Abstract
Background
Geographic stomatitis is an uncommon oral lesion that presents similar clinical, histopathological and genetic features as those of psoriasis. These findings suggest that this lesion may actually represent an oral manifestation of psoriasis. We report one case of geographic stomatitis and discuss a possible connection between this condition and psoriasis.
Main observations
A 37-year-old woman presented with red patches, surrounded by a white border on the labial mucosa and a positive family history of psoriasis. Histopathological examination, immunohistochemical analysis with antibodies against CD4, CD8, CD20, CD68, CD31, and Ki-67 and HLA-A*, -B*, -C*, -DRB1*, -DQA1* and -DQB1* genotyping were performed. Histopathological examination revealed parakeratosis, marked elongation of rete ridges with acanthosis and clubbing, exocytosis, Munro microabscesses, pustule of Kogoj, dilated tortuous vessels at the tip of dermal papillae, and predominant superficial and perivascular lymphocytic chronic inflammatory cell infiltrate. Immunohistochemistry analysis revealed a predominant T-cell subepithelial infiltrate. Based on the referred clinicopathological findings and in the absence of cutaneous lesions, the diagnosis of geographic stomatitiswas confirmed.
Conclusions
This case and theoretical data indicate that geographic stomatitis may be an oral manifestation of psoriasis. Moreover, to improve our understanding, psoriatic patients should routinely undergo a detailed oral examination and patients with geographic stomatitis should routinely be submitted to a cutaneous routine examination.
Keywords: geographic stomatitis, geographic tongue, HLA genotyping immunohistochemistry, oral psoriasis, psoriasis
Introduction
Geographic stomatitis, first described in 1955 under the term erythema migrans, can occur in any area of the oral mucosa.[1] Geographic stomatitis is an uncommon oral lesion and can occur in any area of the oral mucosa.[2] Geographic stomatitis etiology is unknown, although when it comes to its clinical aspects, lesions appear as red areas that are surrounded by a white border with the lesions demonstrating a migrating pattern. When lesions are found only on the tongue, the lesion is referred to as geographic tongue.[2,3] Some authors have observed a relation between geographic stomatitis and immune mediated diseases, including Reiter’s syndrome, atopy and psoriasis[3,4] Geographic stomatitis may present with similar clinical, histopathological and genetics patterns to psoriasis, suggesting that this lesion may represent an oral manifestation of psoriasis.[4–9] The purpose of this article is to report one case of geographic stomatitis and to discuss a possible connection between this condition and psoriasis.
Case Report
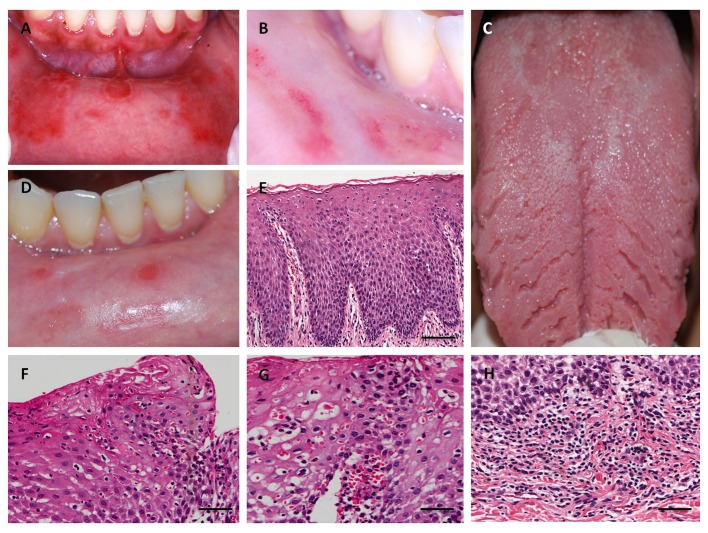
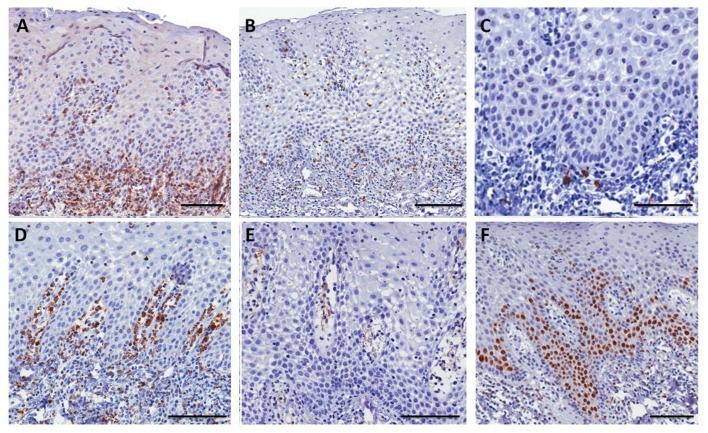
A 37-year-old white woman was referred to the Oral Medicine Service at the Fluminense Federal University with a 5-month history of multiple lesions on the inferior labial mucosa. The patient reported that the lesions regressed completely in 7-10 days, and reappeared after 15 days. The patient’s medical history was non-contributory and with regards to her familial history, her maternal aunt had psoriasis. Extra oral lesions were not observed. Oral examination revealed symptomatic multiple atrophic red patches, surrounded by a raised white circinate border on the inferior labial mucosa [Fig. 1A]. Additionally, the clinical presentation included multiple deep fissures on the lingual dorsum [Fig. 1C]. After informed