Abstract
Background
Alopecia areata is an autoimmune disease that is affecting anagen hair follicles. The triggers of autoimmunity in patients with alopecia areata remain unknown.
Main observation
A 13-year-old boy developed multiple hairless patches of focal hair loss with typical clinical and trichoscopy features of alopecia areata. Mycology examination of the scalp hair and epidermal scrapings reveled massive growth of Alternaria chlamydospora.
Conclusion
We hypothesize that fungal antigens (e.g. antigens involved in fungal melanin synthesis) may be possible triggers, contributing to autoimmune reactions in patients with alopecia areata. We discuss research data, which may indirectly support this hypothesis, however the concept has yet to be verified.
Keywords: allergy, alopecia areata, Alternaria, autoimmunity, dermoscopy, fluorescence, fungus, hair, IgE, infection, melanin, pathogenesis, phaeohyphomycosis, tines capitis, trichoscopy, Wood’s light
Introduction
Alopecia areata (AA) is the most frequent cause of inflammation- induced hair loss, affecting 0.1 to 0.2% of population worldwide.[1,2] The lifetime risk of developing the disease is about 2% in general population.[1] Despite its prevalence, there is little known about the underlying etiology and pathogenesis.
Several data indicate a role of genetic susceptibility to develop the disease.[3] Multiple candidate genes have been suggested.[4–7] However, the triggering antigen(s) responsible for inducing autoimmune phenomena in these individuals remain unknown.[8,9] In this article we report a case of a child with clinically and trichoscopically obvious alopecia areata, in whom fungal culture showed presence of Alternaria chlamydospora and we discuss the possible role of fungi in triggering autoimmunity.
Case Report
A 13-year-old, otherwise healthy, boy presented with multiple hairless patches from 4x4 cm to 9x4 cm [Fig. 1A], which developed 3 years prior to admission during a vacation in Tunisia. According to anamnesis, the lesions were slowly progressing, with no features of regrowth.
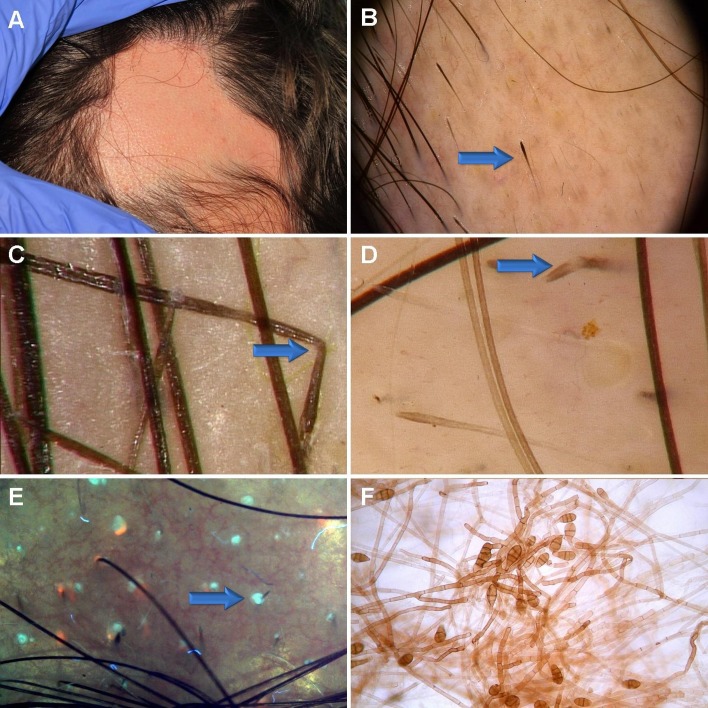
Figure 1.
