Abstract
Neurotrophin-BDNF can be effectively encapsulated in nanoporous poly(L-glutamic acid) particles prepared via mesoporous silica templating. The loaded BDNF can be released in a sustained manner with maintained biological activity. Animal experiments demonstrate the released BDNF can efficiently rescue the auditory neurons (as indicated by the arrows) in the cochlea of guinea pigs with sensorineural hearing loss.
Keywords: Mesoporous silica, Nanoporous polymer particle, Drug delivery, Neurotrophic factors, Sensorineural hearing loss
Neurotrophic factofrs are secreted signaling proteins that are essential for the survival of neurons.[1] Among these factors is a neurotrophin known as brain-derived neurotrophic factor (BDNF) which has a widespread and profound action on the nervous system, affecting many physiological processes such as learning, memory consolidation, sensory circuit maturation, myelination and neuronal survival.[2] Neurodegenerative diseases such as Alzheimer’s disease, nerve deafness, and Huntington’s syndrome are associated with an impaired production of BDNF.[3] Consequently, therapeutic approaches using viruses, genetically modified cells or pump-based delivery systems have been designed to provide an exogenous source of BDNF to prevent or reduce the rate of neurodegeneration.[4]
In the past decade, particle-based therapeutic delivery systems have generated considerable interest because engineered particles can provide unparalleled advantages in many aspects of drug delivery, examples of which include improving the solubility of poorly water-soluble compounds, prolonging the half-life of therapeutic drugs in blood circulation, providing a variety of controlled release profiles (e.g., sustained release, stimuli-responsive release), and delivering a drug locally or in a targeted manner to minimize adverse side effects.[5] In this communication, we report the first investigation of the use of nanoporous peptide particles as a nanocarrier to deliver BDNF to rescue neuronal cells that have lost their endogenous neurotrophin supply. The efficacy of the released BDNF was evaluated both in neuronal cell cultures and in an animal model of sensorineural hearing loss. Compared with the complexity and/or risks of infection associated with the currently available therapeutic approaches (i.e., using genetically modified cells or pump-based delivery systems) to boost BDNF levels,[4] salient potential advantages of using nanoporous particles as reservoirs for prolonged BDNF supply include simplicity and safety, which are essential properties regarding clinical application.
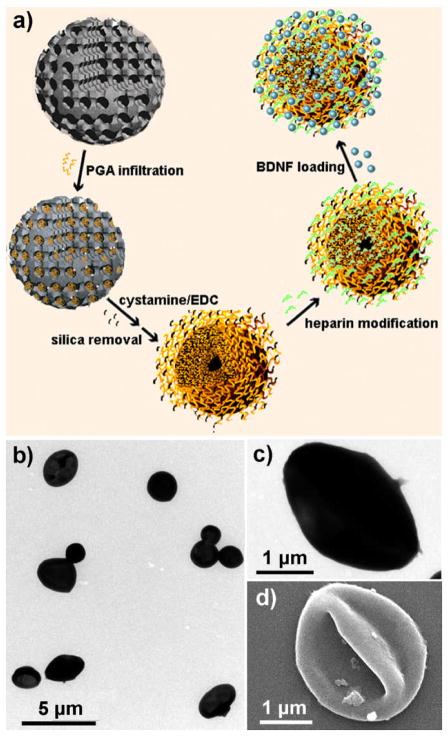
The nanoporous peptide particles are prepared with a mesoporous silica (MS) template using our previously reported protocol.[6] This approach has been shown to be versatile in terms of polymer type and cross-linking strategy, and can be fine-tuned to manipulate the composition, morphology, and porosity of the nanostructures required for various applications.[7] Due to the nanoporosity and high density of functional groups (in the polymer chains), particles prepared via this procedure have demonstrated excellent adsorption capacity to proteins.[6a] In this work, poly(L-glutamic acid) (PGA) was chosen as the polymer building block for preparing the particulate carriers for BDNF delivery because it is a biodegradable polypeptide with excellent biocompatibility.[8] The preparation of PGA replica particles begins with the infiltration of PGA (Mw 60 000 Da) into amine-functionalized, positively charged MS particles with a bimodal pore structure (~3 nm and 10–40 nm), and subsequent cross-linking of the adsorbed PGA chains using a homobifunctional crosslinker, cystamine (Fig. 1a). The cystamine reacts with the substrate carboxyl groups to form an amide linkage which interconnects two PGA chains. Replicated PGA particles are obtained after removal of the MS template.
