Abstract
Auditory neurons provide the critical link between a cochlear implant and the brain in deaf individuals, therefore their preservation and/or regeneration is important for optimal performance of this neural prosthesis. In cases where auditory neurons are significantly depleted, stem cells (SCs) may be used to replace the lost population of neurons, thereby re-establishing the critical link between the periphery (implant) and the brain. For such a therapy to be therapeutically viable, SCs must be differentiated into neurons, retained at their delivery site and damage caused to the residual auditory neurons minimized. Here we describe the transplantation of SC-derived neurons into the deaf cochlea, using a peptide hydrogel to limit their dispersal. The described approach illustrates that SCs can be delivered to and are retained within the basal turn of the cochlea, without a significant loss of endogenous auditory neurons. In addition, the tissue response elicited from this surgical approach was restricted to the surgical site and did not extend beyond the cochlear basal turn. Overall, this approach illustrates the feasibility of targeted cell delivery into the mammalian cochlea using hydrogel, which may be useful for future cell-based transplantation strategies, for combined treatment with a cochlear implant to restore function.
Keywords: cochlear implant, hydrogel, stem cell, auditory neuron, deafness
Introduction
Cochlear implants electrically stimulate auditory neurons (ANs) in patients with severe-to-profound hearing loss. However, there is often a lengthy delay between the loss of hearing and cochlear implantation, resulting in ongoing degeneration of ANs which comprise the auditory nerve. The health and integrity of the auditory nerve is considered to be one of the factors affecting cochlear implant performance [Shepherd and Javel, 1997], and thus, preserving a population of robust neurons is an important factor in improving outcomes with this neural prosthesis. Many laboratories have now published studies describing the delivery and survival of murine stem cells (SCs) into the mammalian cochlea for the replacement of degenerating ANs, using a variety of delivery techniques [Hu et al., 2004a, Hu et al., 2005, Regala et al., 2005, Coleman et al., 2006, Corrales et al., 2006, Sekiya et al., 2006, Ahn et al., 2008, Altschuler et al., 2008, Lang et al., 2008, Reyes et al., 2008].
Despite differences in SC differentiation protocols and transplantation technique adopted, the results from these studies support four main conclusions: 1) SCs can survive in the deaf mammalian cochlea, 2) SCs are capable of extensive migration/dispersal following their delivery into the mammalian cochlea, and 3) a proportion of transplanted SCs express neuronal and glial proteins in vivo. Initial attempts to transplant exogenous cells into the cochlea adopted a conservative surgical approach, that is, cells were delivered directly into the scala tympani in an attempt to minimize trauma to the delicate cochlear structures. While minimizing trauma, the delivery of cells into the scala tympani frequently resulted in the extensive dispersal of cells within the cochlea, and/or low detection of transplanted cells in the target site [Olivius et al., 2003, Hu et al., 2004b, Coleman et al., 2006].
Recent attempts at delivering SCs to the cochlea have investigated a more invasive approach into the modiolus or auditory nerve [Hu et al., 2004a, Okano et al., 2005, Regala et al., 2005, Corrales et al., 2006]. While successful in terms of improving the numbers of exogenous cells detected within the target site, these studies also report the extensive dispersal of cells following transplantation. Although some dispersal of transplanted cells is likely to be necessary for effective replacement of ANs, extensive dispersal has the potential disadvantage of transporting the cells to regions distal to their target site. In addition, the effect of the surgical procedure on the endogenous AN population and the inflammatory tissue response in the cochlea are also important to evaluate when considering these potential therapies. Whilst a variable proportion of ANs will likely have degenerated after deafness, it will be important to preserve those ANs that remain. An effective cell replacement strategy is therefore likely to incorporate both the targeted delivery strategy which minimizes damage to the existing cochlear structures, in combination with the retention of transplanted cells in a matrix that restricts their widespread dispersal until they can become integrated.
Cell encapsulation is one solution which may minimize cell dispersal following transplantation. Effective cell encapsulation for in vivo studies would need to incorporate biocompatible materials and encourage cell growth. A product which meets these specifications is PuraMatrix™ peptide hydrogel. This hydrogel forms synthetic three dimensional matrices at a physiological pH, and can be customized to create specific microenvironments for cell growth and differentiation in vitro and in vivo. Hydrogel is biocompatible comprising >99% water and mimicking a bare extracellular matrix, thereby enabling three dimensional growth analogous to the in vivo scenario http://www.puramatrix.com/index.html. Several reports have described improved growth and differentiation of neurons in hydrogel scaffolds [Holmes et al., 2000, Semino et al., 2004], including neurite outgrowth and formation of functional synapses [Holmes et al., 2000, Ellis-Behnke et al., 2006]. However, the application of hydrogels in conjunction with SC delivery into the inner ear has not yet been explored.
The current study explored the delivery of SCs into the deafened mammalian cochlea, using hydrogel to limit their dispersal (Fig. 1). The efficacy of this delivery procedure was examined based upon the location of transplanted SCs, the density of residual ANs, and the inflammatory tissue response elicited by the procedure. These data were then considered in terms of future cell therapy for combined cochlear implant/SC transplantation.
Figure 1. Transplantation approach for stem cell delivery into Rosenthal’s canal.