Clinical appereance of the hairless areas in our patient were consistent with alopecia areata. Few small pustules were present within these areas (A). Trichoscopy of the hair-bearing margin showed typical features characteristic of alopecia areata, such as exclamation-mark hairs (arrow), yellow dots, black dots, and short, upright regrowing hairs and Pohl-Pinkus constrictions. A characteristic feature of exclamation mark hairs in AA is hypigmentation of the proximal end of the hair shaft, showing that increased disease activity in AA may be associated with decreased melanin content in the hair shaft (B). Additional trichoscopy findings included zigzag-like hairs (arrow, C). Some hairs were bent at a site of hemitranslucent nodules along the hair shaft, what may be consistent with trichorrhexis nodosa (arrow, D). UV-enhanced trichoscopy showed dots of light turquoise fluorescence, associated with hair follicle openings (arrow, E). Fungal culture showed prominent growth of Alternaria chlamydospora (F). Note the brownish color of these melanin-producing fungi.
Trichoscopy was performed with FotoFinder II videodermoscope (Germany) and showed regularly distributed yellow dots, sparse black dots, micro-exclamation mark hairs with hypopigmented proximal end, tapered hairs, broken hairs and upright regrowing hairs, consistent with the diagnosis of alopecia areata.[10–12] Trichoscopy of the hair bearing margin also showed sparse zigzag hairs and hairs with features of trichorrhexis nodosa, which are rarely observed in alopecia areata, and may be also present in tinea capitis.[10,13] Trichoscopy images of this patient are presented in Figure 1 B-D.
Wood’s light examination showed no significant abnormalities, but sparse areas of delicate turquoise fluorescence were visible. Ultarviolet-enhanced trichoscopy (UVET, Bomtech, Kong UV camera, Korea) of the hairless areas showed sparse, drop-like, structureless turquoise fluorescence, associated with hair follicle openings. Some of these drop-like areas contained short hair shafts [Fig. 1E].
Mycology culture of the scalp hair obtained from the hairbearing margin of the patches and epidermal scrapings from the boarder of a hairless patch reveled massive growth of Alternaria chlamydospora [Fig. 1F]. Mycoline® (bioMérieux Clinical Diagnostics, France), a double-sided agar-coated slide for the transport and culture of yeasts and dermatophytes with Sabouraud gentamicin chloramphenicol medium on one side and Sabouraud chloramphenicol actidione on the other was used for the fungal culture. The examination was repeated twice.
Discussion
The hair follicle area is an immunologically privileged tissue, which is sheltered from immune surveillance by autoreactive T cells. Failure of such immune privilege and development of organ-specific autoimmune reactions directed against anagen hair follicles seems to play a key role in the pathogenesis of alopecia areata.[14] The triggering antigens responsible for inducing autoimmune phenomena in alopecia areata remain unknown.[8,9] It has been suggested that hair follicle melanocytes, dermal papilla cells, or keratinocytes may contain the triggering antigens.[8,9,15] Viral, bacterial or fungal pathogens have been implied as possible triggering factors of autoimmune reactions.[16]
It has been documented that active alopecia areata is associated with presence of perifollicular inflammatory infiltrates, including predominantly CD8+ T lymphocytes, but also other mononuclear cells and eosinophils. The role of eosinophils in the pathogenesis of alopecia areata remains unclear. Zhao et al[17] showed that in most patients with diffuse AA the onset of disease was observed in spring and summer, suggesting that diffuse AA may be triggered by an increase in seasonal allergens. Authors indicate that common scalp pruritus before first hair shedding, increased serum IgE level, and prominent eosinophilic infiltration may confirm this hypothesis.[17]
Most authors suggest that AA is a T-cell mediated disease with prominent up-regulation of Th1 cytokines and downregulation of Th2 cytokines.[18] It has also been suggested that immune responses are regulated by Th1 cytokines in alopecia universalis and by Th2 cytokines in the patchy form of AA.[19] There is, however, no direct proof that these cytokines are key players in the pathogenesis of AA. Moreover, despite broad use of cytokine inhibiting antibodies in medicine (anti-TNF-alpha, anti-IL6R, anti-IL17, anti-IL12, anti IL-23)[20] no successful therapy of AA with anti-cytokine biological drugs was described yet. Conversely, cases of AA development during anti-cytokine therapy have been reported.[21]