Figure 1.
Schematic illustration of BDNF encapsulation in nanoporous PGA particles produced via mesoporous silica templating (a). TEM images of the PGA particles at low (b) and high (c) magnifications. SEM images of the PGA particles (d).
Transmission electron microscopy (TEM) reveals the PGA particles have a diameter of ca. 1.8–3.2 μm (Fig. 1b), which is slightly smaller than the original templating MS particles (ca. 2–4 μm). This size difference can be attributed to the shrinkage of the particles after removal of the sacrificial silica template. At higher magnification, the particles showed a collapsed structure (Fig. 1c), which is also evidenced from the scanning electron microscopy (SEM) images (Fig. 1d). The collapse of the particle is likely caused by the relatively low density of PGA molecules infiltrated inside the particles; hence the spherical structures are unable to be maintained upon drying of the particles for electron microscope examination. Zeta-potential measurements reveal that the PGA particles are slightly positively charged (ca. +9 mV) in Milli Q water. This is possibly caused by some side amine groups in the cystamine that are not conjugated to a PGA chain.
Like other neurotrophins, BDNF is a basic protein with a molecular weight of 13 kDa and isoelectronic point of pH 10. To effectively load the positively charged BDNF into the as-prepared PGA particles, the polysaccharide heparin sulphate (Mw. 12 000–15 000 Da) is infiltrated in the PGA particles to reverse their charge because heparin sulphate has the highest negative charge density of any known biological molecule.[9] It has been used previously to control the release of heparin-like growth factors and a nerve growth factor.[10] However, to the best of our knowledge, it has not previously been shown to prolong release of non-heparin-like growth factors such as BDNF. Successful modification of the PGA particles by heparin was indicated by a highly negatively charged particle (ca. −40 mV) and visualized by confocal laser scanning microscopy using AF488-labelled heparin (Figure S1). The efficient and nearly quantitative loading of BDNF in the heparin-modified PGA particles were indicated by the dramatic decrease of the BDNF from an initial concentration of 50 μg mL−1 to 130 ng mL−1 after overnight incubation. This corresponds to sequestering of 99.74% of the protein from the incubation solution. The amount of encapsulated BDNF in each PGA particle is ca. 1.5 × 10−2 pg, corresponding to a BDNF concentration of ca. 20 mg mL−1 in the nanoporous PGA particles (assuming an average particle size of 2.5 μm). This efficient loading can be attributed to the strong electrostatic interactions between the residual amine group on the protein, the carboxyl group on the PGA polymer and the highly sulfonated glycosaminoglycan branches on the heparin polymer.
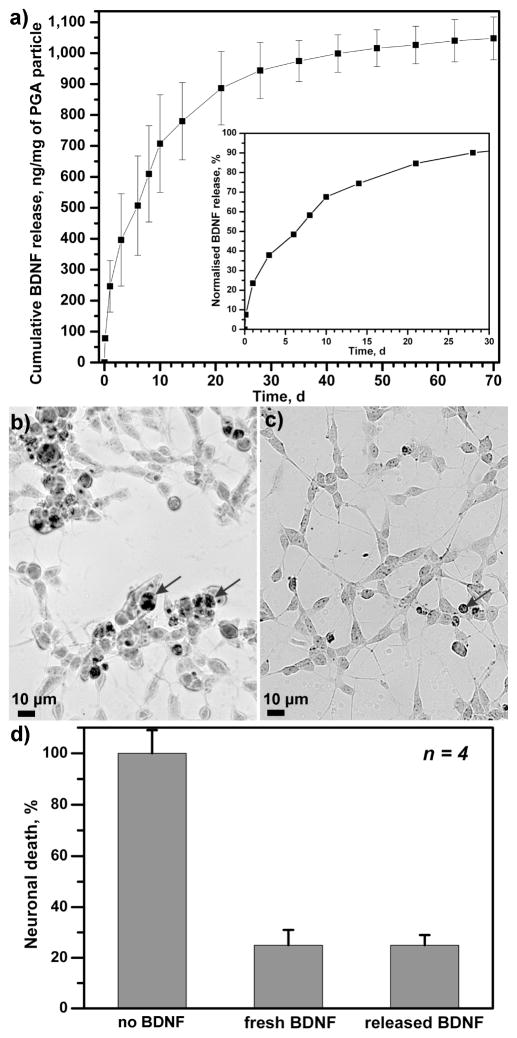
The in vitro release profiles of the encapsulated BDNF were investigated by incubating BDNF-loaded PGA particles (3.4 × 108 particles in each experiment) in phosphate-buffered saline (PBS) at 37 °C. The amount of BDNF released was monitored by an enzyme-linked immunosorbent assay (ELISA). It is worth noting that minimal BDNF leakage (ca. 0.06%) out of the particles was found during storage in Milli Q water, indicating significant affinity between the BDNF protein and the heparin-modified nanoporous PGA particles. The cumulative release of BDNF in PBS over a period of 70 days is depicted in Figure 2a. Overall, the BDNF release profile indicated three phases: a robust release within the first 3 days, a modest release over the next 20 days and a minimal release to the end of the incubation period. Although the majority (ca. 90%) of the BDNF was released by day 30, there was still detectable BDNF released up to day 70. The mechanism of BDNF release is likely attributed to the hydrolytic disruption of the electrostatic interactions between BDNF and PGA particles due to changes in pH, salt concentration and incubation temperature.
