Abstract
While neuropsychological deficits are evident among methamphetamine (meth) addicts, they are often unrelated to meth exposure parameters such as lifetime consumption and length of abstinence. The notion that some meth users develop neuropsychological impairments while others with similar drug exposure do not, suggests that there may be individual differences in vulnerability to the neurotoxic effects of meth. One source of differential vulnerability could come from genotypic variability in metabolic clearance of meth, dependent on the activity of cytochrome P450-2D6 (CYP2D6). We compared neuropsychological performance in 52 individuals with a history of meth dependence according with their CYP2D6 phenotype. All were free of HIV or hepatitis C infection and did not meet dependence criteria for other substances. Extensive metabolizers showed worse overall neuropsychological performance and were three times as likely to be cognitively impaired as intermediate/poor metabolizers. Groups did not differ in their demographic or meth use characteristics, nor did they evidence differences in mood disorder or other substance use. This preliminary study is the first to suggest that efficient meth metabolism is associated with worse neurocognitive outcomes in humans, and implicates the products of oxidative metabolism of meth as a possible source of brain injury.
Keywords: Substance abuse, CYP2D6, Polymorphisms, Neurotoxicity, Metabolism, Cognition
INTRODUCTION
Heavy exposure to amphetamines has been associated with central nervous system (CNS) disturbances involving primarily dopamine (DA), but also serotonin, gamma-aminobutyric acid (GABA), and glutamate-dependent systems, leading to cerebrovascular (Citron, Halpern, McCarron, Lundberg, McCormick, & Pincus, 1970; Rumbaugh, Bergeron, Scanlan, Teal, Segall, & Fang, 1971) and neural pathology. Proposed processes for neurotoxicity include quinone formation, induction of transcription factors and oxidative stress, hyperthermia, and activation of neurochemical pathways implicated in neuronal apoptosis (Cadet, Jayanthi, & Deng, 2003; Quinton & Yamamoto, 2006). Methamphetamine (meth) use has become increasingly more prevalent throughout the United States and has been a commonly abused drug in Japan and other parts of Asia. Meth has been linked to abnormalities on brain imaging (Iyo, Namba, Yanagisawa, Hirai, Yui, & Fukui, 1997), decreased DA receptor and transporter densities (McCann, Wong, Yokoi, Villemagne, Dannals, & Ricaurte, 1998; Sekine, Iyo, Ouchi, Matsunaga, Tsukada, & Okada, 2001; Volkow, Chang, Wang, Fowler, Ding, & Sedler, 2001), and neuropsychological (NP) deficits consistent with alterations in abilities subserved by frontostriatal systems (Kalechstein, Newton, & Green, 2003; McKetin & Mattick, 1997, 1998; Rippeth, Heaton, Carey, Marcotte, Moore, & Gonzalez, 2004; Sim, Simon, Domier, Richardson, Rawson, & Ling, 2002; Volkow, Chang, Wang, Fowler, Leonido-Yee, & Franceschi, 2001). A recent meta-analysis by our group showed that meth dependence is most consistently associated with deficient executive functions, attention, information processing speed, episodic memory, verbal fluency, and motor skills (Scott, Woods, Matt, Meyer, Heaton, & Atkinson, 2007).
In a cohort of abstinent meth-dependent subjects, our group found that meth use characteristics, such as lifetime exposure, chronicity of use, mode of delivery, etc, did not predict who was found to have cognitive impairment (Cherner, Heaton, Gonzalez, Rippeth, Carey, & Grant, 2002; Cherner, Suarez, Casey, Deiss, Letendre, & Marcotte, 2010). The low predictive value of meth exposure parameters suggests that there are individual differences in vulnerability to meth-related neurocognitive deficits. Thus, the identification of factors that render some individuals vulnerable and others protected under conditions of similar drug exposure deserves investigation.
One such factor may be genetic differences in meth metabolism. The enzyme Cytochrome P450, family 2, sub-family D, polypeptide 6 (CYP2D6) is responsible for oxidative metabolism of several psychoactive substances, including methamphetamine (Lin, Di Stefano, Schmitz, Hsu, Ellis, & Lennard, 1997; Wu, Otton, Inaba, Kalow, & Sellers, 1997). In humans, depending on urinary pH, approximately 30–50% of meth is excreted unchanged. Hydroxylation by CYP2D6 yields the most abundant metabolite: 4-hydroxymethamphetamine, both as sulfate and glucuronide conjugates. N-methylation by CYP2D6 yields amphetamine, which is further metabolized into 4-hydroxyamphetamine and its conjugates, norephedrine, phenylacetone, benzoic acid, and hippuric acid (Caldwell, Dring, & Williams, 1972; Shima, Kamata, Katagi, & Tsuchihashi, 2006).
Variants of the CYP2D6 gene have been well characterized, with over 80 polymorphisms identified (Dorado, Berec, Caceres, Gonzales, Cobaleda, & Llerena, 2005). These variants can make their carrier a “poor metabolizer” (PM) “intermediate metabolizer” (IM), or “extensive metabolizer” (EM). Ultra-rapid metabolizer (UM) phenotypes have also been described. Although research to date is not definitive, in humans some of these polymorphisms have been associated with motor neuron disease (Skvortsova, Slominskii, Shadrina, Levitskii, Levitskaia, & Alekhin, 2006), tardive dyskinesia (de Leon, Susce, Pan, Koch, & Wedlund, 2005; Tiwari, Deshpande, Rao, Bhatia, Lerer, & Nimgaonkar, 2005), and extrapyramidal symptoms in association with higher neuroleptic concentrations in plasma (Inada, Senoo, Iijima, Yamauchi, & Yagi, 2003), as well as vulnerability to Parkinson's disease (Singh, Khan, Shah, Shukla, Khaanna, & Parmar, 2008), each implicating effects on dopaminergic systems. Therefore, its role in methamphetamine metabolism and potential dopaminergic involvement makes CYP2D6 a candidate for explaining individual differences in susceptibility to meth exposure that are manifested as cognitive impairment.