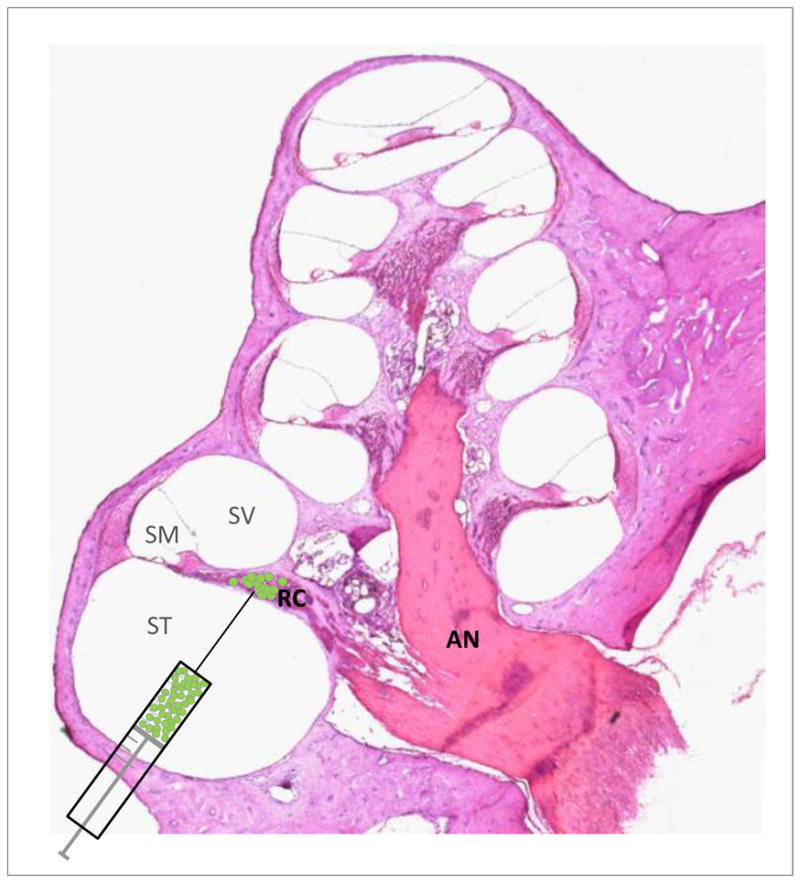
A post-auricular approach was used to transplant green fluorescent protein-positive SCs directly into Rosenthal’s canal in the deafened guinea pig cochlea. ST: scala tympani; SM: scala media; SV: scala vestibuli; AN: auditory nerve; drawing not to scale.
Materials and Methods
Experimental animals
Ten adult guinea pigs (400–600g) from a mixed gender and gene pool were used for the current study. Half the cohort of animals were ototoxically deafened, allowed to recover for two weeks and then transplanted with 11-day differentiated mouse embryonic SCs (n=5). Untreated contralateral cochleae served as untreated, deafened controls (n=5). Age-matched, untreated, normal hearing animals served as additional controls (n=5). All procedures were conducted in accordance with the guidelines set by the Royal Victorian Eye and Ear Hospital Animal Research and Ethics Committee (approval numbers: 02/090A and 05/122A).
Deafening procedure
The hearing status of all guinea pigs was evaluated using standard click-evoked auditory brainstem responses [Hardie and Shepherd, 1999]. Five normal hearing guinea pigs were bilaterally deafened by co-administration of the ototoxic aminoglycoside kanamycin monophosphate and the loop diuretic frusemide, as previouslydescribed [Coleman et al., 2006]. These animals were confirmed profoundly deaf via auditory brainstem responses, one week post-deafening (click-evoked auditory brainstem response thresholds ≥ 98dB peak equivalent sound pressure level; [Hardie and Shepherd, 1999]) and also immediately prior to termination.
Differentiation of stem cells
The mouse embryonic SC line used in this study [R1 B5-EGFP (Tg(GFPU)5 Nagy/J)], was obtained from Dr A. Nagy (Mt Sinai Hospital, Toronto) and expressed enhanced green fluorescent protein (GFP). Stem cells were maintained in standard medium and differentiated in vitro using retinoic acid and conditioned media (collected from organ of Corti explants cultures [Coleman et al., 2007]). Briefly, following differentiation in retinoic acid, SC neurospheres were collected and washed twice for 5 minutes in 10% sterile sucrose in phosphate buffered saline (PBS; Invitrogen). After the final wash, all of the solution was removed and 5 μL of the neurospheres were carefully mixed with 5 μL of liquid PuraMatrix™ Peptide Hydrogel (BD Biosciences) in 4 well chamber slides (Nunc; one pellet per well), to give a neurosphere/hydrogel pellet of 10 μL total volume. The neurosphere/hydrogel pellet underwent several gentle washes by emersion in 0.5 mL of sterile PBS for 2 minutes, followed by 0.5 mL of sterile DMEM/F12 media (Invitrogen) for 5 minutes, before being immersed in conditioned media collected from post-natal day 5 organ of Corti cultures. Each day, the media was removed and replaced with conditioned media from the organ of Corti cultures. Following 3 days growth in conditioned media, neurosphere/hydrogel matrices were ready for transplantation (Fig. 2). Additional neurosphere/hydrogel matrices were fixed in 4% paraformaldehyde (PFA; BDH Laboratories), and embedded in OCT freezing medium on the day of transplantation. These were later cryosectioned and stained for the neural marker βIII tubulin (Millipore AB9354, 1:1000).