Figure 2.
Release profile and biological activity of BDNF-encapsulated PGA particles. (a) Incubating BDNF-encapsulated PGA particles in PBS solution at 37 °C triggered the release of BDNF robustly within the first 3 days (inset), more modestly in the next 20 days and slowly toward the end of incubation period. Data are the mean ± the standard error of three independent release experiments. (b) In the absence of fetal calf serum, differentiated SH-SY5Y neurons underwent apoptosis, as indicated by the presence of darkly stained, TUNEL-positive neurons (arrows), but significantly fewer neurons died when 2 nM of BDNF released from BDNF-encapsulated PGA particles was added (c), demonstrating that BDNF maintained its bioactivity following release from the PGA particles. In four different experiments, BDNF released from BDNF-encapsulated PGA particles or fresh BDNF significantly reduced apoptotic death in serum-deprived differentiated SHSY-5Y neurons (d).
The biological activity of the released BDNF from the PGA particles was confirmed through its ability to prevent programmed cell death (PCD) or apoptosis in SH-SY5Y cells. These neuroblastoma cells readily differentiate into neurons upon the addition of retinoic acid.[11] In the absence of fetal calf serum, these neurons undergo PCD.(Encinas, Iglesias et al. 1999)[12] However, death can be prevented by the addition of 2 nM of BDNF.[12] Following the release of BDNF from PGA particles, we measured its concentration and added the equivalent of 2 nM of BDNF into differentiated, serum-deprived SH-SY5Y cells. Serum-deprived SH-SY5Y cells underwent apoptosis, as indicated by the presence of darkly stained nuclei (Fig. 2b). The addition of 2 nM of BDNF released from PGA particles significantly reduced the number of apoptotic cells in the culture (Fig. 2c). This effect was found to be statistically significant (Fig. 2d, Student-Newman-Keuls pairwise comparison, p<0.001). In addition, the rescue effect was comparable to 2 nM of fresh BDNF added in the culture (Fig. 2d). A previous study has found that BDNF bioactivity is also retained when it is delivered from multilayered polyelectrolyte films.[13]
To assess the potential of BDNF-loaded PGA particles in small animal models, we delivered these particles (3.4 × 108 particles per animal) into the cochlea of guinea pigs that were systemically deafened with high doses of aminoglycoside antibiotics.[14] Anatomical features of the cochlea are shown in Figure S2. These ototoxic drugs destroy hair cells and surrounding cells in the organ of Corti, the receptor organ of hearing in the cochlea.[15] These hair cells are also the targets of primary auditory neurons (PAN) and secrete neurotrophic factors to promote the survival of these neurons.[16] The loss of hair cells diminishes this neurotrophic support, causing secondary degeneration of these neurons.[3b] The BDNF-loaded PGA particles were deposited at the most basal turn of the snail-shaped cochlea, just past the round window. This turn was selected because of three distinctive features: (i) it has a large acellular cavity, known as scala tympani that can hold these particles; (ii) the scala tympani is adjacent to the bony Rosenthal’s canal where the PANs are situated, so delivery at this site increases access of the neurotrophic factor to these neurons; and (iii) this region of the scala tympani is surgically accessible. For each animal, one cochlea was treated for 20 days with BDNF-loaded PGA particles whereas the untreated, contralateral cochlea served as a deafened control. This duration of treatment was selected because we observed relatively higher rates of BDNF released during the first 3 weeks in the in vitro release experiments (Fig. 2a).
