 15 | |
| Female/female | 37,993d | 24,050e |
| n = 11 | n = 13 | |
| Female/male | 21,654 | 18,454f |
| n = 26 | n = 24 |
Urinary NGAL/creatinine ratios at 6 weeks and 6 months after transplantation according to the four combinations of donor and recipient gender. At both time points, differences between the four combinations were significant (a P = 0.041 and b P < 0.001; the Kruskal Wallis test). Posttesting identified differences to the male donor/male recipient group (c P = 0.035; d P = 0.017; e P < 0.001; f P = 0.002).
4. Discussion
Acute tubular injury (ATI) is a frequent finding in biopsies of renal allograft recipients and is linked to an inferior long-term allograft function [1]. In this study, we tested whether urinary NGAL (uNGAL) may be applicable as a sensitive and noninvasive biomarker to monitor ATI in renal allograft recipients.
NGAL can be produced locally in the kidney by injured tubular cells and can be secreted by activated neutrophils/macrophages or inflamed vasculature [8]. In a recent experimental study, Paragas et al. demonstrate that uNGAL originates predominately from the kidney. This group generated an NGAL reporter mouse, which showed a close correlation between tubular stress and uNGAL [9]. Thus, uNGAL levels could reflect kidney damage in real time, as discussed by Mori and Nakao [10]. In animal models, NGAL has shown to be the most upregulated gene and overexpressed protein with a high predictive value for acute kidney injury [11–13].
We established the baseline uNGAL excretion in our transplanted patient cohort without findings of ATI. uNGAL levels in this group were comparable to healthy controls and remained stable 6 weeks, 3 months, and 6 months after transplantation. As proper controls for patients with a renal allograft, we collected sample material from healthy individuals with one kidney (donors with normal kidney function), in addition to healthy controls with 2 kidneys. Our observation of a tendency towards a higher NGAL excretion in healthy individuals with 2 kidneys versus 1 kidney may point to a confounding effect by the kidney mass. This finding should be confirmed with a larger cohort.
In patients with ATI, median uNGAL ratios were significantly higher at 6 weeks after transplantation than in patients without signs of ATI. While these findings may indicate a correlation of uNGAL excretion to ATI in patients early after transplantation, we could not demonstrate increased uNGAL levels in patients with ATI at 6 months after transplantation. On the contrary, median uNGAL levels in patients with ATI were even lower at 6 months compared to the controls without ATI. Further analyses showed no differences of NGAL excretion depending on the frequency of ATI or the distinction between diffuse versus focal ATI. NGAL levels were weakly inversely correlated to the eGFR; however, no link could be established between uNGAL levels and acute rises in serum creatinine at the time of ATI diagnosis. Urinary NGAL levels have been shown to correlate with histopathological alterations in IgAN patients [14]. Tubulointerstitial lesions in these patients were characterized by tubular atrophy, interstitial fibrosis, and inflammatory cell infiltration. Acute tubular injury in our transplanted patients differed from these features as it was defined by epithelial swelling with lucency of the cytoplasm, loss of brush border, and/or luminal dilatation with flattening of the epithelium and cytoplasmatic vacuolization [1]. Furthermore, even though a 2-fold increase in NGAL was present in our patients at 6 weeks, the substantial overlap with values from patients without ATI precludes the use of NGAL as a sensitive marker for kidney injury in transplant patients. Hollmen et al. found that high urinary NGAL levels until day 14 after transplantation in patients with early graft function (EGF) were associated with worse kidney function at 3 weeks, but not at 3 months or 1 year after kidney transplantation [15]. Most studies validating NGAL as a marker for allograft function emphasize its predictive value first and foremost in early urine or serum samples, up to 2 weeks after transplantation [4, 5]. NGAL showed to be an early marker for AKI and subsequent dialysis or DGF in transplanted patients by means of immuneohistochemical staining, urinary excretion levels, and serum levels [5, 6, 15, 16]. In our patient population with ATI findings, a considerable proportion (68.8%) had delayed graft function and need for posttransplantation, yet this was not reflected by a relevantly elevated urinary NGAL value at 6 weeks posttransplantation. Further, in patients with stable graft function, subclinical tubulitis was associated with higher NGAL levels [17]. In our regression analyses, uNGAL ratios correlated significantly with isometric vacuolization 6 weeks after transplantation, which could be related to immunosuppressant toxicity and damage [18], whereas isometrical vacuolization at 6 months was associated with lower NGAL levels. This raises the question whether uNGAL ratios in transplanted patients really reflect the ongoing tubular damage or whether NGAL may be more of an indicator for regenerative capacities of the kidney.