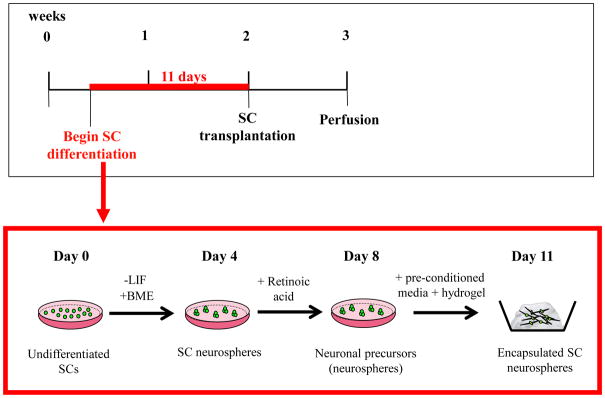
Figure 2. Timeline of stem cell differentiation and encapsulation in hydrogel.
Detailed timeline showing differentiation of stem cells (SCs) into neuronal precursors in vitro. LIF: leukemia inhibitory factor; BME: β-mercaptoethanol.
Mechanical properties of PuraMatrix™ peptide hydrogel
Hydrogels have tissue-like mechanical properties, providing a hydrated environment which is both biocompatible and biodegradable and making them ideally suited as tissue regeneration scaffolds [Mawad et al., 2012b]. Hydrogels possess a degree of flexibility very similar to natural tissue, due to their significant water content. Adjusting the water content can therefore change the mechanical properties of the gels slightly, as can the wide range of polymers from which they can be fabricated. The PuraMatrix™ hydrogel consists of standard amino acids (1% w/v) and 99% water, which is synthesized in small (16 amino acids long, 5 nanometers) oligopeptide fragments that self-assemble into nanofibers on a scale similar to the in vivo extracellular matrix (a fibrous structure with an average pore size of 50–200 nm). The material comprises amphiphilic peptides that have alternating repeating units of positively-charged lysine or arginine and negatively-charged aspartate and glutamate residues. These peptides contain 50% charged residues and are characterised by their periodic repeats of alternating ionic hydrophilic and hydrophobic amino acids; thus, the interaction between the distinct polar and non-polar surfaces facilitates self-assembly of the material into a nanofiber hydrogel scaffold which can coat surfaces or encapsulate cells as a 3-D weak gel (http://www.puramatrix.com/technology/tec1.html).
Transplantation of stem cells
Two weeks post-deafening, guinea pigs were anaesthetized with ketamine (60 mg/mL, Parnell Laboratories) and xylazil (4 mg/mL; Troy Laboratories), and 2% Lignocaine (Troy Laboratories) was administered subcutaneously to the incision site. The left tympanic bulla was exposed under aseptic surgical conditions via a post-auricular incision. The bulla was drilled open and the basal turn of the cochlea visualized under a microscope. A small cochleostomy was made into the scala tympani at the level of the basal turn and a fine probe used to puncture the osseous spiral lamina wall overlying Rosenthal’s canal. The prepared SC neurospheres embedded in hydrogel were then delivered to Rosenthal’s canal using the fine tip of a micro-electrode. Following cell delivery, the cochleostomy was sealed with a muscle plug and the wound sutured in two layers. All transplantation surgeries were performed by a trained otologist using aseptic technique.
Processing of tissues for histology and immunohistology
All guinea pigs were transcardially perfused one week post-transplantation with 4% PFA. The cochleae were quickly removed, the apex gently punctured with a 30 gauge needle, and the cochleae post-fixed for a further 90 minutes in separate vials containing 4% PFA, and maintained on gentle rotation at room temperature. The cochleae were then decalcified in 10% EDTA for approximately two weeks (confirmed via radiography), and were trimmed and embedded for immunohistology as previously described [Coleman et al., 2009]. All cochleae were cryosectioned in the modiolar plane at 12 μm increments.
To examine AN density and the extent of any tissue response every second slide was stained with Hematoxylin and Eosin (H&E). Stem cell identification and survival in vitro and in vivo was detected by immunochemical labeling using antibodies to GFP and neurofilament 68 kDa (NFL) or βIII tubulin, in combination with the nuclear counterstain DAPI, in order to determine the location and differentiation status of transplanted cells. Briefly, cryosections were rinsed in PBS and post-fixed for 5 minutes in a weak solution of PFA (1.5%). After 2 rinses in PBS, sections were placed in blocking solution (comprising 3% bovine serum albumin, 4% goat serum, diluted in 0.1% Triton-X in PBS) for 2 hours. The primary antibodies (mouse anti-GFP, 1:200, Millipore; rabbit anti-neurofilament 200 kDa (NFH), 1:800, Millipore, Chicken anti-βIII tubulin, 1:1000, Millipore) were diluted in the blocking solution and tissues immersed overnight in an humidified chamber at 4°C. Any unbound primary antibody was removed by thorough rinsing in 0.1% Tween 20 (Promega) in PBS and the fluorescent secondary antibodies applied (Alexa Fluor 488 goat anti-mouse; Alexa Fluor 594 goat anti-rabbit; Alexa Fluor goat anti-chicken 647) at a 1:500 dilution in 3% BSA, 4% goat serum in 0.1% Tween 20 in PBS, for 4 hours at room temperature. Unbound secondary antibody was removed by thorough rinsing in PBS and then sections mounted in DAPI ProLong Gold fluorescent mounting medium (Molecular Probes). Sections were evaluated under a Zeiss Axioplan Microscope (Zeiss, Victoria, Australia) using a fluorescent lamp with appropriate filters (Zeiss filter set 00; 488000-0000, Zeiss filter set 02; 488002-0000 and Zeiss filter set 13; 488013-0000). Photomicrographs were taken using a Zeiss, AxioCam 12V monochrome digital camera and the computer software AxioVision 4.7.