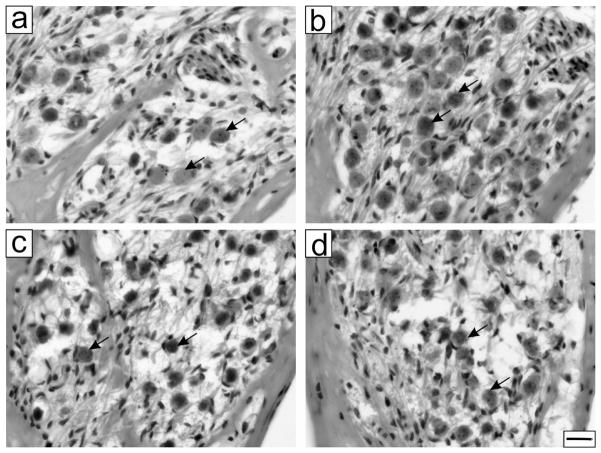
Comparing the neuronal density at the most basal turn, also known here as T1, more neurons were observed in the treated cochlea (Fig. 3b) compared to the untreated cochlea from the same animal (Fig. 3a). From four treated animals, the mean neuronal density of PANs was 948 ± 113 neurons/mm2 in the treated cochlea and 647 ± 92 neurons/mm2 in the untreated cochlea, a significant 47% increase due to the treatment (paired Student’s t-test, p=0.0126). To verify that the rescue effects observed were specific to BDNF treatment, three deafened animals received only heparin-modified PGA particles (3.4 × 108) in one cochlea in control experiments. The mean neuronal density of PANs was 845 ± 161 neurons/mm2 in the treated cochlea and 757 ± 221 neurons/mm2 in the untreated cochlea. As expected, no apparent differences were found between cochlear sections from the particle-treated cochleae (Fig. 3d) and sections from the contralateral, untreated cochleae (Fig. 3c). Differences between both cochleae were not significant (paired Student’s t-test, p=0.6).
Figure 3.
Effects of BDNF-encapsulated PGA particles in the inner ear. (a) After exposure to aminoglycoside antibiotics, fewer primary auditory neurons survived (indicated by arrows) but treatment with BDNF-encapsulated PGA particles in one ear increased the survival of these neurons (b). Treatment with the same amount of non-encapsulated PGA particles did not improve the survival of these neurons in the treated ear (d), compared to the untreated (c). Scale bar is 20 μm for (a)–(d).
In conclusion, we have demonstrated that nanoporous PGA particles could be synthesized via mesoporous silica templating and that the prepared particles could be used effectively as nano-carriers for BDNF delivery. In vitro release experiments showed that the loaded BDNF could be sustainably discharged from the PGA particles in a period over two months with a relatively quicker release rate in the first three weeks. Biological activity of the discharged BDNF was demonstrated by its ability to support the survival of differentiated SH-SY5Y neurons. Chronic animal experiments demonstrated that BDNF released from the particles could efficiently rescue primary auditory neurons in the cochlea of guinea pigs with sensorineural hearing loss. Considering the present need of safer therapeutic approaches to treat neurodegenerative diseases, the use of nanoporous peptide particles as biocarriers for neurotrophin delivery provides a promising method for clinical application in the treatment of damaged nerves. Future studies using this delivery technique include the potential delivery of neurotrophins to sites within the central nervous system for the treatment of a variety of neurodegenerative disorders, and developing the particles as a nanocarrier to co-deliver other therapeutic agents such as anti-inflammatory drugs.
