Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive, adult-onset neurodegenerative disease characterized by degeneration of motor neurons, resulting in paralysis and death. A pathological hallmark of the degenerating motor neurons in most ALS patients is the presence of cytoplasmic inclusions containing the protein TDP-43. The morphology and type of TDP-43 pathological inclusions is variable and can range from large round Lewy body-like inclusions to filamentous skein-like inclusions. The clinical significance of this variable pathology is unclear. Intermediate-length polyglutamine (polyQ) expansions in ataxin 2 were recently identified as a genetic risk factor for ALS. Here we have analyzed TDP-43 pathology in a series of ALS cases with or without ataxin 2 intermediate-length polyQ expansions. The motor neurons of ALS cases harboring ataxin 2 polyQ expansions (n=6) contained primarily skein-like or filamentous TDP-43 pathology and only rarely, if ever, contained large round inclusions, whereas the ALS cases without ataxin 2 polyQ expansions (n=13) contained abundant large round and skein-like TDP-43 pathology. The paucity of large round TDP-43 inclusions in ALS cases with ataxin 2 polyQ expansions suggests a distinct pathological subtype of ALS and highlights the possibility for distinct pathogenic mechanisms.
Keywords: TDP-43, ataxin 2, polyglutamine, amyotrophic lateral sclerosis
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, adult-onset neurodegenerative disease that is characterized by degeneration of motor neurons, resulting in paralysis and death [10]. Degenerating motor neurons in most ALS patients contain abundant cytoplasmic inclusions containing ubiquitin, as well as the protein TDP-43 [41]. TDP-43 pathology is found in almost all non-SOD1 ALS patients and mutations within the TDP-43 gene (TARDBP) have been identified in rare sporadic and familial ALS cases [12,22,41,48]. The pathological and genetic findings implicate TDP-43 as an important contributor to ALS pathogenesis [28]. In addition to being localized to the cytoplasm, TDP-43 within these pathological inclusions contains post-translational modifications associated with disease, including cleavage into C-terminal fragments and phosphorylation [23,40,41].
In addition to the characteristic round neuronal cytoplasmic inclusions, TDP-43 inclusions can be observed as dash- or dot-like inclusions, skein-like filamentous inclusions, nuclear cat-eye inclusions, neuritic tangles, and cytoplasmic and nuclear glial inclusions [23,37,40,41]. A number of initial reports examining TDP-43 pathology noted variation in the types of TDP-43 positive inclusions between patients [23,38,40,41,51]. However, the clinical significance of the different morphologies of TDP-43 pathological inclusions remains unclear [30].
Intermediate-length polyglutamine (polyQ) expansions in ataxin 2 were recently identified as a genetic risk factor for ALS [8,13-15,21,29,31,43,47,53,54]. The subset of ALS patients harboring ataxin 2 polyQ expansions represents a cohort with the same genetic risk factor and perhaps the same mechanistic underpinnings to their disease. Do these patients exhibit differences in TDP-43 pathology compared to ALS cases without ataxin 2 polyQ expansions?
Here we have analyzed TDP-43 pathology in a series of ALS cases with or without ataxin 2 intermediate-length polyQ expansions. The motor neurons of ALS cases harboring ataxin 2 polyQ expansions contained primarily skein-like or filamentous TDP-43 pathology and only rarely, if ever, contained large round inclusions, whereas the ALS cases without ataxin 2 polyQ expansions contained abundant large round and skein-like TDP-43 pathology. The paucity of large round TDP-43 inclusions in ALS cases with ataxin 2 polyQ expansions suggests a distinct pathological subtype of ALS and highlights the possibility for distinct pathogenic mechanisms.
Materials and Methods
Study subjects
Our cohort included 19 cases with a clinical diagnosis of ALS in accordance with the modified El Escorial Criteria [6] and a neuropathological diagnosis of ALS who underwent autopsy in the Center for Neurodegenerative Disease Research at the University of Pennsylvania from 2001 to 2010 (Table 1). Detailed clinical characteristics (gender, age at onset, disease duration, site of onset, and ALS global disease severity as measured by a functional rating score (ALSFRS-R) [7]) were ascertained when available by retrospective chart review of clinical data within the University of Pennsylvania Health System. ALS patients with ataxin 2 intermediate-length polyQ expansions were previously identified [14] and all those who had also donated brain and spinal cord for research at the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania were included (n = 6). ALS cases without ataxin 2 intermediate-length polyQ expansions were selected to represent a control cohort with similar age, gender and disease duration. Of the ALS cases included in this study, 3 (15%) had a family history of disease (fALS), while 16 cases were sporadic (sALS). All cases were screened for TARDBP, SOD1, FUS and C9ORF72 mutations and 3 of the cases without ataxin 2 intermediate-length polyQ harbored C9ORF72 hexanucleotide repeat expansions [5], consistent with this mutation representing a fairly common cause of ALS. The genetic analysis of C9ORF72 status was performed retrospectively to the case selection and did not influence inclusion or exclusion in our autopsy series. None of the 6 ALS cases with ataxin 2 polyQ expansions contained C9ORF72 hexanucleotide repeat expansions.