Quantitative analysis
Detection of transplanted stem cells
Immunolabeled sections were examined for the presence of GFP-positive SCs. Surviving SCs were identified based upon their co-expression of GFP and DAPI. Results were expressed qualitatively to show whether cells were present in the basal, middle or apical cochlear turns.
Auditory neuron density measurements
Sections stained with H&E were examined using light microscopy. To minimize the potential for bias, twenty random sections were selected and counted blindly. Neural density measurements (cells/mm2) were calculated using NIH Image software (http://rsb.info.nih.gov/nih-image/). The number of ANs with a visible nucleus was divided by the cross-sectional area of Rosenthal’s canal, in both the lower basal turn and upper basal turn of the deaf treated, deaf untreated, and normal hearing cochleae. A non-parametric Kruskal-Wallis one-way analysis of variance was used to detect significant differences between these groups.
Inflammatory tissue response
The same cohort of H&E stained sections described above was then used to quantify the tissue response in the basal, middle and apical turns in the cochlea, in each group. The inflammatory tissue response was expressed as a percentage of the total area of the perilymphatic compartment that it occupied, and mean values expressed ± standard error of the mean (SEM). Sections in which a clear cochleostomy or perforation in the osseous spiral lamina wall was visible were deemed “surgical sites” and compared against adjacent sections. A Mann-Whitney Rank Sum Test was used to compare the tissue response measured between treated and untreated groups.
Results
Transplanted stem cells retain a neural phenotype during transplantation
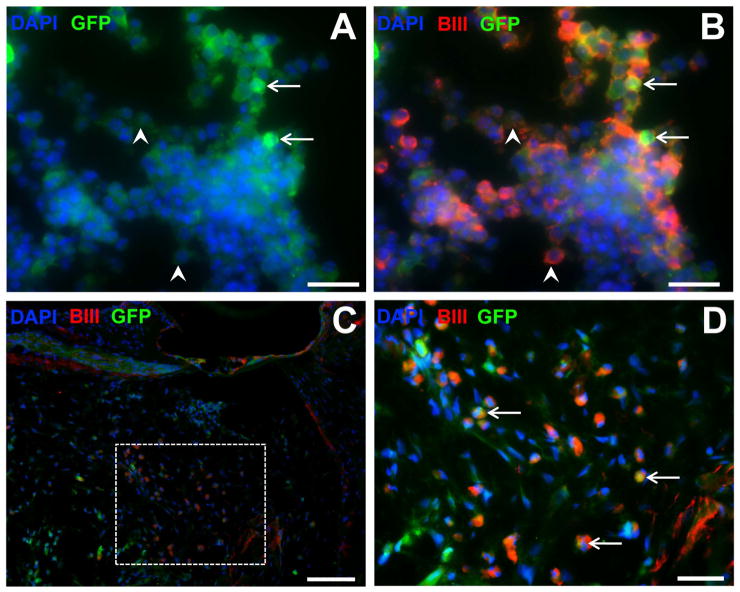
Differentiated SC neurospheres retained their neural phenotype for the duration of the implant period, illustrated by expression of βIII tubulin both before (Fig. 3B) and after (Fig. 3D) transplantation into the deaf cochlea. Furthermore, weak expression of the neuronal marker NFH was detected within transplanted SC neurospheres at the end of the implant period (Fig. 4). Note, the variable luminescence of GFP expression in the R1 B5-EGFP SC line both in vitro (Fig 3. A,B) and in vivo (Fig 3. C,D).
Figure 3. βIII tubulin labeling of stem cells in vitro and in vivo.
Immunofluorescence photomicrographs of differentiated stem cells embedded in hydrogel in vitro (A,B) and in vivo (C, D), expressing green fluorescence protein (GFP, green), βIII tubulin (BIII, red) and counterstained with the nuclear marker DAPI (blue). In vitro preparations (A,B) illustrate the variable expression of GFP within this cell line (low expression, arrowheads; high expression, arrows). Low magnification image in (C) showing location of stem cells in the cochlea, and higher magnification of inset in (D) showing immunopositive labeling. Scale bars = 20 μm (A,B,D); 50 μm (C).
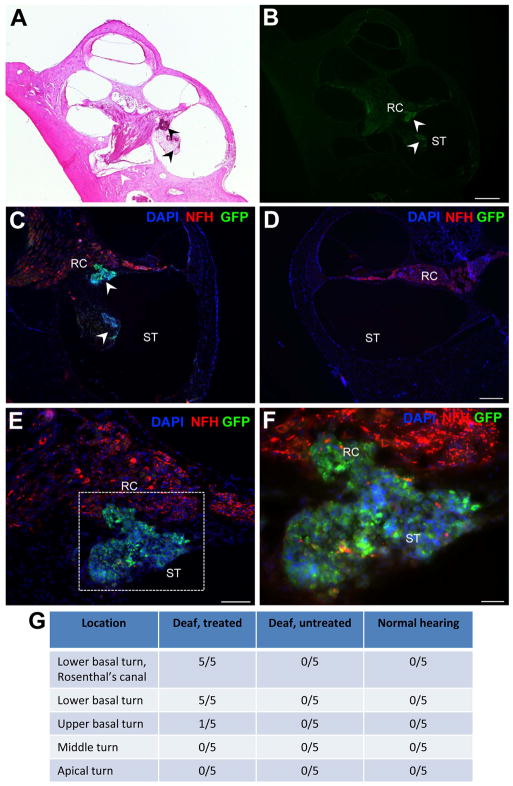
Figure 4. Detection of GFP-positive stem cells in the deaf cochlea.