Experimental
PGA Particles Preparation and BDNF Loading
The polypeptide PGA particles were prepared by incubating 2 mL PGA stock solutions (5 mg mL−1 PGA in 50 mM MES buffer at pH 5.5) with 10 mg of APTS-modified positively charged MS particles for 12 h, followed by removal of excess polypeptide. The covalent cross-linking of the polypeptides was performed by separately adding 50 μL of aqueous cystamine (10 mg mL−1) and 200 μL of EDC (60 mg mL−1) to the particle suspension and incubating at room temperature for 6 h. After washing with Milli Q water, the mesoporous silica template particles were removed by exposure to a solution of 2 M hydrofluoric acid/8 M ammonium fluoride buffer (pH 5) to obtain the replicated PGA particles, which were then dispersed in 1 mL of Milli Q water. From flow-cytometry, we determined that the number concentration of the particles was 1.7 × 109 mL−1. 1 mL of heparin solution (2 mg mL−1) was added to the PGA particle suspension and gently shaken at room temperature overnight, followed by three cycles of spinning down/supernatant removal/washing with Milli Q water to remove the unbound heparin. The particles were then stored in 250 μL of Milli Q water. 50 μL of BDNF (Millipore, GF029) stock solution with a BDNF concentration of 0.1 mg mL−1 was added to the PGA-heparin suspension (50 μL) and allowed to incubate at room temperature overnight. The particles were then centrifuged and washed once to remove excess BDNF.
In Vitro BDNF Release
The BDNF-loaded particles were centrifuged to remove the supernatant and this was kept frozen for analysis to ascertain any leakage of the protein during storage. 100 μL of 150 mM phosphate-buffered saline (PBS, pH 7.3) at 37 °C was added to the centrifugation tube and vortexed gently to lift the particles. The solution containing the particles and PBS was placed in an incubator at 37 °C and shaken gently. At various time intervals, the particles were spun down, supernatant removed for BDNF immunoassay and fresh PBS added to continue the incubation step. For each time-point, BDNF measurements were performed in duplicates using BDNF Emax ImmunoAssay according to the manufacturer’s protocol.
Cell Culture
SH-SY5Y cells were grown in Dulbecco’s modified Eagle’s medium, supplemented with L-glutamate (2 mM), penicillin (20 units mL−1), streptomycin (20 mg mL−1) and 10% fetal bovine serum. They were plated at a density of ~2000 cells cm−2 in each well of a Nunc Lab-Tek II 8-well chamber slide and incubated at 37 °C in a saturated humidity atmosphere containing 95% air and 5% CO2. Cells differentiated into neurons after 5 days incubation in culture medium containing 10 μM retinoic acid. These cells were subsequently exposed to serum-deprived culture medium only (negative control) or culture medium containing only 2 nM of human recombinant BDNF (positive control) or 2 nM of BDNF released from PGA particles. After 1 week, cells were fixed for 10 min in 1% paraformaldehyde in PBS, rinsed 3 times with PBS and permeabilized for 5 min in a 2:1 ethanol:acetic acid mixture, which was precooled to −20 °C. Next, an ApopTag Peroxidase apoptosis detection kit (Millipore Australia, cat #S7100) was used to quantify dying cells based on a TUNEL assay that labels fragmented DNA of dying cells.
Animal Surgery
The St Vincent’s Hospital Melbourne Animal Ethics Committee approved the use of Dunkin-Hartley (strain) guinea pigs in this study. Guinea pigs were deafened with frusemide (130 mg per kg of body weight) and kanamycin (420 mg per kg of body weight) using procedures previously described.[14] One week later, hearing thresholds were determined in anaesthetized animals by recording the auditory brainstem response elicited from a click stimulus, as described elsewhere.[14] Surgery was performed under aseptic conditions 2 weeks after deafening. Animals were anaesthetized with an intramuscular injection of ketamine (60 mg per kg body weight) and xylazine (4 mg per kg body weight). An incision was performed above the external ear to expose the bulla which is a bony chamber protecting the inner ear. A hole was drilled through the bulla to expose the round window of the cochlea. The thin membrane covering this window was ruptured with a 30G needle and traces of exuding perilymphatic fluids were drained using light suction. Particles were then soaked in small pieces of surgical grade Gelfoam® (Pfizer, Germany) made from gelatin sponge and carefully inserted into the cochlea via this round window. Finally, muscle tissues were used to plug the round window and the hole in the bulla was sealed with dental cement.