Table 1.
Demographic and clinical features of cases (NR=not reported).
| ALS CASE # |
ALS patient ataxin 2 polyQ length |
Sex | Family History |
Onset (years) |
Age at Death (years) |
Duration (years) |
Site of onset | Dementia | ALSFRS- R |
C9Orf72 status |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 | 24 | M | Yes | 41 | 42 | 1 | Bulbar | No | 26 | Expansion |
| 2 | 22 | F | No | 47 | 50 | 3 | Extremity | No | 15 | |
| 3 | 22 | M | No | 75 | 76 | 1 | Extremity | No | 24 | |
| 4 | 22 | M | No | 56 | 74 | 18 | Extremity | No | 12 | |
| 5 | 22 | F | No | 79 | 81 | 2 | Bulbar | No | 27 | |
| 6 | 22 | M | No | 41 | 44 | 3 | Extremity | No | 14 | |
| 7 | 22 | M | No | 45 | 54 | 9 | Bulbar | No | NR | |
| 8 | 22 | F | No | 55 | NR | NR | Extremity | No | NR | |
| 9 | 22 | M | Yes | 59 | 62 | 3 | Bulbar | No | 23 | Expansion |
| 10 | 22 | F | No | 50 | 51 | 1 | Cognitive/Bulbar | Yes | 24 | |
| 11 | 22 | F | No | 48 | 51 | 3 | Extremity | No | 14 | |
| 12 | 22 | F | No | 73 | 74 | 1 | Bulbar | No | 24 | |
| 13 | 22 | M | No | 39 | NR | NR | Bulbar | No | NR | Expansion |
| 14 | 27 | F | No | 54 | 56 | 2 | Extremity | No | 29 | |
| 15 | 27 | F | Yes | 30 | 43 | 13 | Extremity | No | 15 | |
| 16 | 27 | M | No | 78 | 78 | 0 | Cognitive | Yes | NR | |
| 17 | 31 | F | No | 65 | 62 | 3 | Extremity | No | 17 | |
| 18 | 32 | F | No | NR | 48 | NR | Extremity | No | NR | |
| 19 | 32 | M | No | NR | 56 | NR | Extremity | No | NR | |
Immunohistochemistry
Patient brain tissue was deparaffinized before pretreatment using heat antigen retrieval with Bull’s Eye Decloaker (BioCare Medical). Endogenous peroxidase was then blocked with 3% hydrogen peroxide in PBS for 10 minutes. After washing with 0.1% PBST (phosphate-buffered saline+0.1% Tween20) and blocking with 10% goat serum in 0.5% PBST for 30-60 minutes at 25°C, sections (6-7 μm) were incubated with α-TDP-43 rabbit polyclonal antibody (Proteintech Group), 1:500; α-phosphorylated TDP-43 (409/410-1) mouse monoclonal antibody (Cosmo Bio Co., ltd), 1:100 in 0.1% PBST overnight at 4°C. After washing with 0.1% PBST, sections were incubated with biotinylated goat anti-mouse or rabbit IgG (1:200; Vector Laboratories) for 1 hour at 25°C. After washing with 0.1% PBST, sections were then incubated with Vectastain ABC (Vector Laboratories) for 45 minutes. After washing with 0.1% PBST followed by 0.1M Tris (pH 7.5) and 0.3M NaCl. Peroxidase activity was then detected with DAB (Sigma). Detailed immunohistochemistry protocols are available at http://www.med.upenn.edu/mcrc/histology_core/.