Low magnification images show implanted stem cells in the lower basal turn of the cochlea (arrowheads in A: H&E stained, and B: GFP-stained). Sequentially higher magnification images of the lower basal turn are shown (C–F) illustrating implanted stem cells (GFP, green, arrowheads C) and endogenous auditory neurons and processes (NFH, red) in the lower basal turn Rosenthal’s canal (RC) and scala tympani (ST). The same region in the deaf untreated cochlea is shown in (D), with no stem cells observed. High magnification image (F) of inset region in (E), illustrating stem cells in RC. Note implanted stem cell sphere is weakly positive for NFH. All images are counterstained with the nuclear marker DAPI (blue; C–F). Table in (G) gives summary of location of stem cells detected in each cochlea examined. Scale bars = 500 μm (B, relative to A); 200 μm (D, relative to C); 100 μm (E), 50 μm (F).
Hydrogel retains transplanted stem cells in the basal turn of the cochlea
Stem cells encapsulated in hydrogel were retained in the basal turn of implanted cochleae (arrowheads, Fig. 4A,B; see also Fig. 6B), effectively limiting their dispersal throughout the perilymphatic compartments for the implantation period. Transplanted cells were identified via co-expression of GFP and DAPI (Fig. 4C–F). GFP-positive SCs were delivered to and detected within Rosenthal’s canal and also in the scala tympani, in the lower basal turn of treated cochleae (arrowheads, Fig. 4C). No GFP-positive SCs were observed in the contralateral control cochleae (Fig. 4D). In one instance, a small number of SCs were observed in the upper basal turn scala tympani, proximal to the surgical site, however no SCs were observed in the middle or apical turns of the SC treated cochleae. Weak expression of the neuron-specific NFH protein was detected within transplanted GFP-positive SC neurospheres (red, Fig. 4F). The location of transplanted SCs within the treated versus untreated cochleae is summarized in Fig. 4G.
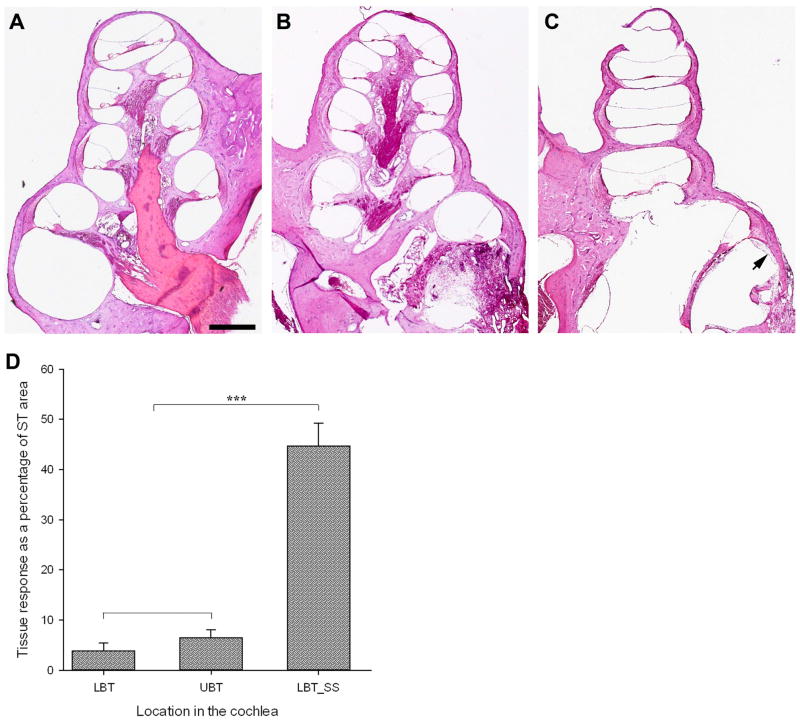
Figure 6. Tissue response measurements and histology.
No tissue response was observed in untreated, normal hearing cochleae (A), however a localized areolar tissue response was observed at the surgical site in deafened, SC-treated animals (arrows B,C) along with a small amount of new bone growth (arrowheads, B). The most aggressive response observed is shown in (B); mean response measured across all five treated animals was 0.5691 mm2 ± 0.0658 mm2. Importantly, only a tiny response was observed in sections adjacent to the surgical site (arrow, C). Measurements and statistical analyses of the tissue response in the treated cochleae showed a small and non-significant reaction in the lower and upper basal turns (LBT and UBT, respectively), but a significant response which was localized to the surgical site (LBT_SS; D). Scale bar in (A) 1000 μm (relative to A–C).