consent was given, an incisional biopsy of the labial mucosa was performed. Histopathological examination revealed parakeratosis, marked elongation of rete ridges with acanthosis and clubbing, suprapapillary thinning, exocytosis of polymorphonuclear leukocytes, Munro microabscesses, pustule of Kogoj, dilated tortuous vessels at the tip of dermal papillae, and predominant superficial and perivascular lymphocytic chronic inflammatory cell infiltrate [Fig. 1E-H]. Spores or hyphae of Candida spp were not identified (PAS stain) and cytopathological analysis was negative for candidiasis. Samples were stained with monoclonal antibodies against CD4 (T helper cells), CD8 (T suppressor cells), CD20 (B lymphocytes), CD68 (macrophages), CD31 (endothelial cell marker), and Ki-67 (epithelial cell proliferation marker). Immunohistochemistry analysis revealed a predominant T-cell subepithelial infiltrate. These cells were also observed within the epithelium. Most of the T-cells were CD4-positive and developed a subepithelial and epithelial basal layer band of inflammatory infiltrate. T CD8 lymphocytes were scarce and localized predominantly at the basal epithelial layer and at the epithelial-connective tissue junction. Very few CD20 lymphocytes were observed and some macrophages were observed in the subepithelial infiltrate predominantly in the papillary corium. The superficial dilated vessels at the papillary corium were visualized with anti-CD31. There was a balance between the proliferative activity and squamous cell differentiation through the moderate increase of positively stained nuclei of basal and parabasal keratinocytes, as confirmed by Ki-67 [Fig. 2]. HLAA*, -B*, -C*, -DRB1*, -DQA1* and -DQB1* genotyping were performed. DNA was extracted from peripheral blood by GFX Genomic Blood DNA Purification Kit (GE Health Care Indianapolis, IN, USA). HLA typing was assessed by PCRSSO technique (One Lambda Inc., Canoga Park, CA, USA). PCR products were denatured and rehybridized to complementary DNA probes conjugated with fluorescently coded microspheres. The flow analyzer LABScan 100 (Luminex Corporation, Austin, TX, USA) was used to identify HLA types based on the reaction pattern compared to patterns associated with published HLA genes sequences. The alleles found were HLA-A*03, A*25, B*51, B*55, C*03, C*14, DRB1*04, DRB1*13, HLA-DQA1*01:03, DQA1*03:01/ 03:02/03:03, DQB1*03:04, and DQB1*0603. Based on the referred clinicopathological findings and in the absence of cutaneous lesions, the final diagnosis of geographic stomatitis was confirmed. The patient received topical steroid until remission of the symptoms. The patients’ monthly followup has reached 1 year with no symptomatic lesions [Fig. 1B,D].
Figure 1.
Clinical and histopathological aspects of geographic stomatitis. (A) Erythematous lesions surrounded by a raised white circinate on the labial mucosa. (B) Partial regression of the lesions was achieved six months after the administration of topics corticosteroids. (C) Fissures on the lingual dorsum. (D) Aspect of lesion after one year. (E) Histopathological features of labial mucosa exhibiting parakeratosis, acanthosis, elongation, and fusion of rete ridges, suprapapillary thinning, and dilated tortuous vessel at the tip of dermal papillae (hematoxylin and eosin (H&E) scale bar in 200µm). (F) Exocytosis of polymorphonuclear leukocytes and Munro microabscesses (H&E, bar in 100 µm). (G) Dilated tortuous vessels and pustule of Kogoj (H&E, bar in 100 µm). (H) Superficial and perivascular lymphocytic chronic inflammatory cell (H&E, bar in 100 µm).