Histology
After 20 days of treatment, animals were sacrificed with a lethal dose of sodium pentobarbitone and perfused intracardially with ice-cold PBS. Cochleae were rapidly dissected out and the middle ear ossicles removed to allow penetration of the fixative – 4% paraformaldehyde dissolved in PBS. Cochleae were fixed overnight at 4 °C with shaking. Following fixation, they were decalcified in 10% ethylene diamine tetraacetic acid dissolved in PBS (pH 8.0) to soften the tissues for sectioning. After decalcification, they were incubated for 7 h in 30% sucrose dissolved in PBS before overnight infiltration and final embedding in Optimum Cutting Temperature (OCT) compound (Sakura, Tokyo, Japan). For each animal, both cochleae were fixed and decalcified for the same duration. Cochleae were cryo-sectioned along the plane of the vertical, central axis at a thickness of 12 μm and stained with haematoxylin and eosin, as described. Five representative sections were chosen for neuronal counting. In this series, each section was separated from its consecutive section by a distance of 96 μm between sections. Histological images and counting were performed using the Axio Imager M2 microscope (Carl Zeiss MicroImaging, Goettingen, Germany). Counting was performed under 40× magnification using fine focusing to identify neurons with clear nuclei.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) Project Grant APP1005071 (R.K.S., F.C., J.T., Y.W.), by the Australian Research Council under the Federation Fellowship scheme (F.C.), the Royal Victorian Eye and Ear Hospital (F.G.) and the National Institutes of Health (USA) HHS-N-263-2007-00053-C (R.K.S.). The Bionics Institute acknowledges the support it receives from the Victorian government through its Operational Infrastructure Support Program. We thank Genevieve Evin, Jacqueline Andrews, Dimitra Stathopoulos, and Alison Neil for advice and assistance. Supporting Information is available online from Wiley InterScience or from the author.
Contributor Information
Dr. Justin Tan, Bionics Institute, East Melbourne, Victoria 3002 (Australia). Department of Otolaryngology, The University of Melbourne, East Melbourne, Victoria 3002 (Australia).
Dr. Yajun Wang, Department of Chemical and Biomolecular Engineering, The University of Melbourne Parkville, Victoria 3010 (Australia).
Xiaopei Yip, Bionics Institute, East Melbourne, Victoria 3002 (Australia).
Dr. Fergal Glynn, Bionics Institute, East Melbourne, Victoria 3002 (Australia)
Prof. Robert K. Shepherd, Bionics Institute, East Melbourne, Victoria 3002 (Australia). Department of Otolaryngology, The University of Melbourne, East Melbourne, Victoria 3002 (Australia)
Prof. Frank Caruso, Email: fcaruso@unimelb.edu.au, Department of Chemical and Biomolecular Engineering, The University of Melbourne Parkville, Victoria 3010 (Australia).