Auditory neuron density is not significantly decreased following stem cell transplantation proceudres
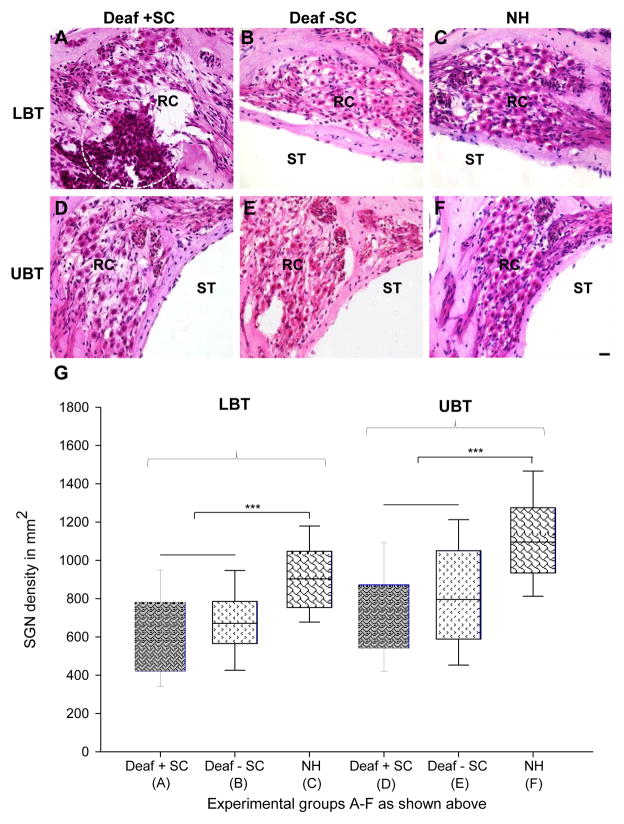
To quantify the extent of AN loss following stem cell delivery, we calculated the density of neurons in the basal turn (Rosenthal’s canal) for each experimental group. The basal turn was chosen due to the localization of the surgery and cochleostomy site to this region. Photomicrographs of Rosenthal’s canal (Fig. 5A–F) illustrate the degree of AN loss in the lower (Fig. 5A–C) and upper (Fig. 5D–F) basal turns in deaf treated, deaf untreated and normal hearing animals. The box plot shows quantified data and statistically different groups (Fig. 5G). Statistical evaluation of data revealed that there was no significant difference in AN density in the lower basal turn of deaf treated (5A; 601 ± 37 cells/mm2) versus deaf untreated (5B; 681 ± 27 cells/mm2) animals. Both groups did however, have statistically lower AN densities in the lower basal turn than those calculated for normal hearing controls (5C; 912 ± 27 cells/mm2; P≤0.001). The same trend was observed in the upper basal turn, which showed no statistical difference in AN density measurements between deaf treated (5D; 737 ± 32 cells/mm2) and deaf untreated (5E; 821 ± 32 cells/mm2) animals. Again, both upper basal turn AN densities in the treated and untreated groups were significantly lower than those measured in normal hearing controls (5F; 1115 ± 33 cells/mm2; P≤0.001).
Figure 5. Auditory neuron density measurements and histology.
Representative histological sections (A–F) from the upper and lower basal turns (UBT and LBT, respectively) from each treatment (Deaf + SC; Deaf − SC; NH), illustrating surviving auditoryneurons (ANs) and transplanted stem cells (circled, A). Auditory neuron densities from each treatment group (A–F above) were measured and graphed (G), and no significant difference was detected between deaf treated and deaf untreated animals in either the upper basal or lower basal turns. There were significantly fewer ANs in deaf cochleae (A,B, D,E) in comparison to normal hearing cochleae (P≤0.001; ***). SC: stem cells; NH: normal hearing; ST: scala tympani; RC: Rosenthal’s canal. Scale bar in (F) 20 μm (relative to A–F).
Transplantation of stem cells causes a localized tissue response, which is restricted to the surgical site
The tissue reaction at the surgical site was dominated by a lose areolar fibrous tissue (arrows; Fig. 6B, C). This tissue response reduced significantly away from the surgical site but did extend into other regions of the basal turn scala tympani (Fig. 6C). The extent of the tissue response was expressed as a percentage of the perilymphatic compartment occupied. As expected, no tissue response was observed in the lower, middle, or upper turns of any of the normal hearing (Fig. 6A) or deaf untreated cochleae (not illustrated). Conversely, animals that received SC transplants showed evidence of an extensive tissue reaction (44.7% ± 4.5%), that was confined to the surgical site in the lower basal turn (Fig. 6B, D). A small tissue response was measured in sections adjacent to the surgical site in the lower (3.9% ± 1.5%; arrow Fig. 6C) and upper (6.9% ± 1.8%; arrow Fig. 6B) basal turns of transplanted animals (illustrated graphically in Fig. 6D). These minor responses were not statistically different to one another (P = 0.817), but were significantly smaller than the tissue response measured at the surgical site (P ≤ 0.001; Fig. 6D). The tissue reaction measured at the surgical site was variable between treated cochleae, with some showing a minimal response (7.3% ± 1.4%) and others a much greater reaction (76.5% ± 9.8%; illustrated in Fig. 6B). Moreover, when quantified in mm2 and compared to surgery only controls from a previous study [Backhouse et al., 2008], no significant difference was detected (0.5691 mm2 ± 0.0658 mm2 versus 0.5231 mm2 ± 0.0478 mm2, respectively).
Discussion
Some of the challenges associated with transplantation of SCs into the deaf mammalian cochlea include choice of delivery site and dispersal of transplanted cells after injection. The purpose of this study was primarily to evaluate the effectiveness of hydrogel at retaining SCs at their site of implantation. The study illustrated that hydrogel was effective at retaining cells at the implant site for the duration of the study, and that the density of ANs was not significantly altered following the delivery of SCs into Rosenthal’s canal. Furthermore, a localized inflammatory tissue reaction was observed in treated cochleae, however this did not prevent the survival of SCs in the lower basal turn, and the localized nature of the tissue response supports our previous findings describing these surgical procedures in guinea pigs [Backhouse et al., 2008].