Figure 2.
Immunohistochemical characterization of geographic stomatitis. (A) Intense presence of T lymphocytes CD4+ at the subepithelial inflammatory infiltrate (bar in 200 µm). (B) Scarce T lymphocytes CD8+ at the epithelial-connective tissue junction (bar in 200 µm). (C) Very few B lymphocytes CD20+ permeating the T lymphocytic inflammatory infiltrate (bar in 200 µm). (D) Macrophages CD68+ at the subepithelial infiltrate predominantly at the papillary corium (bar in 200 µm). (E) CD31+ dilated vessels at the papillary corium (bar in 100 µm) (bar in 100 µm). (F) Increase of positively stained nuclei of basal and parabasal keratinocytes were seen for antibody Ki-67 (bar in 200 µm).
Discussion
Geographic stomatitis is uncommon with an incidence rate of about 1% of the population.[2] This lesion is also known as geographic tongue or benign migratory glossitis when the tongue is involved.[2,3] In contrast to most studies, there was no association between geographic stomatitis and geographic tongue. Geographic stomatitis can occur in children and adults, most commonly in females as in our case report.[2] The most frequently reported sites of geographic stomatitis are the buccal mucosa, lower labial mucosa and mucobuccal fold. The characteristic mucosal and tongue lesions are well demarcated, circular, red patches that are surrounded by a yellowish-white, slightly elevated border with the lesions demonstrating a migrating pattern.[10] Some patients may complain of pain or burning, especially when eating spicy food. For these cases several treatments were proposed, including topical steroids, cyclosporine and topical tacrolimus.[2,3,10,11] The patient examined here reported burning, requiring treatment with topical steroids, which lessened the symptoms but did not resolve the onset of new lesions. Diagnosis of this oral disease is usually made only based in clinical signs and symptoms.[3] If the pattern is atypical and other chronic mucocutaneous conditions cannot be excluded, then an incisional biopsy is recommended for a definitive diagnosis.[10] The etiology is unknown; however, geographic stomatitis and geographic tongue may be oral manifestations of psoriasis, where clinical, genetic and histological findings support this hypothesis.[6–10] In a controlled study, geographic stomatitis was found in 5.4% of patients with psoriasis compared with 1% of control patients. Geographic tongue was identified in 10.3% of patients with psoriasis and 2.5% of control patients.[12] Picciani et al[13] also observed a higher frequency of geographic tongue (12.1%) in 203 patients with psoriasis. The histological appearance of the biopsy from inferior labial mucosa showed several features typical of psoriasis, as outlined by Espelid et al,[10] Femiano[7] and Murphy et al,[14] namely parakeratosis, marked elongation of rete ridges with acanthosis and clubbing, exocytosis of polymorphonuclear leukocytes, Munro microabscesses, suprapapillary thinning, dilated tortuous vessels at the tip of dermal papillae, predominantly superficial and perivascular lymphocytic chronic inflammatory cell infiltrate.[7,10,14] In psoriatic lesions, an infiltrate consisting predominantly of T-cells, mainly CD4, with rare B lymphocytes and macrophages are regularly present.[10,15,16] Our immunohistochemical analyses revealed a cellular distribution similar to that of psoriasis.[10,15,16] Tortuous and dilated dermal capillaries stained with anti-CD31 antibody, commonly observed in psoriatic lesions, were detected in this case. The nuclear proliferation, highlighted with Ki-67 marker, is increased with psoriasis, as reported here, where an increase in proliferation of basal and parabasal cells was observed.[15] However, without the presence of skin lesions, the diagnosis of oral psoriasis is controversial, despite the fact that some cases of oral psoriasis without skin involvement have been documented.[5] The association of several HLA classes I and II molecules with psoriasis vulgaris has been extensively investigated in different ethnic groups. HLA-B*13, HLA-B*17, HLA-C*06, and HLADR7 were already considered to be associated with psoriasis vulgaris in Caucasian populations, mainly in the earlyonset type of the disease.[16] Reviewing the literature, we found an increase in the frequency of HLA-B*15, C*06, DR3 or DR4, DR5, and DR6 in patients with geographic tongue.[8] There are no studies with association of HLA typing and geographic stomatitis that the authors of this study are ware. The HLA results do not correspond to those reported for the Caucasian population, which could be explained by the notable miscegenation of the Brazilian population.[17]
Conclusion
A new research endeavor on the subject is necessary to pursue a better understanding of the immunogenetic profile of the Brazilian psoriatic patients, geographic tongue and geographic stomatitis. This case and theoretical data indicate that geographic stomatitis may be an oral manifestation of psoriasis. Moreover, to improve our understanding, psoriatic patients should routinely undergo a detailed oral examination.