CYP2D6 Phenotypes
CYP2D6 phenotyping is increasingly indicated clinically to determine optimal dosing of pharmaceutical agents that use this metabolic pathway. Alteration of alleles from the normal wild-type (EMs) fall into several categories: one amino acid change or deletion, frameshift, splicing defect, stop codon, insertion, and entire gene deletion (Gonzalez, Vilbois, Hardwick, McBride, Nebert, & Gelboin, 1988; Gough, Miles, Spurr, Noss, Gaedigk, & Eichelbaum, 1990; Kimura, Umeno, Skoda, Meyer, & Gonzalez, 1989; Marez, Legrand, Sabbagh, Guidice, Spire, & Lafitte, 1997). PMs have no active CYP2D6 alleles or only one that is partially active. As a result, they are at greater risk of drug-induced side effects due to diminished drug elimination. Approximately 5 to 14% of Caucasians are poor metabolizers. The four most common mutant alleles are CYP2D6*3, CYP2D6*4, CYP2D6*5, and CYP2D6*6 and account for 93–97% of the PM phenotypes in Caucasian populations. Individuals who are homozygous for PM alleles do not display CYP2D6 enzyme activity, nor do any of those who carry combinations of these inactive alleles (Sachse, Brockmoller, Bauer, & Roots, 1997). IMs have one active and one inactive CYP2D6 allele or two partially active alleles. Approximately 30% of Caucasians fall in the IM category (Raimundo, Fischer, Eichelbaum, Griese, Schwab, & Zanger, 2000). EMs correspond to the normal functional activity alleles, designated CYP2D6*1 and CYP2D6*2. Genotypes consistent with the EM phenotype include two active CYP2D6 alleles or one active and one partially active allele. This phenotype represents approximately 65 to 71% of Caucasians (Bradford, 2002). Ultra-rapid metabolizers have higher than normal rates of drug metabolism, and have three or more active alleles due to duplication or multi-duplication. Between 1 and 3% of Europeans fall in this category (Dahl, Johansson, Bertilsson, Ingelman-Sundberg, & Sjoqvist, 1995; Johansson, Lundqvist, Bertilsson, Dahl, Sjoqvist, & Ingelman-Sundberg, 1993). Ethnic and racial differences in the prevalence (Aklillu, Herrlin, Gustafsson, Bertilsson, & Ingelman-Sundberg, 2002; Aklillu, Persson, Bertilsson, Johansson, Rodrigues, & Ingelman-Sundberg, 1996; Bernal, Sinues, Johansson, McLellan, Wennerholm, & Dahl, 1999; Cascorbi, 2003; Dahl, Yue, Roh, Johansson, Sawe, & Sjoqvist, 1995; Gaedigk, Bhathena, Ndjountche, Pearce, Abdel-Rahman, & Alander, 2005), and possibly functionality (Gaedigk, Bradford, Marcucci, & Leeder, 2002; Inada et al., 2003) of specific alleles have been described in the literature. However, as the majority of the current study participants are of European Caucasian origin, we are limiting the description of population rates for the various phenotypes to those for that racial group.
In the present study, we set out to examine whether CYP2D6 phenotype is related to cognitive impairment among meth-dependent individuals. We hypothesized that those with PM phenotype would exhibit worse neuropsychological performance and greater likelihood of cognitive impairment than phenotypes corresponding to higher CYP2D6 activity because it was speculated that low or delayed clearance of meth would result in greater net exposure in poor metabolizers for the same actual amount consumed, compared with extensive metabolizers. To our knowledge, this is the first investigation of this relationship.
MATERIALS AND METHODS
Participants
We analyzed retrospective data and fluids collected on 52 study participants who were evaluated at the HIV Neurobehavioral Research Center (HNRC) in San Diego, California, USA, as part of a federally funded, institutionally approved project on neuroAIDS effects of methamphetamine. Subjects were selected from a larger sample to be free of HIV or hepatitis C infection, as well neurologic, metabolic, or psychiatric conditions that might confound interpretation of neuropsychological findings. All gave written informed consent to participate in accordance with our Institutional Review Board requirements. To be eligible for the parent study, participants had to meet lifetime criteria for meth dependence, with use within the previous 18 months. Other substance dependence, except alcohol or cannabis, within 5 years, or abuse within the past 12 months was an exclusion. Alcohol dependence within 12 months was also exclusionary. No restrictions were placed on cannabis use, given its high prevalence in this population and minimal long-term effects on neuropsychological function (Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003). Participants were requested to be abstinent for at least 10 days before testing and show negative urine toxicology for any nonprescribed substances except cannabis, as well as negative Breathalyzer test for alcohol on the day of NP testing.
Neurobehavioral and Drug Use Characterization
The methods of neurobehavioral and drug use characterization have been described elsewhere (Gonzalez, Rippeth, Carey, Heaton, Moore, & Schweinsburg, 2004; Rippeth et al., 2004). Briefly, participants were characterized as meth (and other substance) dependent based on DSM-IV criteria using a structured psychiatric interview (First, Spitzer, Gibbon, & Williams, 1994; Robins, Wing, Wittchen, Helzer, Babor, & Burke, 1988). History of mood disorder, attention deficit/hyperactivity disorder, and antisocial personality disorder were also evaluated according to DSM-IV criteria. A detailed history of meth and other substance use was gathered with a semistructured instrument covering onset, quantity, frequency, duration, and route of drug use over the participant's lifetime, previous 12 months, and previous 30 days. NP functioning was determined with a validated comprehensive battery of tests covering 7 ability domains (Learning, Memory, Attention/Working Memory, Verbal Fluency, Processing Speed, Abstraction/Problem Solving, and Motor Speed) with measures that have shown sensitivity to meth-related impairments. The specific tests in the battery are listed in the appendix. Raw scores were converted to demographically adjusted T-scores (M = 50, SD = 10), including adjustments for age, education, gender, and ethnicity as available for each test (Cherner, Suarez, Casey, Deiss, Letendre, & Marcotte, 2007; Heaton, Miller, Taylor, & Grant, 2004; Heaton, Taylor, & Manly, 2003). T-scores for each test were then converted into deficit scores based on half standard deviation (SD) increments, which reflect degree impairment by setting performances within the normal range at zero. The deficit scores range from 0 (T-score > 39; no impairment) to 5 (T-score < 20; severe impairment). The individual deficit scores were averaged to derive the Global Deficit Score (GDS), which reflects the number and the severity of deficits across the test battery (Carey, Woods, Gonzalez, Conover, Marcotte, & Grant, 2004; Heaton, Grant, Butters, White, Kirson, & Atkinson, 1995). For example, a GSD of 0.5 corresponds to scoring −1 SD on half the tests in the battery. Domain-specific deficit scores were also derived by averaging tests within an area of functioning. This method of data reduction is useful in avoiding multiple comparisons, as would be the case when considering individual tests, and has shown robust relationships with documented brain injury (Moore, Masliah, Rippeth, Gonzalez, Carey, & Cherner, 2006). Finally, level of premorbid ability was estimated with the Reading subtest of the Wide Range Achievement Test-3.
Genotyping and Phenotyping
CYP2D6 phenotype characterization was performed by an accredited commercial laboratory (Genelex, Seattle, WA, CLIA No. 50D0980559), using their standard CYP2D6 mutation panel (Table 1). DNA was extracted from peripheral blood mononuclear cells that were stored at −70°C, using a commercially available DNA extraction kit, QIAamp DNA Mini kit (Qiagen, Valencia, CA; Catalog #51185). Specimens were analyzed using the Tag-It™ Mutation Detection System for P450-2D6, which detects 12 nucleotide variants and two gene rearrangements in a multiplex polymerase chain reaction and allele-specific primer extension format. This method identifies 93–97% of PM phenotypes. Genelex provided the genotype, as well as the interpreted phenotype for each participant (see Appendix).