Plasma NGAL measurements can be influenced by numerous clinical variables, such as hypertension [19], systemic infections [20], age [21], and a higher validity in children. In our linear regression analyses, we confirmed that in patients with concomitant urinary tract infection at 6 weeks and at 6 months posttransplantation uNGAL ratios were significantly higher. This could be related to the leukocytes present in the urine as shown by Decavele et al. who demonstrated a correlation of one urinary leukocyte to an increase of 12 pg of uNGAL [22]. One other important but unexpected finding of our study was that female patients with grafts from female kidney donors as well as female graft recipients with male or female donors had higher uNGAL ratios compared to male recipients with kidneys from male donors. Further parameters such as coronary heart disease and blood transfusion before transplantation could be correlated to higher NGAL levels after 6 months but not at 6 weeks after transplantation. These variables showed no significant association to uNGAL levels in subsequent multivariate analyses but may indicate other potential confounders in transplanted patients.
The limitations to our study are that we present a single-centre study and that urine samples were stored at −80°C for more than a year and some protein degradation may have occurred during the freeze-thaw cycle thus, this study should be confirmed with immediate uNGAL measurements in fresh urine samples. In addition we do not have information about the uNGAL levels in our patients prior to transplantation or in the donors and some patients did not provide urine samples at all time points when protocol biopsies were taken. Thus, further studies are necessary to evaluate the diagnostic value of uNGAL measurements in patients after kidney transplantation.
In conclusion, even though NGAL may be useful in classifying patients with established AKI or glomerulonephritis in native kidneys or may be a predictor for delayed graft function and recovery early after kidney transplantation [14–16, 23], it is not a sensitive tool for monitoring for ATI at later times after transplantation.
Supplementary Material
Variables without statistical significance after correlation to urinary NGAL ratios in an explorative analysis.
Acknowledgments
The authors appreciate the help of K. Worthmann and S. Zachura in the collection of sample materials. Abstracts were accepted at the ASN Renal Week and American Transplant Congress 2010. J. K. Kaufeld performed study, wrote the paper, and collected data, W. Gwinner designed the study and analyzed data, I. S. cheffner analyzed data, H. G. Haller designed study, M. Schiffer designed study.
References
- 1.Gwinner W, Hinzmann K, Erdbruegger U, et al. Acute tubular injury in protocol biopsies of renal grafts: prevalence, associated factors and effect on long-term function. American Journal of Transplantation. 2008;8(8):1684–1693. doi: 10.1111/j.1600-6143.2008.02293.x. [DOI] [PubMed] [Google Scholar]
- 2.Devarajan P. Emerging biomarkers of acute kidney injury. Contributions to Nephrology. 2007;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 3.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. American Journal of Transplantation. 2006;6(7):1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatric Nephrology. 2006;21(6):856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 6.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. Journal of the American Society of Nephrology. 2010;21(1):189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. American Journal of Transplantation. 2004;4(10):1562–1566. doi: 10.1111/j.1600-6143.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clinical Journal of the American Society of Nephrology. 2010;5(12):2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nature Medicine. 2011;17(2):216–223. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney International. 2007;71(10):967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 11.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney International. 2003;63(5):1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 12.Mishra J, Qing MA, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 13.Devarajan P, Mishra J, Supavekin S, Patterson LT, Potter SS. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Molecular Genetics and Metabolism. 2003;80(4):365–376. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, He Y, Li K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clinical Immunology. 2007;123(2):227–234. doi: 10.1016/j.clim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Hollmen ME, Kyllönen LE, Inkinen KA, Lalla MLT, Salmela KT. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney International. 2011;79(1):89–98. doi: 10.1038/ki.2010.351. [DOI] [PubMed] [Google Scholar]
- 16.Kusaka M, Kuroyanagi Y, Mori T, et al. Serum neutrophil gelatinase-associated lipocalin as a predictor of organ recovery from delayed graft function after kidney transplantation from donors after cardiac death. Cell Transplantation. 2008;17(1-2):129–134. doi: 10.3727/000000008783907116. [DOI] [PubMed] [Google Scholar]
- 17.Schaub S, Mayr M, Hönger G, et al. Detection of subclinical tubular injury after renal transplantation: comparison of urine protein analysis with allograft histopathology. Transplantation. 2007;84(1):104–112. doi: 10.1097/01.tp.0000268808.39401.e8. [DOI] [PubMed] [Google Scholar]
- 18.Gaspari F, Cravedi P, Mandalà M, et al. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron—Clinical Practice. 2010;115(2):c154–c160. doi: 10.1159/000312879. [DOI] [PubMed] [Google Scholar]
- 19.Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Pawlak K, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in hypertensive and normotensive patients with coronary artery disease. Nephrology. 2008;13(2):153–156. doi: 10.1111/j.1440-1797.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 20.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. Journal of the American Society of Nephrology. 2008;19(3):547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and Meta-analysis. American Journal of Kidney Diseases. 2009;54(6):1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Decavele ASC, Dhondt L, De Buyzere ML, Delanghe JR. Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clinical Chemistry and Laboratory Medicine. 2011;49(6):999–1003. doi: 10.1515/CCLM.2011.156. [DOI] [PubMed] [Google Scholar]
- 23.Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney International. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variables without statistical significance after correlation to urinary NGAL ratios in an explorative analysis.