The present study demonstrated that SCs survive in vivo delivery into the deaf mammalian cochlea within a hydrogel matrix, and retain expression of the neural protein βIII tubulin. In addition, the data illustrate that hydrogel was effective at retaining SCs in the lower basal turn for the duration of the study. Transplanted cells encapsulated in hydrogel were observed both within Rosenthal’s canal and the scala tympani of the basal turn of the cochlea (Fig. 4A; Fig. 5A), an observation supported by others describing this surgical approach [Lang et al., 2008]. This is likely to be a consequence of cell delivery through the fluid-filled scala tympani, which makes precise cell delivery (even using micro-pipettes) challenging. The promising combination of using hydrogel with the Rosenthal’s canal approach, is that even if some cells are delivered into the scala tympani during transplantation, they are retained at the site of implantation. Prevention of cell dispersal has been previously reported in vitro [Semino et al., 2004], and in vivo [Davis et al., 2005], however, this is the first report using hydrogel to minimize dispersal of SCs following delivery of SCs into the deaf cochlea.
In future, hydrogels may confer the benefit of providing a three-dimensional scaffold for new neurite outgrowth, before naturally biodegrading after 6–8 weeks in vivo. Because hydrogels comprise >99% water they are readily biodegradable and biocompatible (http://www.puramatrix.com/index.html). In this way, peptide hydrogels could provide an environment whereby newly transplanted SCs could more readily establish connections with endogenous neurons [Holmes et al., 2000, Ellis-Behnke et al., 2006], providing a scaffold until such time as connections could be made. Moreover, they can be fabricated from natural polysaccharides such as chitosan (cationic), alginate (anionic) or dextran (neutral), natural proteins such as collagen or gelatin, hyaluronan and fibrin, or, from a range of synthetic polymers [Mawad et al., 2012a, Pakulska et al., 2012], making them suitable to a variety of in vivo niches. For cell delivery or encapsulation for example, it is important that the hydrogel matrix be capable of solute transportation, to ensure allow effective exchange of nutrients and waste between exogenous cells and the surrounding environment. Moreover, the natural polymers agarose and chitosan have functional groups available for chemical modification [Pakulska et al., 2012], facilitating the design of tailor-made hydrogels for specific in vivo niches. The incorporation of specific neurotrophins and growth promoting molecules for example, may in future allow for sustained growth and specific differentiation of SCs in vivo, including the possibility of directed neurite outgrowth toward central and peripheral targets in the cochlea.
A fundamental consideration in transplanting SCs into Rosenthal’s canal is to preserve the existing population of ANs, particularly given the persistence of a proportion of these neurons in deaf human subjects [Fayad and Linthicum, 2006]. An effective cell-based therapy would ideally avoid depleting the cells that it intended to replace, therefore the density of ANs was an important indicator of the efficacy of this approach. We observed that the density of ANs in deaf treated animals was not significantly different to the density of ANs in deaf untreated animals, in both the lower and upper basal turns of the cochlea. These findings illustrate the feasibility of this approach for future cell-based therapies given that the trauma from such invasive surgery does not result in a significant loss of endogenous neurons and is localized to the surgical site. With many studies now aimed at regenerating/replacing the ANs following deafness, it will be important to quantify the survival of endogenous ANs in order to compare the efficacy of various cell replacement strategies in the deafened cochlea in future.
The delivery of SCs into the deaf cochlea in the present study involved a cochleostomy into the lower basal turn scala tympani, followed by the perforation of the osseous spiral lamina wall overlying Rosenthal’s canal. The inflammatory tissue response observed was moderate considering the invasive nature of this approach, and most importantly, was localized to the surgical site. The tissue response was identified histologically and comprised primarily areolar fibrous tissue which was limited to the surgical site. New bone was also evident in the vicinity of the surgical site (arrowheads; Fig. 6B) and was likely associated with the presence of bone chips that entered the scala tympani during the drilling to create the cochleostomy [Clark et al., 1995]. In the treated cochleae, the mean cross-sectional area that inflammatory cells occupied was less than 50% of the perilymphatic space in the lower basal turn scala tympani. This was reduced to <10% in sections adjacent to the surgical site and in the upper basal turn, demonstrating the localized nature of this response. This is consistent with previous experimentation describing localized trauma to the osseous spiral lamina wall using the same approach [Backhouse et al., 2008], and during the insertion of a cochlear implant electrode array [Shepherd et al., 1983, Shepherd et al., 1995, Shepherd et al., 2005]. Such a response is undesirable clinically, due to the fibrous tissue which can surround the cochlear implant electrode array and contribute to increased impedance and, as a result, increased power consumption, of this electrical device [Xu et al., 1997, Newbold et al., 2004]. The tissue response observed in the current investigation is likely to resolve into mature fibrous tissue over more extended periods, and it may assist to retain transplanted cells within the perilymphatic space after the eventual degradation of the hydrogel. Using the described approach, SCs could be delivered at the same time as a cochlear implant electrode array. This may prove important, given the likelihood of a localized tissue response to SC implantation, and the effect this might have on electrode insertion at a later time.