Table 1.
Details of cytochrome P450-2D6 genetic analysis
| Cytochrome P-450 2D6 mutations detected |
||
|---|---|---|
| CYP2D6 allele | Nucleotide change | Effect on enzyme metabolism |
| *1 | None (wild-type) | Normal |
| *2 | 2850C>T | Normal |
| *3 | 2549A>del | Inactive |
| *4 | 1846G>A | Inactive |
| *5 | Gene deletion | Inactive |
| *6 | 1707T>del | Inactive |
| *7 | 2935A>C | Inactive |
| *8 | 1758G>T | Inactive |
| *9 | 2613–2615 delAGA | Partially active |
| *10 | 100C>T | Partially active |
| *11 | 883G>C | Inactive |
| *12 | 124G>A | Inactive |
| *17 | 1023C>T | Partially active |
| *41 | 2988G>A | Partially active |
| Gene duplication | Duplication | Increased or decreased dependent on which allele is duplicated |
Statistical Analyses
Group differences in NP domain performance were analyzed with Wilcoxon Rank Sum tests, given the non-normal distribution of the variables. As individual NP test data were reduced by combining into ability domains, and given the exploratory nature of the study, we did not make experiment-wise adjustments for multiple comparisons. Other continuous variables were analyzed with Student's t tests. Group differences in the proportions of NP impaired participants and discrete background variables were analyzed using Fisher's exact tests and χ2 tests. Nonparametric correlations were computed between CYP2D6 activity and NP performance.
RESULTS
Analyses yielded genotypes consistent with three meth metabolism phenotypes: EM (n = 32), IM (n = 17), and PM (n = 3). Given the small sample sizes and preliminary nature of the study, the IM and PM groups were combined for analyses. There were no significant differences in meth use characteristics between the groups (Table 2), with the exception of primary route of administration: The most prevalent mode among EMs was smoking, whereas the IM/PM group more often reported intranasal administration. Additionally, the IM/PM group had a greater proportion of injection users. Values for the PM group alone were comparable to those of the IM group alone, and results were essentially unchanged when PMs were excluded from analyses.
Table 2.
Methamphetamine use parameters by cytochrome P450-2D6 metabolic phenotype
| Mean (SD) or % | Extensive n=32 | Intermediate/poor n=20 |
|---|---|---|
| Age of onset of meth use | 22 (9) | 23 (6) |
| Total years of meth use | 12 (5) | 12 (6) |
| Days abstinent from meth use | 134 (107) | 111 (75) |
| Density of meth use (grams/year) | 419 (327) | 342 (412) |
| Lifetime meth consumed (grams) | 4875 (4295) | 3690 (3876) |
| Meth in last 12 months (grams) | 350 (318) (n=29/32) | 277 (304) (n=20/20) |
| Binge use predominant | 4% | 5% |
| Primary administration route* | ||
| Injection | 6% | 25% |
| Intranasal | 27% | 50% |
| Smoke | 67% | 25% |
Overall Chi Square p < .05.
The EM and combined IM/PM groups were comparable with respect to demographic characteristics and estimated premorbid cognitive ability (Table 3).
Table 3.
Participant characteristics by cytochrome P450-2D6 metabolic phenotype
| Mean (SD) or proportion | Extensive n=32 | Intermediate/Poor n=20 | p value |
|---|---|---|---|
| Age | 35.5 (9.6) | 37.9 (10.6) | NS |
| Education | 12.5 (1.7) | 12.6 (2.1) | NS |
| n (%) Male | 24 (75%) | 15 (75%) | NS |
| n (%) Non-white | 8 (25%) | 4 (20%) | NS |
| WRAT-3 Reading Quotient | 98.4 (9.8) | 101.5 (10.6) | NS |
| n (%) Lifetime alcohol dependenceaa | 11 (33%) | 8 (40%) | NS |
| Average daily alcohol (drinks, mean, SD) | 6 (4) | 8 (6) | NS |
| Years of alcohol use (mean, SD) | 7.0 (6) | 8.7 (6.0) | NS |
| n (%) Lifetime cannabis dependence | 3 (9%) | 8 (40%) | .009 |
| Lifetime cannabis (grams, mean, SD) | 4927 (6761) | 7181 (6769) | NS |
| n (%) Lifetime cocaine dependenceb | 5 (16%) | 4 (20%) | NS |
| n (%) Lifetime opioid dependenceb | 1 (3%) | 0 | NS |
| n (%) Lifetime sedative dependenceb | 0 | 0 | – |
| n (%) Lifetime hallucinogen dependenceb | 0 | 0 | – |
| n (%) Lifetime bipolar disorder | 2 (6%) | 1 (5%) | NS |
| n (%) Lifetime major depression | 8 (27%) | 9 (47%) | NS |
| n (%) Current major depression | 2 (6%) | 2 (10%) | NS |
| n (%) Ever on serotonin reuptake inhibitor | 4 (12%) | 7 (35%) | .05 |
| n (%) ASPD | 10 (31%) | 4 (20%) | NS |
| n (%) ADHD/ADD | 2 (7%) | 3 (16%) | NS |
Greater than 12 months prior to assessment.
Greater than 5 years prior to assessment and episodic in nature.
ASPD = antisocial personality disorder; ADHD/ADD = attention deficit disorder with/without hyperactivity; SSRI = Selective serotonin reuptake inhibitor; WRAT-3 = Wide-Range Achievement Test-3, estimate of premorbid ability; NS = not significant.
Contrary to the initial hypothesis, EMs showed significantly worse overall NP performance, including significantly poorer scores in the areas of processing speed, abstraction/executive functioning, and learning (Table 4).
Table 4.
Global and domain-specific neuropsychological performance by cytochrome P450-2D6 phenotype
| Extensive | Intermediate/poor | Probability | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Mean | (SD) | Mean | (SD) | >|Z| | |
| Neuropsychological Deficit Score | |||||
| Global | 0.45 | (0.43) | 0.22 | (0.25) | .04 |
| Processing Speed | 0.31 | (0.43) | 0.14 | (0.33) | .13 |
| Attention/Working Memory | 0.39 | (0.67) | 0.18 | (0.37) | .19 |
| Verbal Fluency | 0.36 | (0.70) | 0.10 | (0.21) | .10 |
| Learning | 0.82 | (0.73) | 0.43 | (0.55) | .05 |
| Delayed Recall | 0.53 | (0.71) | 0.33 | (0.77) | .33 |
| Abstraction/Executive Functioning | 0.69 | (1.00) | 0.18 | (0.49) | .04 |
| Motor | 0.69 | (1.21) | 0.45 | (0.71) | .40 |
Note. Where differences are present, extensive metabolizers show worse performance.