We describe a case of a clinically typical alopecia areata with multiple patches of focal hair loss and no macroscopic features indicative of cutaneous inflammation or infection. The diagnosis was confirmed by trichoscopy, which showed regularly distributed yellow dots, sparse black dots, microexclamation mark hairs, tapered hairs, broken hairs and upright regrowing hairs. These observations were consistent with the diagnosis of active alopecia areata. Trichoscopy of alopecia areata may differ depending on disease activity. These differences were evaluated in multiple studies in recent years. Lacarrubba et al[22] identified three features of acute alopecia areata: micro-exclamation marks, black dots and vellus hairs. The results of Inui et al[12] show that presence of black dots, tapering hairs, and broken hairs correlates with disease activity, but yellow dots tend to be more commonly present in patients with lower diseases activity. Thus, trichoscopy features in our case are characteristic of alopecia areata in an active phase of the disease. Some concomitant trichoscopy features, such as very sparse zigzag hairs or trichorrhexis nodosa-like hairs may be occasionally observed in AA, but may be also indicative of tinea capitis. Trichoscopy performed with UV light (UV-enhanced trichoscopy, UVET)[13] was inconclusive, except the presence of sparse, drop-like, structureless turquoise fluorescence, associated with hair follicle openings. To further investigate this abnormality a mycological examination was performed. Mycology culture of the scalp hair and epidermal scrapings from the scalp reveled absence of dermatophytes, but massive growth of Alternaria chlamydospora was observed.
Alternaria is a large genus of species. These are mainly saprophytic indoor and outdoor airborne fungi. These fungi have pathogenic capacities over a broad range of hosts. Most species are plant pathogens. In humans, over 200 cases of alternarioses have been published. The most frequent clinical manifestations in humans were cutaneous and subcutaneous infections (74.3%), followed by oculomycosis (9.5%), rhinosinusitis (8.1%) and onychomycosis (8.1%).[23] Most of these cases were opportunistic infections in immunosuppressed individuals. Our literature search indicates that our patient is the first described case of scalp infection with an Alternaria mold.
Alternaria species are keratinophilic and keratinolylic fungi, found commonly on hair shafts of domestic animals, such as cats, dogs or horses.[24] It is not unusual to observe subtle growth of Alternaria species in cultures from human scalp hair or epidermal scraping cultures. However, presence of the saprophytic fungi is considered insignificant for clinical practice and consequently may not be reported by mycology labs. In our patient, an unusual, massive growth of the fungus was observed.
Sensitization to Alternaria species has been associated with asthma and other forms of atopy.[25] Kobayashi et al[26] documented that Alternaria activates dendritic cells and produces potent Th2 adjuvant activity. It has also been shown that the serum concentration of Alternaria-specific IgE is increased in patients with asthma and allergic rhinitis.[27] Moreover, acute exacerbations of asthma have been associated with increased airborne concentrations of Alternaria spores.[27] Analyzes of outdoor air routinely show presence of Alternaria spores, showing that most people are exposed to these fungi, but only few develop disease. Exposure to Alternaria results most commonly from inhalation or minor trauma. Little is known about the pathogenic mechanisms by which these fungi cause disease, particularly in immunocompetent individuals.[28]
Among the Alternaria species Alternaria alternata was most extensively studied.[25] Literature related to Alternaria chlamydospora is sparse. Alternaria chlamydospora are keratinolytic, dematiaceous (melanin-producing) fungi that are only occasionally implicated in opportunistic human diseases, such as skin and nail infections.[29]
Considering that antigens associated with melanogenesis have been discussed as potential triggers of autoimmunity in AA[30–32], is may be of interest that several fungi, including Alternaria species are melanin-producing microorganisms.[33,34]
Melanins are a large group of diverse substances, which share similar properties. Their exact chemical structure remains unknown, but pathways leading to melanin production have been extensively studied. There are two main pathways of melanin production: the dihydroxynaphthalene (DHN)-melanin biosynthesis pathway, which predominates in fungi and the dihydroxyphenylalanine (DOPA)-melanin biosynthesis pathway in animals and humans. There are several common elements in both pathways, reviewed in detail by Plonka and Grabacka.[34] It may be speculated that exposure of predisposed individuals to fungal substances involved in melanin biosynthesis may contribute to development autoimmune reactions directed against human peptides involved in biosynthesis of follicular melanin. This would be possible in the mechanism of molecular mimicry, bystander activation or epitope spreading.