Evaluation of TDP-43 pathology
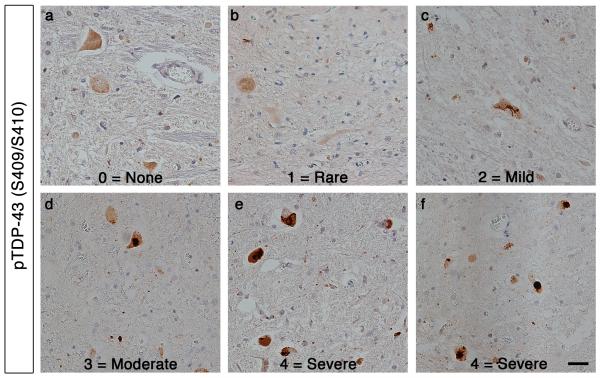
The presence of total TDP-43 pathology, filamentous or skein-like TDP-43 pathology, or large round TDP-43 pathology was scored in cervical and lumber spinal cord and motor cortex using a semi-quantitative 5-point ordinal scale (0, none; 1, rare; 2, mild; 3, moderate; 4, severe) by two of us, blinded to the genetic data and each other’s assessment (M.P.H and J.B.). See Fig. 2 for examples of this scoring system. This same scoring approach has been used effectively in several similar comparative pathology studies. [4,5,19,20]. We utilized this system rather than numeric image analysis-based quantification because an ordinal scale acknowledges the sequential nature of stages of increasing severity, reflecting the spread of pathology throughout a given section, or throughout the brain, which has been shown in all major neurodegenerative diseases. Indeed, ordinal data also provides information about the relation ofseverity stages since 1 stage follows continuously into the other, representing sequential classes rather than values on a numerical scale [19,20,33].
Figure 2. Semiquantitative staging of ALS TDP-43 pathology.
The presence of TDP-43 pathology was graded using a semi-quantitative 5-point ordinal scale. Representative images of the anterior horn of spinal cord sections stained with phospho-specific TDP-43 (S409/S410) antibody. Pathology grading: 0, none (a); 1, rare (b); 2, mild (c); 3, moderate (d); 4, severe (e,f). Scale bar = 20 μm.
Statistical analyses
All statistical comparisons represent p-values calculated using two-sample Mann–Whitney U (Wilicoxon) test on R software. The significance level for all comparisons was set at the 0.05 level.
Results
Genetic and clinical features of ALS cases in this study
The ataxin 2 polyQ length, though variable, is most frequently 22Q. PolyQ expansions greater than 34 cause spinocerebellar ataxia 2 (SCA2) [25,32,42,44]. Intermediate-length ataxin 2 polyQ expansions (27-33Q) are associated with increased risk for ALS [8,13-15,21,29,31,43,47,53,54]. We examined 19 subjects including 6 ALS patients harboring ataxin 2 intermediate-length polyQ expansions (3 cases with 27Q, 1 case with 31Q, 2 cases with 32Q) and 13 ALS patients with normal ataxin 2 polyQ lengths (13 with 22Q and 1 with 24Q). The mean age of onset of ataxin 2 polyQ-expanded cases was 55.2 years (median = 55, range = 30-78) and the mean age of onset of the normal-length ataxin 2 group was 55.6 years (median = 52, range = 39-81)(Table 1). One of the intermediate-length ataxin 2 polyQ ALS patients and two of the normal-length ataxin 2 ALS patients had disease duration longer than 4 years, and would meet the usual definition of progressive muscular atrophy (PMA) as recently described by Geser et al [20].
Comparing TDP-43 pathology in ALS cases with and without ataxin 2 polyQ expansions
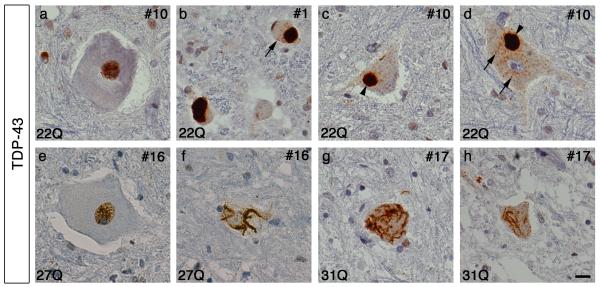
To determine if there are differences in TDP-43 pathology in cases with or without ataxin 2 polyQ expansions, we examined autopsy tissue from 6 ALS cases with ataxin 2 polyQ expansions and 13 ALS cases with normal-length ataxin 2. TDP-43 inclusions were initially identified using a polyclonal TDP-43 antibody as well as a monoclonal phosphorylation-specific TDP-43 (pTDP-43) antibody. For ease in distinguishing pathological TDP-43 from normal nuclear TDP-43 we used the pTDP-43 antibody for the majority of cases in our study. The patterns of pathology recognized by both antibodies were similar (Figs. 1,3). In a subset of cases stained with the polyclonal TDP-43 antibody we found that the pathology of ALS cases with ataxin 2 polyQ expansions was characterized primarily by filamentous cytoplasmic TDP-43 inclusions within motor neurons of the anterior horn (Fig. 1). ALS patients with normal ataxin 2 polyQ length, as previously reported, were characterized by cytoplasmic inclusions in motor neurons that tended to be large, dense round inclusions or dense pre-inclusions, although filamentous skein-like inclusions could also be found (Fig. 1). Thus, there was a lack of large round TDP-43 inclusions in ALS cases harboring ataxin 2 polyQ expansions.