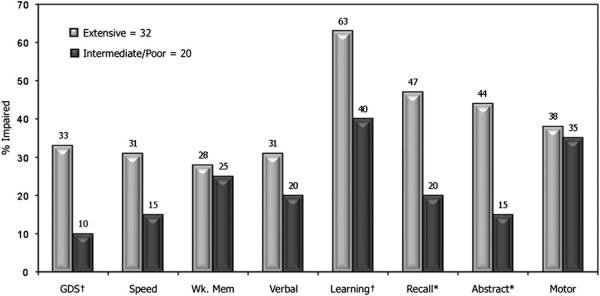
EMs were also more likely to obtain scores in the impaired range of cognitive functioning (Figure 1) compared with the combined IM/PM group, with significant differences in abstraction/executive functioning, and delayed recall, as well as trend level differences in learning, and global functioning. Although several of the comparisons did not reach statistical significance (which may be attributable to low power from small sample sizes), there was a consistent trend in the same direction in all domains, both in terms of level of performance, as well as proportion of subjects performing in the impaired range. Individual test T-scores and the proportion of participants in each group that performed at least one standard deviation below the mean appear in Table 5. In every case where statistically significant differences were detected, EMs showed worse performance.
Fig. 1.
Proportion of subjects performing within the impaired range of global and domain specific neuropsychological performance. Extensive metabolizers tend to have a greater likelihood of neuropsychological impairment than intermediate/poor metabolizers. *p < .02 †p < .10. GDS = Global Deficit Score; Speed = Speed of information processing; Att/Wk. Mem = Attention/working memory; Verbal = Verbal fluency; Abstr = Abstraction/problem-solving/executive functioning.
Table 5.
Demographically adjusted T-scores for neuropsychological tests and proportion performing within the impaired range (T-score < 40) in each group
| EM Mean (SD) % < 40 | IM/PM Mean (SD) % < 40 | p value | |
|---|---|---|---|
| Learning | |||
| Brief Visuospatial Memory Test-Revised (BVMT-R) | 44.4(11.9) | 47.9(10.1) | NS |
| 36% | 20% | NS | |
| Hopkins Verbal Learning Test-Revised (HVLT-R) | 41.7 (6.4) | 47.5 (8.9) | .008 |
| 24% | 20% | NS | |
| Story Memory Test | 44.0 (12.0) | 47.6 (10.8) | NS |
| 45% | 15% | .019 | |
| Figure Memory Test | 37.6 (9.0) | 41.5 (7.5) | NS |
| 70% | 60% | NS | |
| Memory | |||
| BVMT-R Delayed Recall | 47.8 (12.8) | 49.0 (12.0) | NS |
| 24% | 15% | NS | |
| HVLT-R Delayed Recall | 40.3 (7.7) | 45.2 (8.8) | .039 |
| 36% | 30% | NS | |
| Story Memory Test Retention | 49.1 (12.4) | 53.9 (10.1) | NS |
| 30% | 10% | .073 | |
| Figure Memory Test Retention | 53.0 (7.0) | 51.0(10.7) | NS |
| 3% | 10% | NS | |
| Attention/Working Memory | |||
| Paced Auditory Serial Addition Task-200 item | 41.9 (9.6) | 49.2 (9.4) | .009 |
| 30% | 20% | NS | |
| WAIS-III Letter-Number Sequencing | 47.4 (7.6) | 54.7 (10.1) | .004 |
| 9% | 10% | NS | |
| Processing Speed | |||
| Trail Making Test A | 51.1 (9.3) | 52.2 (7.8) | NS |
| 6% | 5% | NS | |
| WAIS-III Symbol Search | 49.1 (10.2) | 52.6(8.1) | NS |
| 24% | 11% | .051 | |
| WAIS-III Digit Symbol | 46.8 (9.3) | 49.8 (9.8) | NS |
| 27% | 15% | NS | |
| Executive/Abstraction | |||
| Trail Making Test B | 47.7 (10.3) | 58.0 (10.9) | .001 |
| 18% | 5% | NS | |
| Halstead Category Test Errors | 43.9 (10.0) | 50.7 (7.8) | .012 |
| 39% | 5% | .003 | |
| WCST-64 Perseverations | 45.0 (12.4) | 41.8 (9.3) | NS |
| 27% | 30% | NS | |
| Stroop Interference Ratio | 48.0 (7.5) | 47.3 (8.3) | NS |
| 12% | 7% | NS | |
| Verbal Fluency | |||
| Letter Fluency (FAS) | 48.5 (10.0) | 49.6 (9.3) | NS |
| 15% | 15% | NS | |
| Category Fluency (Animals) | 49.1 (11.2) | 50.9 (8.4) | NS |
| 24% | 5% | .051 | |
| Motor | |||
| Grooved Pegboard Dominant Hand | 46.0 (12.9) | 48.1 (9.0) | NS |
| 33% | 20% | NS | |
| Grooved Pegboard Nondominant Hand | 42.3 (9.1) | 43.4 (8.5) | NS |
| 27% | 30% | NS |
EM = Extensive metabolizer; IM/PM = Intermediate/poor metabolizer; NS = not significant; WAIS III = Wechsler Adult Intelligence Scale-III; WCST-64 = Wisconsin Card Sorting Test 64-item computerized version.
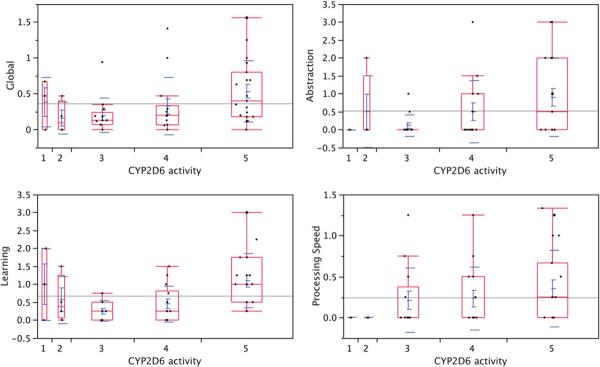
As a post hoc exploratory analysis, we also investigated the strength of the relationship between participants' neuropsychological performance and their theoretical metabolic activity based on the combination of active, partially active, or inactive alleles present in their genotype. We recognize that this approach is speculative given the absence of data on the subjects' actual metabolic activity, but we believed that this exploration could be fruitful in corroborating our general finding that higher metabolic activity is associated with worse NP outcome. To this end, we used the data generated by Zanger, Raimundo, and Eichelbaum (2004) to rank-order metabolic activity from lowest to highest (1 to 5), as follows: 1 = two non-functional alleles; 2 = one decreased function and one non-functional allele; 3 = one normal function and one non-functional allele, or two decreased function alleles; 4 = one normal function and one decreased function allele; 5 = two normal function alleles. The appendix shows the number of participants with the various genotypes, corresponding phenotypes, and metabolic activity ranks. As shown in Table 6, and illustrated in Figure 2, higher purported metabolic activity was associated with worse cognitive functioning overall and in the areas of processing speed, learning, and abstraction/executive functioning.
Table 6.