This hypothesis may be supported by studies, which have shown that melanin and enzymes involved in melanin biosynthesis (e.g. tyrosinase) are highly immunogenic.[34,35] Various kinds of melanins revealed immunomodulatory activity by regulating cytokine production by T-lymphocytes and monocytes, as well as fibroblasts and endothelial cells.[34] It was even reported that the edible mushrooms, as they are a rich source of melanin and tyrosinase, may induce immunity to these antigens in humans.[35] The possible role of melanin- related antigens in human autoimmunity remains, however, a field of research.
In conclusion, we hypothesize that fungal antigens (eg. antigens involved in melanin synthesis) may trigger autoimmunity in alopecia areata. In this paper we discuss research data, which may indirectly support this hypothesis, however the concept has yet to be verified.
Acknowledgments
We thank Ms. Maria Grodecka for her mycology expertise.
References
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- Alkhalifah A. Alopecia areata update. Dermatol Clin. 2013;31:93–108. doi: 10.1016/j.det.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Blaumeiser B, van der Goot I, Fimmers R, Hanneken S, Ritzmann S, Seymons K, Betz RC, Ruzicka T, Wienker TF, De Weert J, Lambert J, Kruse R, Nöthen MM. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627–632. doi: 10.1016/j.jaad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Petukhova L, Cabral RM, Mackay-Wiggan J, Clynes R, Christiano AM. The genetics of alopecia areata: What's new and how will it help our patients? Dermatol Ther. 2011;24:326–336. doi: 10.1111/j.1529-8019.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- Alzolibani AA, Zari S, Ahmed AA. Epidemiologic and genetic characteristics of alopecia areata (part 2) Acta Dermatovenerol Alp Panonica Adriat. 2012;21:15–19. [PubMed] [Google Scholar]
- Redler S, Albert F, Brockschmidt FF, Herold C, Hanneken S, Eigelshoven S, Giehl KA, Kruse R, Lutz G, Wolff H, Blaumeiser B, Böhm M, Becker T, Nöthen MM, Betz RC. Investigation of selected cytokine genes suggests that IL-2RA and the TNF/LTA locus are risk factors for severe alopecia areata. Br J Dermatol. 2012;167:1360–1365. doi: 10.1111/bjd.12004. [DOI] [PubMed] [Google Scholar]
- Jagielska D, Redler S, Brockschmidt FF, Herold C, Pasternack SM, Garcia Bartels N, Hanneken S, Eigelshoven S, Refke M, Barth S, Giehl KA, Kruse R, Lutz G, Wolff H, Blaumeiser B, Böhm M, Blume-Peytavi U, Becker T, Nöthen MM, Betz RC. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192–2197. doi: 10.1038/jid.2012.129. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Keren A, Shemer A, d'Ovidio R, Ullmann Y, Paus R. Autoimmune Disease Induction in a Healthy Human Organ: A Humanized Mouse Model of Alopecia Areata. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.365. [DOI] [PubMed] [Google Scholar]
- Bertolini M, Gilhar A, Paus R. Alopecia areata as a model for T cell-dependent autoimmune diseases. Exp Dermatol. 2012;21:477–479. doi: 10.1111/j.1600-0625.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Czuwara J. Alopecia areata. In: Rudnicka L, Olszewska M, Rakowska A (ed). Atlas of Trichoscopy. Dermoscopy in hair and scalp disease. Springer; 2013. [Google Scholar]
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040–1048. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Slowinska M. richoscopy update 2011. J Dermatol Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists' guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916–926. doi: 10.1111/j.1365-2133.2012.10955.x. [DOI] [PubMed] [Google Scholar]
- Kim IH, Jo HY, Cho CG, Choi HC, Oh CH. Quantitative image analysis of hair follicles in alopecia areata. Acta Derm Venereol. 1999;79:214–216. doi: 10.1080/000155599750011002. [DOI] [PubMed] [Google Scholar]