References
- Cooke BE. Erythema migrans affecting the oral mucosa. Oral Surg Oral Med Oral Pathol. 1955;8:164–167. doi: 10.1016/0030-4220(55)90189-4. [DOI] [PubMed] [Google Scholar]
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. Erythema migrans. Gen Dent. 2007;55:163. [PubMed] [Google Scholar]
- Zadik Y, Drucker S, Pallmon S. Migratory stomatitis (ectopic geographic tongue) on the floor of the mouth. J Am Acad Dermatol. 2011;65:459–460. doi: 10.1016/j.jaad.2010.04.016. [DOI] [PubMed] [Google Scholar]
- van der Wal N, van der Kwast WA, van Dijk E, van der Waal I. Geographic stomatitis and psoriasis. Int J Oral Maxillofac Surg. 1988;17:106–109. doi: 10.1016/s0901-5027(88)80161-9. [DOI] [PubMed] [Google Scholar]
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42–45. doi: 10.1111/j.1600-0714.1995.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Yesudian PD, Chalmers RJ, Warren RB, Griffiths CE. In search of oral psoriasis. Arch Dermatol Res. 2012;304:1–5. doi: 10.1007/s00403-011-1175-3. [DOI] [PubMed] [Google Scholar]
- Femiano F. Geographic tongue (migrant glossitis) and psoriasis. Minerva Stomatol. 2001;50:213–217. [PubMed] [Google Scholar]
- Gonzaga HF, Torres EA, Alchorne MM, Gerbase-Delima M. Both psoriasis and benign migratory glossitis are associated with HLA-Cw6. Br J Dermatol. 1996;135:368–370. [PubMed] [Google Scholar]
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61–67. doi: 10.1016/s1079-2104(97)90297-3. [DOI] [PubMed] [Google Scholar]
- Espelid M, Bang G, Johannessen AC, Leira JI, Christensen O. Geographic stomatitis: report of 6 cases. J Oral Pathol Med. 1991;20:425–428. doi: 10.1111/j.1600-0714.1991.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Tojo G, Watanabe M, Tamabuchi T, Masu T, Aiba S. Geographic tongue treated with topical tacrolimus. J Dermatol Case Rep. 2010;4:57–59. doi: 10.3315/jdcr.2010.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LF, Phillips CM, Binnie WH, Sander HM, Silverman AK, Menter MA. Oral lesions in patients with psoriasis: a controlled study. Cutis. 1992;49:339–344. [PubMed] [Google Scholar]
- Picciani BL, Silva-Junior GO, Michalski-Santos B, Avelleira JC, Azulay DR, Pires FR, Dias EP, Cantisano MH. Prevalence of oral manifestations in 203 patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25:1481–1483. doi: 10.1111/j.1468-3083.2010.03936.x. [DOI] [PubMed] [Google Scholar]
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524–528. doi: 10.1016/j.clindermatol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, Langewouters AM, van de Kerkhof PC. Dimethylfumarate for psoriasis: Pronounced effects on lesional T-cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. Am J Clin Dermatol. 2010;11:343–350. doi: 10.2165/11533240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Christophers E, Mrowietz U. The inflammatory infiltrate in psoriasis. Clin Dermatol. 1995;13:131–135. doi: 10.1016/0738-081x(95)93819-a. [DOI] [PubMed] [Google Scholar]
- Cassia FF, Carneiro SC, Marques MT, Pontes LF, Filgueira AL, Porto LC. Psoriasis vulgaris and human leukocyte antigens. J Eur Acad Dermatol Venereol. 2007;21:303–310. doi: 10.1111/j.1468-3083.2006.02008.x. [DOI] [PubMed] [Google Scholar]