Non-parametric correlations between neuropsychological deficit Scores and CYP2D6 metabolic activity based on combination of alleles coded ordinally between 1 and 5
| Deficit Score | Spearman ρ | Probability > |ρ| |
|---|---|---|
| Global | 0.36 | 0.009 |
| Processing Speed | 0.31 | 0.025 |
| Attention/Working Memory | 0.16 | NS |
| Verbal Fluency | 0.18 | NS |
| Learning | 0.39 | 0.004 |
| Delayed Recall | 0.20 | NS |
| Abstraction/Executive | 0.35 | 0.010 |
| Motor | 0.02 | NS |
Note. Positive correlations indicate that higher metabolic activity is related to greater neurocognitive deficit. NS = not statistically significant.
Fig. 2.
Examples of the relationship between cytochrome P450-D6 (CYP2D6) activity and neurocognitive performance. The panel on the upper left shows increasing global impairment (GDS) with increasing CYP2D6 activity. The remaining panels show a similar relationship in learning, abstraction, processing speed.
Because the IM/PM group had a greater prevalence of lifetime cannabis dependence, as well as somewhat greater lifetime exposure (not statistically significant), we explored the possible effects of cannabis on cognitive performance. In addition, we modeled the effects of meth exposure, given that EMs tended to have consumed greater amounts over their lifetime (again, not statistically significant). In linear regressions with phenotype, lifetime grams of meth consumption, and lifetime grams of marijuana consumption, only phenotype was a significant predictor of the global deficit score (t = 2.05; p <.05).
DISCUSSION
To our knowledge, this study is the first to suggest differences in vulnerability to methamphetamine-associated brain dysfunction linked to CYP2D6 genotype in human users. The finding that the genotype associated with high metabolic activity is related to poorer cognitive performance was not expected, but it is consistent with the possibility that the metabolic products of methamphetamine oxidation may be a greater source of neurotoxicity than the parent compound. In fact, this has been demonstrated in vitro, where the metabolite 4-hydroxymethamphetamine showed significantly more cytotoxicity than unmetabolized meth (Clement, Behrens, Moller, & Cashman, 2000). In cultures exposed to other substituted amphetamines typically sold as “ecstasy” (methylenedioxy-methamphetamine: MDMA, methylthioamphetamine: MTA), cells expressing the active form of CYP2D6 showed significantly greater toxicity than cells with less active forms or those devoid of CYP2D6 activity. In these studies, toxicity was dependent on the formation of the oxidative metabolite N-methyl-α-methyldopamine, which was found to be 100-fold more cytotoxic than the parent substance (Carmo, Brulport, Hermes, Oesch, de Boer, & Remiao, 2007; Carmo, Brulport, Hermes, Oesch, Silva, & Ferreira, 2006). Furthermore, it has been demonstrated that stimulation of the P450 system in mice not only potentiates metabolism of MDMA but also increases the magnitude of neurotoxicity that can be observed (Monks, Jones, Bai, & Lau, 2004). These findings are discordant with results derived from an investigation of Dark Agouti rats, in which the females, considered a model for PM phenotype, exhibited greater acute MDMA-induced toxicity than males (Colado, Williams, & Green, 1995), and similarly in PM rats exposed neonatally to meth (Vorhees, Morford, Inman, Reed, Schilling, & Cappon, 1999f). However, translation of CYP2D6 neurotoxicity findings from animals to humans has been criticized (de la Torre & Farre, 2004) as a result of evidence linking metabolism of amphetamines in rats to CYP2D1, which, while homologous to human CYP2D6, may be functionally different (Kobayashi, Murray, Watson, Sesardic, Davies, & Boobis, 1989). Additionally, significant inter-species differences have been described in the proportion of the various metabolites that are excreted in urine (Caldwell, Dring, Franklin, Koster, Smith, & Williams, 1977; Dring, Smith, & Williams, 1970; Shima et al., 2006). Thus, extrapolation of neurotoxicity findings involving the P450 system from animals to humans must be done cautiously. While no studies, to our knowledge, have investigated links between amphetamine metabolite concentrations and neurotoxicity in humans, it has been demonstrated that EM have greater urinary excretion of the hydroxy metabolite, followed by IM, and then by PM (Miranda, Sordo, Salazar, Contreras, Bautista, & Rojas Garcia, 2007).
Although our findings are intriguing, several limitations must be considered. First, the small sample size makes our results preliminary. For instance, because our sample only included three truly poor metabolizers, we were not able to test whether there is a “U” shaped function in CYP2D6 effects on meth-related neurocognition. It could be that extensive metabolism is deleterious because it results in the formation of large quantities of toxic metabolites, while complete lack of CYP2D6 activity could also be harmful because there is delayed clearance of the parent compound. Additionally, while meth consumption differences were not statistically significant, there tended to be a stair step increase in density of use (grams/year) with increasing metabolic efficiency. This raises the possibility that, although meth exposure was not related to NP deficits in these and previous analyses (Cherner et al., 2010), EMs evidence more impairment because they are indeed consuming larger amounts of meth. Future studies with larger samples will be required to address these possibilities with confidence.
Second, the lack of a drug-free control group precludes testing the possibility that CYP2D6 genotype affects neurocognitive performance independently of meth use, for example, through some developmental effect. While it cannot be ruled out, there is no clear a priori reason to suspect such an effect, particularly because the EM phenotype is the most commonly occurring. Using our methods for determining cognitive impairment, which are based on the normal distribution of test performance, we would expect approximately 16% of a healthy normal population to perform in the impaired range (i.e., 1 SD below the mean). If the affected phenotype were the more rare poor metabolizers then we could not rule out that those members of a normative sample performing below 1 SD did so because of an underlying genotype effect alone (i.e., in the absence of methamphetamine). However, between 65 and 70% of a Caucasian normative population would be expected to be extensive metabolizers. Thus, the norms that we use to interpret test performance ought to already reflect an underlying effect of genotype, given that EM would compose a majority of the normative sample. Nevertheless, future studies would benefit from including a control group to increase confidence in the findings.
Third, although several possible confounders of neuropsychological effects were controlled by use of demographic adjustments and careful exclusion criteria, factors that could potentially affect meth pharmacokinetics or pharmacodynamics, such as tobacco, herbal supplement, and prescription and non-prescription drug consumption, as well as diet (Wijnen, Op den Buijsch, Drent, Kuipers, Neef, & Bast, 2007) were not accounted for. These extrinsic factors may significantly affect the absorption, distribution, metabolism, and/or excretion of meth and thus should be examined in future work. For example, there was a higher proportion of lifetime depression in the IM/PM group, with an accompanying higher lifetime prevalence of serotonin reuptake inhibitor (SSRI) use. Because most SSRIs are substrates and inhibitors of CYP2D6, it would be important to determine the effects of concomitant meth and SSRI use, as this would presumably result in lower formation of meth metabolites. We unfortunately did not have the information required for this type of analysis but hope to tackle this question in future work.