- Delogu LG, Deidda S, Delitala G, Manetti R. Infectious diseases and autoimmunity. J Infect Dev Ctries. 2011;5:679–687. doi: 10.3855/jidc.2061. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang B, Caulloo S, Chen X, Li Y, Zhang X. Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J Dermatol Venereol Leprol. 2012;78:709–714. doi: 10.4103/0378-6323.102361. [DOI] [PubMed] [Google Scholar]
- Barahmani N, Lopez A, Babu D, Hernandez M, Donley SE, Duvic M. Serum T helper 1 cytokine levels are greater in patients with alopecia areata regardless of severity or atopy. Clin Exp Dermatol. 2010;35:409–416. doi: 10.1111/j.1365-2230.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis) Acta Derm Venereol. 1996;76:421–423. doi: 10.2340/0001555576421423. [DOI] [PubMed] [Google Scholar]
- Kurzeja M, Rudnicka L, Olszewska M. New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab. Am J Clin Dermatol. 2011;12:113–125. doi: 10.2165/11538950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Slowinska M, Kardynal A, Warszawik O, Czuwara J, Rudnicka L. Alopecia areata developing paralell to improvement of psoriasis during ustekinumab therapy. J Dermatol Case Rep. 2010;4:15–17. doi: 10.3315/jdcr.2010.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacarrubba F, Dall'Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004;5:205–208. doi: 10.2165/00128071-200405030-00009. [DOI] [PubMed] [Google Scholar]
- Pastor FJ, Guarro J. Alternaria infections: laboratory diagnosis and relevant clinical features. Clin Microbiol Infect. 2008;14:734–746. doi: 10.1111/j.1469-0691.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Moriello KA, DeBoer DJ. Fungal flora of the coat of pet cats. Am J Vet Res. 1991;52:602–606. [PubMed] [Google Scholar]
- Hedayati MT, Arabzadehmoghadam A, Hajheydari Z. Specific IgE against Alternaria alternata in atopic dermatitis and asthma patients. Eur Rev Med Pharmacol Sci. 2009;13:187–191. [PubMed] [Google Scholar]
- Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, Cyong JC, Pease LR, Oguchi K, Kita H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol. 2009;182:2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–291; quiz 292-293. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- Shelton BG, Kirkland KH, Flanders WD, Morris GK. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol. 2002;68:1743–1753. doi: 10.1128/AEM.68.4.1743-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu J. Growing incidence of cutaneous and ungual infections by non-dermatophyte fungi at Jabalpur (M.P.) Indian J Pathol Microbiol. 1993;36:113–118. [PubMed] [Google Scholar]
- Sun J, Silva KA, McElwee KJ, King LE Jr, Sundberg JP. The C3H/HeJ mouse and DEBR rat models for alopecia areata: review of preclinical drug screening approaches and results. Exp Dermatol. 2008;17:793–805. doi: 10.1111/j.1600-0625.2008.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp EH, Sandhu HK, Weetman AP, McDonagh AJ. Demonstration of autoantibodies against tyrosine hydroxylase in patients with alopecia areata. Br J Dermatol. 2011;165:1236–1243. doi: 10.1111/j.1365-2133.2011.10597.x. [DOI] [PubMed] [Google Scholar]
- Nagai H, Oniki S, Oka M, Horikawa T, Nishigori C. Induction of cellular immunity against hair follicle melanocyte causes alopecia. Arch Dermatol Res. 2006;298:131–134. doi: 10.1007/s00403-006-0668-y. [DOI] [PubMed] [Google Scholar]
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonka PM, Grabacka M. Melanin synthesis in microorganisms--biotechnological and medical aspects. Acta Biochim Pol. 2006;53:429–443. [PubMed] [Google Scholar]
- Dorđić M, Matić IZ, Filipović-Lješković I, Džodić R, Sašić M, Erić-Nikolić A, Vuletić A, Kolundžija B, Damjanović A, Grozdanić N, Nikolić S, Pralica J, Dobrosavljević D, Rašković S, Andrejević S, Juranić Z. Immunity to melanin and to tyrosinase in melanoma patients, and in people with vitiligo. BMC Complement Altern Med. 2012;12:109. doi: 10.1186/1472-6882-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]