References
- 1.a) Hans T. Trends Neurosci. 1991;14:165. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]; b) Lewin GR, Barde YA. Annu Rev Neurosci. 1996;19:289. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.a) McAllister AK, Katz LC, Lo DC. Annu Rev Neurosci. 1999;22:295. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]; b) Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Neuron. 1999;24:401. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]; c) Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Proc Nat Acad Sci USA. 2001;98:14661. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Murer MG, Yan Q, Raisman-Vozari R. Prog Neurobio. 2001;63:71. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]; b) Tan J, Shepherd RK. Am J Pathol. 2006;169:528. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Nat Med. 2009;15:331. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pettingill LN, Wise AK, Geaney MS, Shepherd RK. PLoS One. 2011;6:e18733. doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Praetorius M, Limberger A, Muller M, Lehner R, Schick B, Zenner HP, Plinkert P, Knipper M. Audiol Neurootol. 2001;6:250. doi: 10.1159/000046130. [DOI] [PubMed] [Google Scholar]
- 5.a) Farokhzad OC, Langer R. Adv Drug Deliv Rev. 2006;58:1456. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]; b) Emerich DF, Thanos CG. J Drug Target. 2007;15:163. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]; c) Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin Pharmacol Ther. 2008;83:761. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]; d) Roy S, Johnston AH, Newman TA, Glueckert R, Dudas J, Bitsche M, Corbacella E, Rieger G, Martini A, Schrott-Fischer A. Int J Pharm. 2010;390:214. doi: 10.1016/j.ijpharm.2010.02.003. [DOI] [PubMed] [Google Scholar]; e) Wang Y, Yan Y, Cui JW, Hosta-Rigau L, Heath JK, Nice EC, Caruso F. Adv Mater. 2010;22:4293. doi: 10.1002/adma.201001497. [DOI] [PubMed] [Google Scholar]
- 6.a) Wang Y, Yu AM, Caruso F. Angew Chem Int Ed. 2005;44:2888. doi: 10.1002/anie.200462135. [DOI] [PubMed] [Google Scholar]; b) Wang Y, Price AD, Caruso F. J Mater Chem. 2009;19:6451. [Google Scholar]
- 7.a) Wang Y, Caruso F. Adv Mater. 2006;18:795. [Google Scholar]; b) Wang Y, Angelatos AS, Dunstan DE, Caruso F. Macromolecules. 2007;40:7594. [Google Scholar]; c) Wang Y, Bansal V, Zelikin AN, Caruso F. Nano Letters. 2008;8:1741. doi: 10.1021/nl080877c. [DOI] [PubMed] [Google Scholar]; d) Cui JW, Wang Y, Hao JC, Caruso F. Chem Mater. 2009;21:4310. [Google Scholar]; e) Zhang X, Oulad-Abdelghani M, Mendoza C, Zelkin AN, Wang Y, Haikel Y, Mainard D, Voegel JC, Caruso F, Benkirane-Jessel N. Biomaterials. 2010;31:1699. doi: 10.1016/j.biomaterials.2009.11.032. [DOI] [PubMed] [Google Scholar]; f) Yan Y, Wang Y, Heath JK, Nice EC, Caruso F. Adv Mater. 2011;23:3916. doi: 10.1002/adma.201101609. [DOI] [PubMed] [Google Scholar]
- 8.a) Manocha B, Margaritis A. Crit Rev Biotechnol. 2008;28:83. doi: 10.1080/07388550802107483. [DOI] [PubMed] [Google Scholar]; b) Tryoen-Toth P, Vautier D, Haikel Y, Voegel JC, Schaaf P, Chluba J, Ogier J. J Biomedical Mater Res. 2002;60:657. doi: 10.1002/jbm.10110. [DOI] [PubMed] [Google Scholar]
- 9.Cox MM, Nelson DL. In: Lehninger Principles of Biochemistry. Nelson DL, Cox MM, editors. Freeman; 2004. p. 1100. [Google Scholar]
- 10.a) Chung HJ, Kim HK, Yoon JJ, Park TG. Pharm Res. 2006;23:1835. doi: 10.1007/s11095-006-9039-9. [DOI] [PubMed] [Google Scholar]; b) Almodovar J, Bacon S, Gogolski J, Kisiday JD, Kipper MJ. Biomacromolecules. 2010;11:2629. doi: 10.1021/bm1005799. [DOI] [PubMed] [Google Scholar]
- 11.Encinas M, Iglesias M, Liu YH, Wang HY, Muhaisen A, Cena V, Gallego C, Comella JX. J Neurochem. 2000;75:991. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 12.Encinas M, Iglesias M, Llecha N, Comella JX. J Neurochem. 1999;73:1409. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- 13.Vodouhe C, Schmittbuhl M, Boulmedais F, Bagnard D, Vautier D, Schaaf P, Egles C, Voegel JC, Ogier J. Biomaterials. 2005;26:545. doi: 10.1016/j.biomaterials.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd RK, Coco A, Epp SB, Crook JM. J Comp Neurol. 2005;486:145. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forge A, Schacht J. Audiol Neurootol. 2000;5:3. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 16.Rubel EW, Fritzsch B. Annu Rev Neurosci. 2002;25:51. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.