Along the same lines, no study to our knowledge has examined how chronic exposure to meth may affect CYP2D6 metabolic activity. Although we have found that chronicity of meth use does not appear to predict cognitive impairment (Cherner et al., 2002, 2010), it is possible that chronic exposure to meth may alter metabolic activity and consequently our findings may not apply to the current literature, which has focused on acute exposure.
One factor on which the groups differed was history of cannabis dependence, with a greater prevalence among IMs/PMs (although 100% of study participants reported lifetime use). It is possible that cannabis conferred a protective effect on neurocognition. At least one study has shown that among meth-dependent subjects, those with coexisting marijuana dependence had somewhat better neuropsychological performance (Gonzalez et al., 2004). While further research will be needed to elucidate the effects of concurrent or historic cannabis exposure, analyses in our sample did not show evidence relating lifetime amount of cannabis consumed to global neurocognitive performance.
Another difference between groups that should be noted was route of meth administration. Although no study to our knowledge has examined the effect that different routes of administration may have on meth metabolism, recent work (Hendrickson et al., 2008) with pigeons suggests that administration of meth either intramuscularly or intravenously does not affect metabolism. Nevertheless, route of administration should be examined in future investigations, given that it results in differences in bioavailability.
Additionally, in this retrospective study of abstinent users, we were unable to test actual metabolic rates or metabolite concentrations. Such information would be useful to substantiate our hypothesis that meth metabolites are responsible for the neuropsychological manifestations observed. Finally, as individuals of Asian and African descent have a higher percentage (40–50%) of reduced function and non-functional CYP2D6 compared with Caucasians (25–30%) (Bradford, 2002), generalization of these results to other racial/ethnic groups is not possible at this time.
These limitations notwithstanding, the current study found clear differences in neurocognitive impairment in meth-dependent adults in relation to their CYP2D6 genotype and corresponding phenotype. While preliminary, our findings suggest differential vulnerability to meth-induced neurocognitive impairment in extensive metabolizers, specifically in learning, delayed recall, and executive ability domains, as well as overall global functioning. This differentiation was further demonstrated for these domains, along with performance in processing speed, during post hoc analysis using a linear measure of hypothetical metabolism. We also observed similar differential vulnerability at the trend level for the remaining ability domains, including attention/working memory, verbal fluency, and motor speed. Failure to find a significant association in these latter ability domains may be a consequence of the small sample size and limited power to detect significant differences. Again, further research with larger sample sizes will be required to determine whether a Type II error was committed.
If replicated, our findings may be of particular importance in guiding future development in the early identification of vulnerability to and prevention of neurocognitive impairment among meth-dependent individuals. Given the relatively high prevalence of extensive metabolizers in the general population and their putative vulnerability to meth-related neurocognitive dysfunction, there is potential public health impact in interventions to address brain injury in meth users. To date, several CYP2D6 inhibitors have been identified, including sertraline, fluoxetine, paroxetine, quinidine and ticlopidine (Hemeryck & Belpaire, 2002). Studies have investigated the efficacy of sertraline (Shoptaw, Huber, Peck, Yang, Liu, & Jeff, 2006), fluoxetine (Batki, Moon, Bradley, Hersh, Smolar, & Mengis, 1999), and paroxetine (Piasecki, Steinagel, Thienhaus, & Kohlenberg, 2002) on reduction of meth use, albeit with no significant effect. Thus, even though CYP2D6 inhibitors may not be efficacious for reducing meth use, future work might examine their influence on neurocognitive functioning.
Finally, future studies seeking to investigate or replicate relationships between meth use and indicators of brain disturbance may benefit from understanding the phenotypic makeup of their study groups to help interpret their findings as well as discrepancies among studies.
ACKNOWLEDGMENTS
This manuscript has never been published either electronically or in print. Portions of the information contained in the manuscript have been previously presented at the International Neuropsychological Society Mid-Year Meeting, July 2008, Buenos Aires, Argentina and the XVth World Congress on Psychiatric Genetics, Oct 2008, Osaka, Japan. The authors wish to acknowledge support from the United States National Institutes of Health (grant numbers R03-DA27513, P01-DA12065, and P30-MH62512) and the contributions of study participants and staff at the HIV Neurobehavioral Research Center (HNRC) and Translational Methamphetamine AIDS Research Center, San Diego, CA, USA.
The HNRC Group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Assistant Center Manager: Jennifer Marquie-Beck; Business Manager: Melanie Sherman; Naval Hospital San Diego: Braden R. Hale, M.D., M.P.H. (P.I.); Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D.; Terry Alexander, R.N.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., Steven Paul Woods, Psy.D., David J. Moore, Ph.D.; Matthew Dawson, Donald Franklin; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., Marc Jacobson, Ph.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Ian Everall, FRCPsych., FRCPath., Ph.D., Cristian Achim, M.D., Ph.D.; Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, MD, Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Clinical Trials Component: J. Allen McCutchan, M.D., J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., Scott Letendre, M.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Tanya Wolfson, MS, Reena Deutsch, Ph.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
APPENDIX
Cytochrome P450-2D6 genotype, phenotype, and rank-ordered hypothetical metabolic activity level for study cases
Metabolic activity was assigned as follows, based on data from Zanger et al (2004):
-
5:
two normal function alleles
-
4:
one normal function and one decreased function allele
-
3:
one normal function and one non-functional allele, or two decreased function alleles
-
2:
one decreased function and one non-functional allele
-
1:
two non-functional alleles
Normal Function Alleles: *1, *2, *33, *35
Decreased Function Alleles: *9, *10, *17, *36, *41
Increased Function Alleles: *1×N, *2×N, *35×N
Non-Functional Alleles: *3, *4, *5, *6, *7, *8, *11, *12, *13, *14, *15, *16, *18, *19, *20, *21, *38, *40, *42
| No. of cases |
CYP2D6 genotype |
Metabolic activity |
|
|---|---|---|---|
| Phenotype | |||
| 6 | *1/*1 | EM | 5 |
| 11 | *1/*2 | EM | 5 |
| 1 | *2/*2 | EM | 5 |
| 2 | *1/*9 | EM | 4 |
| 1 | *1/*10 | EM | 4 |
| 1 | *1/*17 | EM | 4 |
| 5 | *1/*41 | EM | 4 |
| 2 | *2/*10 | EM | 4 |
| 3 | *2/*41 | EM | 4 |
| 5 | *1/*4 | IM | 3 |
| 4 | *2/*4 | IM | 3 |
| 3 | *2/*5 | IM | 3 |
| 1 | *2/*41 | IM | 3 |
| 1 | *3/*41 | IM | 2 |
| 1 | *4/*41 | IM | 2 |
| 1 | *10/*41 | IM | 3 |
| 1 | *5/*9 | IM | 2 |
| 1 | *6/*10 | IM | 2 |
| 3 | *4/*4 | PM | 1 |
Note. EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST The authors declare that this work was funded entirely by NIH grants P01-DA12065 and P30-MH62512. The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest for Mariana Cherner, Chad Bousman, Daniel Barron, Florin Vaida, Robert Heaton, Ian Everall, and Igor Grant. The authors declare that, over the past 3 years, J. Hampton Atkinson has received compensation from Eli Lilly Pharmaceuticals; Scott Letendre is an advisor for Abbott Labs, GlaxoSmithKline, and Schering-Plough, has given CME accredited talks funded by Abbott Labs and GlaxoSmithKline, and has received research funding from GlaxoSmithKline, Schering-Plough, Merk, Tibotec, and Gilead Sciences.