Future studies will investigate the long-term survival and viability of SCs delivered in hydrogel, and these are currently underway in our laboratory. We envisage that the hydrogel will biodegrade over 6–8 weeks in vivo, leaving stem cells at the implantation site. However, we do not expect any subsequent migration of these cells, but rather that they will be contained within the fibrous tissue matrix that develops around the hydrogel. In addition, future studies will need to incorporate the development of delivery techniques that allow SCs transplanted into Rosenthal’s canal to spread along the length of the modiolus. Previous studies have illustrated that widespread migration of SCs within the modiolus is indeed possible and that exogenous SCs survived in the denervated cochlea for the maximum implant period of 14 weeks [Corrales et al., 2006]. Yet to be elucidated is whether these transplanted cells are capable of forming functional and tonotopic connections, a vital step to the long-term success of SC replacement therapy in the cochlea. Combining cell transplantation with electrical stimulation from a cochlear implant, may confer the advantage of maintaining the residual population of auditory neurons whilst depolarizing the newly transplanted cell population. Previous studies have illustrated that chronic depolarization provides important trophic cues for auditory neurons in vitro [Hansen et al., 2001], and in vivo [Leake et al., 1999] and this may improve survivability and connectivity of newly transplanted SC-derived neurons.
Acknowledgments
The authors extend their thanks to the following organizations for their financial support of this work: the Department of Otolaryngology, the University of Melbourne, the Garnett Passe and Rodney Williams Memorial Foundation, the Bionics Institute, the Thomas Wickham Jones Foundation (UK), and the Royal Victorian Eye and Ear Hospital. The Bionics Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program. B.A. Nayagam is supported by an NH&MRC Australian-Based Biomedical Research Fellowship.
Footnotes
Author Disclosure Statement
The authors declare no competing commercial associations or potential conflicts of interest in connection with the submitted manuscript.
References
- 1.Ahn KS, Jeon SJ, Jung JY, Kim YS, Kang JH, Shin S, Choi T, Choi SJ, Chung P, Shim H. Isolation of embryonic stem cells from enhanced green fluorescent protein-transgenic mouse and their survival in the cochlea after allotransplantation. Cytotherapy. 2008;10:759–769. doi: 10.1080/14653240802419286. [DOI] [PubMed] [Google Scholar]
- 2.Altschuler RA, O’Shea KS, Miller JM. Stem cell transplantation for auditory nerve replacement. Hear Res. 2008;242:110–116. doi: 10.1016/j.heares.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhouse S, Coleman B, Shepherd R. Surgical access to the mammalian cochlea for cell-based therapies. Exp Neurol. 2008;214:193–200. doi: 10.1016/j.expneurol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark GM, Shute SA, Shepherd RK, Carter TD. Cochlear implantation: osteoneogenesis, electrode-tissue impedance, and residual hearing. Ann Otol Rhinol Laryngol Suppl. 1995;104:40–22. [PubMed] [Google Scholar]
- 5.Coleman B, Hardman J, Coco A, Epp S, de Silva M, Crook J, Shepherd R. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman B, Rickard NA, de Silva MG, Shepherd RK. A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods. 2009;176:144–151. doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of corti. J Neurobiol. 2006;66:1489–5000. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis-Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci U S A. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlear and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 14.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004a;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol. 2004b;185:7–14. doi: 10.1016/j.expneurol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051:137–144. doi: 10.1016/j.brainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, Schmiedt RA. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9:225–240. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Mawad D, Boughton EA, Boughton P, Lauto A. Advances in hydrogels applied to degenerative diseases. Curr Pharm Des. 2012a;18:2558–2575. doi: 10.2174/138161212800492895. [DOI] [PubMed] [Google Scholar]
- 21.Mawad D, Stewart E, Officeer DL, Romeo T, PW, Wagner K, Wallace GG. A single component conducting polymer hydrogel as a scaffold for tissue engineering. Advanced Functional Materials. 2012b doi: 10.1002/adfm. 20110237318. [DOI] [Google Scholar]
- 22.Newbold C, Richardson R, Huang CQ, Milojevic D, Cowan R, Shepherd R. An in vitro model for investigating impedance changes with cell growth and electrical stimulation: implications for cochlear implants. J Neural Eng. 2004;1:218–227. doi: 10.1088/1741-2560/1/4/005. [DOI] [PubMed] [Google Scholar]
- 23.Okano T, Nakagawa T, Endo T, Kim TS, Kita T, Tamura T, Matsumoto M, Ohno T, Sakamoto T, Iguchi F, Ito J. Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport. 2005;16:1919–1922. doi: 10.1097/01.wnr.0000187628.38010.5b. [DOI] [PubMed] [Google Scholar]
- 24.Olivius P, Alexandrov L, Miller J, Ulfendahl M, Bagger-Sjoback D, Kozlova EN. Allografted fetal dorsal root ganglion neuronal survival in the guinea pig cochlea. Brain Res. 2003;979:1–6. doi: 10.1016/s0006-8993(03)02802-6. [DOI] [PubMed] [Google Scholar]
- 25.Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed Mater. 2012;7:024101. doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- 26.Regala C, Duan M, Zou J, Salminen M, Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193:326–333. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Reyes JH, O’Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiya T, Kojima K, Matsumoto M, Kim TS, Tamura T, Ito J. Cell transplantation to the auditory nerve and cochlear duct. Exp Neurol. 2006;198:12–24. doi: 10.1016/j.expneurol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Semino CE, Kasahara J, Hayashi Y, Zhang S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Eng. 2004;10:643–655. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Physiological and histopathological results. Acta Otolaryngol Suppl. 1983;399:19–31. doi: 10.3109/00016488309105589. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd RK, Clark GM, Xu SA, Pyman BC. Cochlear pathology following reimplantation of a multichannel scala tympani electrode array in the macaque. Am J Otol. 1995;16:186–199. [PubMed] [Google Scholar]
- 32.Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]