Figure 1. Characterization of TDP-43 pathology in the lumbar spinal cord of cases with and without ataxin 2 polyQ expansions.
Case numbers and ataxin 2 polyQ lengths are indicated. Spinal cord sections stained with polyclonal TDP-43 antibody exhibit both nuclear staining (a,e) and cytoplasmic inclusions of TDP-43 (b-d,f-h). Cases without ataxin 2 polyQ expansions show abundant cytoplasmic round Lewy Body-like inclusions (arrow heads) and smaller round pre-inclusions (arrows) (b-d). Cases harboring ataxin 2 intermediate-length polyQ expansions show cytoplasmic filamentous and skein-like inclusions, and lack large round inclusions (e-h). Scale bar = 10 μm.
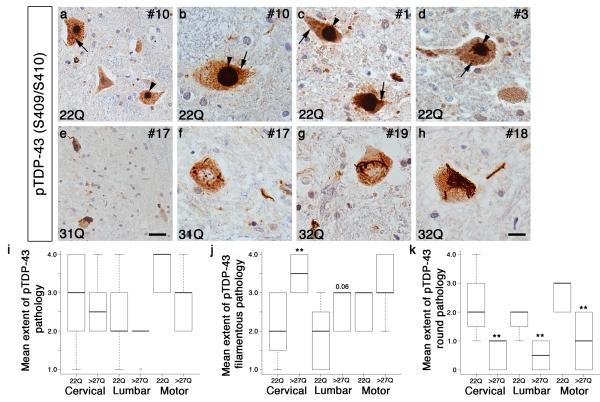
Figure 3. Cases with ataxin 2 intermediate-length polyQ expansions are characterized by distinct pathological TDP-43 features.
Case numbers and ataxin 2 polyQ lengths are indicated. a-h) Representative images of anterior horn of spinal cord from ALS cases with and without ataxin 2 polyQ expansions stained with a phospho-specific TDP-43 (S409/S410) antibody. Cases with normal polyQ length ataxin 2 are characterized primarily by large dense round inclusions (arrow heads), round pre-inclusions (arrows) and occasionally skein or filamentous inclusions (a-d). Cases with ataxin 2 polyQ expansions lack large dense round inclusions and are instead characterized primarily by less compact filamentous inclusions throughout the cytoplasm of motor neurons (e-h). Scale bar = 10 μm for b-d and f-h. Scale bar = 20 μm for a,e. i) Semi-quantitative grading of total TDP-43 pathology showed no difference in the mean extent of pTDP-43 pathology in cervical or lumbar spinal cord, or motor cortex. j) Semi-quantitative grading showed a significant increase in the mean extent of pTDP-43 filamentous inclusions in ataxin 2 polyQ expanded ALS cases compared to cases with normal ataxin 2 in cervical spinal cord (**, P<0.01). k) Semi-quantitative grading showed a significant decrease in the mean extent of pTDP-43 large round inclusions in ataxin 2 polyQ expanded ALS cases compared to cases with normal-length ataxin 2 in cervical and lumbar spinal cord, as well as motor cortex (**, P<0.01).
To further characterize this potential difference in TDP-43 pathology we analyzed relevant brain regions, including cervical and lumbar spinal cord and motor cortex, from the complete group of 19 cases (Table 2). The presence of TDP-43 pathology, as well as the presence of filamentous inclusions or dense round inclusions was graded using a semi-quantitative 5-point ordinal scale (0, none; 1, rare; 2, mild; 3, moderate; 4, severe) (Table 2) (see Fig. 2 for examples of grading system).
Table 2.
Semi-quantitative grading of TDP-43 pathology in ALS cases with and without ataxin 2 polyQ expansions (5-point ordinal scale: 0, none; 1, rare; 2, mild; 3, moderate; 4, severe) (ND=not done).