REFERENCES
- Aklillu E, Herrlin K, Gustafsson LL, Bertilsson L, Ingelman-Sundberg M. Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living in Ethiopia or in Sweden. Pharmacogenetics. 2002;12:375–383. doi: 10.1097/00008571-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M. Frequent distribution of ultra-rapid metabolizers of debrisoquine in an ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. The Journal of Pharmacology and Experimental Therapeutics. 1996;278:441–446. [PubMed] [Google Scholar]
- Batki SL, Moon J, Bradley M, Hersh D, Smolar S, Mengis M. Fluoxetine in methamphetamine dependence-a controlled trial: Preliminary analysis; Paper presented at the 61st Annual Scientific Meeting of the College on Problems of Drug Dependence; Acapulco, Mexico. 1999. [Google Scholar]
- Bernal ML, Sinues B, Johansson I, McLellan RA, Wennerholm A, Dahl ML. Ten percent of North Spanish individuals carry duplicated or triplicated CYP2D6 genes associated with ultrarapid metabolism of debrisoquine. Pharmacogenetics. 1999;9:657–660. [PubMed] [Google Scholar]
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229–243. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: Cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. The FASEB Journal. 2003;17:1775. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Caldwell J, Dring LG, Franklin RB, Koster U, Smith RL, Williams RT. Comparative metabolism of the amphetamine drugs of dependence in man and monkeys. Journal of Medical Primatology. 1977;6:367–375. doi: 10.1159/000459773. [DOI] [PubMed] [Google Scholar]
- Caldwell J, Dring LG, Williams RT. Comparative metabolism of [C14]methamphetamine in man, the guinea pig, and the rat. The Biochemical Journal. 1972;129:11–22. doi: 10.1042/bj1290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carmo H, Brulport M, Hermes M, Oesch F, de Boer D, Remiao F. CYP2D6 increases toxicity of the designer drug 4-methylthioamphetamine (4-MTA) Toxicology. 2007;229:236–244. doi: 10.1016/j.tox.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Carmo H, Brulport M, Hermes M, Oesch F, Silva R, Ferreira LM. Influence of CYP2D6 polymorphism on 3,4-methylenedioxymethamphetamine ('Ecstasy') cytotoxicity. Pharmacogenetics and Genomics. 2006;16:789–799. doi: 10.1097/01.fpc.0000230419.05221.fc. [DOI] [PubMed] [Google Scholar]
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: Genetic background and clinical implication. European Journal of Clinical Investigation. 2003;33(Suppl. 2):17–22. doi: 10.1046/j.1365-2362.33.s2.3.x. [DOI] [PubMed] [Google Scholar]
- Cherner M, Heaton RK, Gonzalez RG, Rippeth J, Carey C, Grant I. Exposure to methamphetamine and neuropsychological functioning. Journal of the International Neuropsychological Society. 2002;8:250. [Google Scholar]
- Cherner M, Suarez P, Casey CY, Deiss R, Letendre S, Marcotte T. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and Alcohol Dependence. 2010;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Lazzaretto D, Fortuny LA, Mindt MR, Dawes S. Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Archives of Clinical Neuropsychology. 2007;22:343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BP, Halpern M, McCarron M, Lundberg GD, McCormick R, Pincus IJ. Necrotizing angiitis associated with drug abuse. New England Journal of Medicine. 1970;283:1003–1011. doi: 10.1056/NEJM197011052831901. [DOI] [PubMed] [Google Scholar]
- Clement B, Behrens D, Moller W, Cashman JR. Reduction of amphetamine hydroxylamine and other aliphatic hydroxylamines by benzamidoxime reductase and human liver microsomes. Chemical Research in Toxicology. 2000;13:1037–1045. doi: 10.1021/tx000043t. [DOI] [PubMed] [Google Scholar]
- Colado MI, Williams JL, Green AR. The hyperthermic and neurotoxic effects of 'Ecstasy' (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. British Journal of Pharmacology. 1995;115:1281. doi: 10.1111/j.1476-5381.1995.tb15037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl ML, Johansson I, Bertilsson L, Ingelman-Sundberg M, Sjoqvist F. Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. The Journal of Pharmacology and Expimental Therapeutics. 1995;274:516–520. [PubMed] [Google Scholar]
- Dahl ML, Yue QY, Roh HK, Johansson I, Sawe J, Sjoqvist F. Genetic analysis of the CYP2D locus in relation to debrisoquine hydroxylation capacity in Korean, Japanese and Chinese subjects. Pharmacogenetics. 1995;5:159–164. doi: 10.1097/00008571-199506000-00004. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): The limitations of scaling from animals to humans. Trends in Pharmacological Sciences. 2004;25:505. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- de Leon J, Susce MT, Pan RM, Koch WH, Wedlund PJ. Polymorphic variations in GSTM1, GSTT1, PgP, CYP2D6, CYP3A5, and dopamine D2 and D3 receptors and their association with tardive dyskinesia in severe mental illness. Journal of Clinical Psychopharmacology. 2005;25:448–456. doi: 10.1097/01.jcp.0000177546.34799.af. [DOI] [PubMed] [Google Scholar]
- Dorado P, Berecz R, Caceres MC, Gonzalez I, Cobaleda J, Llerena A. Determination of debrisoquine and 4-hydroxydebrisoquine by high-performance liquid chromatography: Application to the evaluation of CYP2D6 genotype and debrisoquine metabolic ratio relationship. Clinical Chemistry and Laboratory Medicine. 2005;43:275–279. doi: 10.1515/CCLM.2005.046. [DOI] [PubMed] [Google Scholar]
- Dring LG, Smith RL, Williams RT. The metabolic fate of amphetamine in man and other species. The Biochemical Journal. 1970;116:425–435. doi: 10.1042/bj1160425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM-IV disorders (SCID) Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Gaedigk A, Bhathena A, Ndjountche L, Pearce RE, Abdel-Rahman SM, Alander SW. Identification and characterization of novel sequence variations in the cytochrome P4502D6 (CYP2D6) gene in African Americans. The Pharmacogenomics Journal. 2005;5:173–182. doi: 10.1038/sj.tpj.6500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Bradford LD, Marcucci KA, Leeder JS. Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clinical Pharmacology and Therapeutics. 2002;72:76–89. doi: 10.1067/mcp.2002.125783. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Vilbois F, Hardwick JP, McBride OW, Nebert DW, Gelboin HV. Human debrisoquine 4-hydroxylase (P450IID1): cDNA and deduced amino acid sequence and assignment of the CYP2D locus to chromosome 22. Genomics. 1988;2:174–179. doi: 10.1016/0888-7543(88)90100-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Gough AC, Miles JS, Spurr NK, Moss JE, Gaedigk A, Eichelbaum M. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990;347:773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH. The HNRC 500–neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton R, Miller S, Taylor M, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and caucasian adults. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- Heaton R, Taylor M, Manly J. Demographic effects and use of demographically corrected norms with the WAIS III and the WMS-III. In: Tulsky D, Saklofske D, Heaton RK, Cheline G, Ivnik R, Bornstein RA, Prifitera A, Ledbetter MF, editors. Clinical interpretation of the WAIS-III and WMS-III. Academic Press; San Diego: 2003. pp. 183–210. [Google Scholar]