| ALS CASE # |
ALS patient ataxin-2 polyQ length |
Cervical Spinal Cord |
Lumbar Spinal Cord |
Motor Cortex |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Total TDP-43 |
Skein/ Filamentous |
Round | Total TDP-43 |
Skein/ Filamentous |
Round | Total TDP-43 |
Skein/ Filamentous |
Round | ||
|
| ||||||||||
| 1 | 24 | 4 | 2 | 3 | 2 | 1 | 2 | ND | ND | ND |
| 2 | 22 | 4 | 1 | 4 | 4 | 2 | 2 | ND | ND | ND |
| 3 | 22 | 2 | 1 | 2 | 2 | 0 | 2 | 3 | 2 | 3 |
| 4 | 22 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 1 | 2 |
| 5 | 22 | 3 | 2 | 3 | 1 | 0 | 1 | 4 | 1 | 3 |
| 6 | 22 | 2 | 0 | 2 | 3 | 1 | 2 | ND | ND | ND |
| 7 | 22 | 2 | 0 | 2 | 2 | 1 | 2 | 4 | 2 | 3 |
| 8 | 22 | 3 | 2 | 1 | ND | ND | ND | ND | ND | ND |
| 9 | 22 | 2 | 1 | 1 | 3 | 1 | 2 | 4 | 2 | 3 |
| 10 | 22 | 4 | 1 | 3 | 3 | 2 | 1 | ND | ND | ND |
| 11 | 22 | 4 | 2 | 2 | 4 | 2 | 2 | ND | ND | ND |
| 12 | 22 | 4 | 1 | 3 | 2 | 0 | 2 | 4 | 2 | 2 |
| 13 | 22 | ND | ND | ND | 2 | 1 | 2 | ND | ND | ND |
| 14 | 27 | 4 | 3 | 1 | 2 | 1 | 1 | 3 | 2 | 1 |
| 15 | 27 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 2 |
| 16 | 27 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 |
| 17 | 31 | 3 | 3 | 1 | 2 | 2 | 0 | 3 | 3 | 0 |
| 18 | 32 | 2 | 2 | 0 | 1 | 1 | 0 | 3 | 2 | 1 |
| 19 | 32 | 3 | 3 | 1 | 2 | 2 | 1 | 4 | 3 | 2 |
Comparing the total TDP-43 pathology score present in each case, we found no statistical difference between patients with ataxin 2 polyQ expansions and those without polyQ expansions in cervical and lumbar spinal cord and motor cortex (Fig. 3i and Table 2). However, in the lumbar spinal cord, there was a trend towards less total TDP-43 pathology in the ataxin 2 polyQ expanded cases, but this difference did not reach statistical significance (Fig. 3i and Table 2). In agreement with our earlier observations, we found that ALS patients with ataxin 2 polyQ expansions had a significant increase in the presence of less dense filamentous inclusions within motor neurons of the anterior horn of the spinal cord (Fig. 3j and Table 2). These filamentous inclusions varied in size and density, and were commonly spread throughout the cytoplasm of the cell (Fig. 3 e-h). As expected, it was possible to find filamentous inclusions in patients with normal-length ataxin 2, but was less common (Table 2). When grading the more stereotypical TDP-43 dense, round inclusions, they were rare or entirely absent from ataxin 2 polyQ expanded patients in spinal cord regions examined, and were absent, rare or mild in motor cortex (Fig. 3k and Table 2). This was most noticeable in the anterior horn of spinal cord where these large cytoplasmic inclusions are most obvious (Fig. 3e-h).
While the presence of dense large round inclusions in normal-length ataxin 2 patients was variable between cases, overall there was a significant increase in the presence of these types of inclusions compared to ALS patients with ataxin 2 polyQ expansions (Fig. 3a-d,k and Table 2). This was consistent in all brain regions examined (Fig. 3k). Finally, even if we exclude the 3 ALS cases with C9ORF72 hexanucleotide repeat expansions from the cohort of 13 normal-length ataxin 2 ALS cases it does not affect the results and there are still significant differences in TDP-43 inclusion morphology between cases with and without ataxin 2 polyQ expansions. These findings indicate that the TDP-43 pathology in ALS cases with intermediate-length polyQ expansions differs from that of ALS cases without ataxin 2 polyQ expansions.
Discussion
TDP-43 pathological inclusions are a hallmark characteristic of the majority of ALS cases, however the different types of TDP-43 positive inclusions and their relevance to disease are poorly understood [30,41]. The presence of distinct morphologies of neuronal cytoplasmic TDP-43 inclusions has been noted in a number of reports [23,40], including one which hypothesizes that development of the two most common inclusion types, round and filamentous, may be distinct and manifest separately [38]. We observe that almost all TDP-43 pathology in ALS patients with ataxin 2 polyQ expansions is characterized by filamentous inclusions and a striking paucity of round dense inclusions. To further investigate the pathological features of ALS associated with ataxin 2 polyQ expansions it will be important to expand these analyses to include additional ALS cases. While there is much variability in the types of inclusions seen in a sampling of ALS cases, the group harboring ataxin 2 polyQ expansions shares a characteristic pathology that can be separated from cases without ataxin 2 expansions as a whole.