- Hemeryck A, Belpaire FM. Selective serotoninreup-take inhibitors and cytochrome P-450 mediated drug-drug interactions: An update. Current Drug Metabolism. 2002;3:13. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]
- Hendrickson HP, Hardwick WC, McMillan DE, Owens SM. Bioavailability of (+)-methamphetamine in the pigeon following an intramuscular dose. Pharmacology Biochemistry and Behavior. 2008;90:382–386. doi: 10.1016/j.pbb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Senoo H, Iijima Y, Yamauchi T, Yagi G. Cytochrome P450 II D6 gene polymorphisms and the neuroleptic-induced extrapyramidal symptoms in Japanese schizophrenic patients. Psychiatric Genetics. 2003;13:163–168. doi: 10.1097/00041444-200309000-00005. [DOI] [PubMed] [Google Scholar]
- Iyo M, Namba H, Yanagisawa M, Hirai S, Yui N, Fukui S. Abnormal cerebral perfusion in chronic methamphetamine abusers: A study using 99MTc-HMPAO and SPECT. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1997;21:789–796. doi: 10.1016/s0278-5846(97)00079-1. [DOI] [PubMed] [Google Scholar]
- Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjoqvist F, Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kimura S, Umeno M, Skoda RC, Meyer UA, Gonzalez FJ. The human debrisoquine 4-hydroxylase (CYP2D) locus: Sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. American Journal of Human Genetics. 1989;45:889–904. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Murray S, Watson D, Sesardic D, Davies DS, Boobis AR. The specificity of inhibition of debrisoquine 4-hydroxylase activity by quinidine and quinine in the rat is the inverse of that in man. Biochemical Pharmacology. 1989;38:2795. doi: 10.1016/0006-2952(89)90433-4. [DOI] [PubMed] [Google Scholar]
- Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metabolism and Disposition. 1997;25:1059–1064. [PubMed] [Google Scholar]
- Marez D, Legrand M, Sabbagh N, Guidice JM, Spire C, Lafitte JJ. Polymorphism of the cytochrome P450 CYP2D6 gene in a European population: Characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics. 1997;7:193–202. doi: 10.1097/00008571-199706000-00004. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C] WIN-35,428. The Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug and Alcohol Dependence. 1997;48:235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: Comparison with non-drug-using controls. Drug and Alcohol Dependence. 1998;50:181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Miranda GE, Sordo M, Salazar AM, Contreras C, Bautista L, Rojas Garcia AE. Determination of amphetamine, methamphetamine, and hydroxyamphetamine derivatives in urine by gas chromatography-mass spectrometry and its relation to CYP2D6 phenotype of drug users. Journal of Analytical Toxicology. 2007;31:31–36. doi: 10.1093/jat/31.1.31. [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Therapeutic Drug Monitoring. 2004;26:132. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. Aids. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Piasecki MP, Steinagel GM, Thienhaus OJ, Kohlenberg BS. An exploratory study: The use of paroxetine for methamphetamine craving. Journal of Psychoactive Drugs. 2002;34:301. doi: 10.1080/02791072.2002.10399967. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. The AAPS Journal. 2006;8:E337. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM. Elucidation of the genetic basis of the common `intermediate metabolizer' phenotype for drug oxidation by CYP2D6. Pharmacogenetics. 2000;10:577–581. doi: 10.1097/00008571-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J. The Composite international diagnostic interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rumbaugh CL, Bergeron RT, Scanlan RL, Teal JS, Segall HD, Fang HC. Cerebral vascular changes secondary to amphetamine abuse in the experimental animal. Radiology. 1971;101:345–351. doi: 10.1148/101.2.345. [DOI] [PubMed] [Google Scholar]
- Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: Allele frequencies and phenotypic consequences. American Journal of Human Genetics. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Revies. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. American Journal of Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Shima N, Kamata HT, Katagi M, Tsuchihashi H. Urinary excretion of the main metabolites of methamphetamine, including p-hydroxymethamphetamine-sulfate and p-hydroxymethamphetamine-glucuronide, in humans and rats. Xenobiotica. 2006;36:259–267. doi: 10.1080/00498250600627475. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2006;85:12. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Sim T, Simon SL, Domier CP, Richardson K, Rawson RA, Ling W. Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. Journal of Addictive Diseases. 2002;21:75–89. doi: 10.1300/j069v21n01_07. [DOI] [PubMed] [Google Scholar]
- Singh M, Khan A, Shah P, Shukla R, Khanna V, Parmar D. Polymorphism in environment responsive genes and association with Parkinson disease. Molecular and Cellular Biochemistry. 2008;312:131–138. doi: 10.1007/s11010-008-9728-2. [DOI] [PubMed] [Google Scholar]
- Skvortsova VI, Slominskii PA, Shadrina MI, Levitskii GN, Levitskaia NI, Alekhin AV. [Detoxication gene polymorphism and susceptibility to sporadic motor neuron disease in Russian population] Zhurnal nevropatologii i psikhiatrii imeni S.S. Korsakova (Moscow, Russia: 1952) 2006;106:4–13. [PubMed] [Google Scholar]
- Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Lerer B, Nimgaonkar VL. Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphisms. Schizophrenia Research. 2005;75:21–26. doi: 10.1016/j.schres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD. Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: Possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–181. [PubMed] [Google Scholar]
- Wijnen PA, Op den Buijsch RA, Drent M, Kuipers PM, Neef C, Bast A. Review article: The prevalence and clinical relevance of cytochrome P450 polymorphisms. Alimentary Pharmacology & Therapeutics. 2007;26(Suppl.2):211. doi: 10.1111/j.1365-2036.2007.03490.x. [DOI] [PubMed] [Google Scholar]
- Wu D, Otton SV, Inaba T, Kalow W, Sellers EM. Interactions of amphetamine analogs with human liver CYP2D6. Biochemical Pharmacology. 1997;53:1605–1612. doi: 10.1016/s0006-2952(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn-Schmiedeberg's Archives of Pharmacology. 2004;369:23. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]