Some cases in the control ALS group are also characterized by the same TDP-43 pathology, and it perhaps makes sense that other unidentified genetic risk factors could culminate in the same pattern of pathology. This would also lead us to predict that the round dense inclusions of TDP-43 are formed via a different mechanism or under different cellular conditions (M.P.H. and A.D.G. manuscript in preparation). TDP-43 is an intrinsically aggregation-prone protein [26]; perhaps then different alterations to TDP-43 or different cellular environments can influence its misfolding trajectories towards distinct morphologies (e.g. round or skein-like). This study represents a starting point and follow-up studies are required to extend and confirm these findings in additional ALS autopsy cases harboring ataxin 2 polyQ expansions and to further define the role of ataxin 2 in ALS pathogenesis.
A convergence of neuropathology and genetics has provided fundamental insight into human neurodegenerative diseases [17]. Case in point: the neuropathology of frontotemporal lobar degeneration (FTLD). The subclassification of different types of FTLD based on neuropathological features (e.g. ubiquitin-positive, tau-positive, TDP-43-positive, FUS-positive, etc.) [9,16,35,36] has proven tremendously valuable in understanding disease mechanisms and is supported by the genetic underpinnings of various FTLD subtypes [9,45]. Therefore, a similar stratification of ALS cases based on genetics and neuropathology will hopefully provide additional insight into disease mechanisms. The notion that different genetic causes can result in the same clinical phenotype, but differing neuropathology, has previously been proposed. In ALS alone, pathology has been subtyped into at least 3 exclusive categories, including ALS cases with TDP-43 pathology, FUS pathology or SOD1 pathology [2,27,34,50,55]. Furthermore, chromosome 9-linked ALS and FTD cases, which are caused by a hexanucleotide repeat expansion in the C9ORF72 gene, exhibit distinct pathological features [1,3,5,11,24,39,46,49,52]. In the future it will be of interest to compare the morphology of TDP-43 inclusions, as we have here for ataxin 2 (e.g. skein vs. round morphology), in ALS patients with distinct underlying genetic characteristics (e.g. mutations in VCP, UBQLN2, OPTN, C9ORF72). In the limited number of C9ORF72 ALS cases that we examined here (n=3) we did not observe any significant differences in skein vs. round TDP-43 inclusions compared to ALS cases without C9ORF72 mutations, although a larger follow-up study will be important.
It was recently reported that different mutations in the FUS gene might result in differences in FUS pathology as well as differences in clinical phenotype (i.e. disease severity) [33]. This example underscores the idea that mutations within the same gene can produce distinct pathology, which is relevant to disease and may suggest different underlying pathogenic mechanisms. There are some recent examples in which TDP-43 pathology has been subtyped within ALS [18] and it is commonly split into subtypes within the related disorder, FTLD [36]. Ataxin 2 polyQ expanded cases are another example where TDP-43 pathology can be subtyped in ALS, whereby a distinct difference in TDP-43 inclusion morphology (i.e. absence of large round inclusions and presence of prominent skein-like filamentous inclusions) characterizes disease with different genetic underpinnings.
Acknowledgments
We thank Min Min Lu and Lan Cheng for help with immunohistochemistry. This work was supported by NIH Director’s New Innovator Award 1DP2OD004417 (A.D.G.), NIH grants 1R01NS065317 and 1R01NS073660 (A.D.G.) and AG032953 (J.Q.T. and V.M.Y.L). A.D.G. is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts, and a Rita Allen Foundation Scholar. M.P.H. is supported by a NIH Ruth L. Kirschstein NRSA predoctoral fellowship 5F31NS074848-02. JB is supported by a grant of the Deutsche Forschungsgemeinschaft DFG (AOBJ586910).
References
- 1.Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 2.Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, Blumbergs PC, Vucic S, Kiernan MC, Nicholson GA. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2010;81:20881–20890. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, Dickson DW, Josephs KA, Rush BK, Machulda MM, Fields JA, Ferman TJ, Baker M, Rutherford NJ, Adamson J, Wszolek ZK, Adeli A, Savica R, Boot B, Kuntz KM, Gavrilova R, Reeves A, Whitwell J, Kantarci K, Jack CR, Jr., Parisi JE, Lucas JA, Petersen RC, Rademakers R. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brettschneider J, Libon DJ, Toledo JB, Xie SX, McCluskey L, Elman L, Geser F, Lee VM, Grossman M, Trojanowski JQ. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol. 2012;123:395–407. doi: 10.1007/s00401-011-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, McCluskey L, Irwin DJ, Grossman M, Molina-Porcel L, Lee VM, Trojanowski JQ. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012 doi: 10.1007/s00401-012-0970-z. Doi 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 7.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Huang R, Yang Y, Chen K, Song W, Pan P, Li J, Shang HF. Ataxin-2 intermediate-length polyglutamine: a possible risk factor for Chinese patients with amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:1925, e1–5. doi: 10.1016/j.neurobiolaging.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Chen-Plotkin AS, Martinez-Lage M, Sleiman PM, Hu W, Greene R, Wood EM, Bing S, Grossman M, Schellenberg GD, Hatanpaa KJ, Weiner MF, White CL, 3rd, Brooks WS, Halliday GM, Kril JJ, Gearing M, Beach TG, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Pickering-Brown SM, Snowden J, van Swieten JC, Heutink P, Seelaar H, Murrell JR, Ghetti B, Spina S, Grafman J, Kaye JA, Woltjer RL, Mesulam M, Bigio E, Llado A, Miller BL, Alzualde A, Moreno F, Rohrer JD, Mackenzie IR, Feldman HH, Hamilton RL, Cruts M, Engelborghs S, De Deyn PP, Van Broeckhoven C, Bird TD, Cairns NJ, Goate A, Frosch MP, Riederer PF, Bogdanovic N, Lee VM, Trojanowski JQ, Van Deerlin VM. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch Neurol. 2011;68:488–497. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 11.Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, Martindale J, Hartley J, Walsh T, Gelsthorpe C, Baxter L, Forster G, Fox M, Bury J, Mok K, McDermott CJ, Traynor BJ, Kirby J, Wharton SB, Ince PG, Hardy J, Shaw PJ. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012;135:751–764. doi: 10.1093/brain/awr365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Cruz S, Cleveland DW. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daoud H, Belzil V, Martins S, Sabbagh M, Provencher P, Lacomblez L, Meininger V, Camu W, Dupre N, Dion PA, Rouleau GA. Association of long ATXN2 CAG repeat sizes with increased risk of amyotrophic lateral sclerosis. Arch Neurol. 2011;68:739–742. doi: 10.1001/archneurol.2011.111. [DOI] [PubMed] [Google Scholar]
- 14.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischbeck KH, Pulst SM. Amyotrophic lateral sclerosis and spinocerebellar ataxia 2. Neurology. 2011;76:2050–2051. doi: 10.1212/WNL.0b013e31821f4498. [DOI] [PubMed] [Google Scholar]
- 16.Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nature medicine. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 18.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geser F, Stein B, Partain M, Elman LB, McCluskey LF, Xie SX, Van Deerlin VM, Kwong LK, Lee VM, Trojanowski JQ. Motor neuron disease clinically limited to the lower motor neuron is a diffuse TDP-43 proteinopathy. Acta Neuropathol. 2011;121:509–517. doi: 10.1007/s00401-011-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gispert S, Kurz A, Waibel S, Bauer P, Liepelt I, Geisen C, Gitler AD, Becker T, Weber M, Berg D, Andersen PM, Kruger R, Riess O, Ludolph AC, Auburger G. The modulation of Amyotrophic Lateral Sclerosis risk by Ataxin-2 intermediate polyglutamine expansions is a specific effect. Neurobiol Dis. 2012;45:356–361. doi: 10.1016/j.nbd.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, 3rd, Bigio EH, Caselli R, Baker M, Al-Lozi MT, Morris JC, Pestronk A, Rademakers R, Goate AM, Cairns NJ. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiung GY, Dejesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, Butler R, Leung B, Fok A, Rutherford NJ, Baker M, Rademakers R, Mackenzie IR. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, Weber C, Mandel JL, Cancel G, Abbas N, Durr A, Didierjean O, Stevanin G, Agid Y, Brice A. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 28.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagier-Tourenne C, Cleveland DW. Neurodegeneration: An expansion in ALS genetics. Nature. 2010;466:1052–1053. doi: 10.1038/4661052a. [DOI] [PubMed] [Google Scholar]
- 30.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 2012;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee T, Li YR, Ingre C, Weber M, Grehl T, Gredal O, de Carvalho M, Meyer T, Tysnes OB, Auburger G, Gispert S, Bonini NM, Andersen PM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients. Hum Mol Genet. 2011;20:1697–1700. doi: 10.1093/hmg/ddr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzetti D, Bohlega S, Zoghbi HY. The expansion of the CAG repeat in ataxin-2 is a frequent cause of autosomal dominant spinocerebellar ataxia. Neurology. 1997;49:1009–1013. doi: 10.1212/wnl.49.4.1009. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie IR, Ansorge O, Strong M, Bilbao J, Zinman L, Ang LC, Baker M, Stewart H, Eisen A, Rademakers R, Neumann M. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011;122:87–98. doi: 10.1007/s00401-011-0838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 35.Mackenzie IR, Munoz DG, Kusaka H, Yokota O, Ishihara K, Roeber S, Kretzschmar HA, Cairns NJ, Neumann M. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2010;121:207–218. doi: 10.1007/s00401-010-0764-0. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuno Y, Fujita Y, Takatama M, Okamoto K. Comparison of phosphorylated TDP-43-positive inclusions in oculomotor neurons in patients with non-ALS and ALS disorders. J Neurol Sci. 2012;315:20–25. doi: 10.1016/j.jns.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H, Wakabayashi K. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;116:193–203. doi: 10.1007/s00401-008-0396-9. [DOI] [PubMed] [Google Scholar]
- 39.Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 42.Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, Starkman S, Orozco-Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 43.Ross OA, Rutherford NJ, Baker M, Soto-Ortolaza AI, Carrasquillo MM, DeJesus-Hernandez M, Adamson J, Li M, Volkening K, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Woodruff BK, Knopman DS, White CL, 3rd, Van Gerpen JA, Meschia JF, Mackenzie IR, Boylan K, Boeve BF, Miller BL, Strong MJ, Uitti RJ, Younkin SG, Graff-Radford NR, Petersen RC, Wszolek ZK, Dickson DW, Rademakers R. Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet. 2011;20:3207–3212. doi: 10.1093/hmg/ddr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, Tashiro K, Ishida Y, Ikeuchi T, Koide R, Saito M, Sato A, Tanaka T, Hanyu S, Takiyama Y, Nishizawa M, Shimizu N, Nomura Y, Segawa M, Iwabuchi K, Eguchi I, Tanaka H, Takahashi H, Tsuji S. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14:277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- 45.See TM, LaMarre AK, Lee SE, Miller BL. Genetic causes of frontotemporal degeneration. J Geriatr Psychiatry Neurol. 2010;23:260–268. doi: 10.1177/0891988710383574. [DOI] [PubMed] [Google Scholar]
- 46.Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, Jones M, Gerhard A, Davidson YS, Robinson A, Gibbons L, Hu Q, Duplessis D, Neary D, Mann DM, Pickering-Brown SM. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soraru G, Clementi M, Forzan M, Orsetti V, D’Ascenzo C, Querin G, Palmieri A, Ermani M, Angelini C, Pegoraro E. ALS risk but not phenotype is affected by ataxin-2 intermediate length polyglutamine expansion. Neurology. 2011;76:2030–2031. doi: 10.1212/WNL.0b013e31821e557a. [DOI] [PubMed] [Google Scholar]
- 48.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart H, Rutherford NJ, Briemberg H, Krieger C, Cashman N, Fabros M, Baker M, Fok A, Dejesus-Hernandez M, Eisen A, Rademakers R, Mackenzie IR. Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathol. 2012;123:409–417. doi: 10.1007/s00401-011-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 51.Thorpe JR, Tang H, Atherton J, Cairns NJ. Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J Neural Transm. 2008;115:1661–1671. doi: 10.1007/s00702-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troakes C, Maekawa S, Wijesekera L, Rogelj B, Siklos L, Bell C, Smith B, Newhouse S, Vance C, Johnson L, Hortobagyi T, Shatunov A, Al-Chalabi A, Leigh N, Shaw CE, King A, Al-Sarraj S. An MND/ALS phenotype associated with C9orf72 repeat expansion: Abundant p62-positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathology. 2011 doi: 10.1111/j.1440-1789.2011.01286.x. Doi 10.1111/j.1440-1789.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 53.Van Damme P, Veldink JH, van Blitterswijk M, Corveleyn A, van Vught PW, Thijs V, Dubois B, Matthijs G, van den Berg LH, Robberecht W. Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology. 2011;76:2066–2072. doi: 10.1212/WNL.0b013e31821f445b. [DOI] [PubMed] [Google Scholar]
- 54.Van Langenhove T, van der Zee J, Engelborghs S, Vandenberghe R, Santens P, Van den Broeck M, Mattheijssens M, Peeters K, Nuytten D, Cras P, De Deyn PP, De Jonghe P, Cruts M, Van Broeckhoven C. Ataxin-2 polyQ expansions in FTLD-ALS spectrum disorders in Flanders-Belgian cohorts. Neurobiol Aging. 2012;33:1004e17–20. doi: 10.1016/j.neurobiolaging.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]