Abstract
Recently, lifespan and healthspan have been extended in experimental animals using interventions that are potentially translatable into humans. A great deal of thought and work are needed beyond the usual steps in drug development to advance these findings into clinical application. Realistic pre-clinical and clinical trials paradigms need to be devised. Focusing on subjects with symptoms of age-related diseases or frailty or who are at imminent risk of developing these problems, measuring effects on short-term, clinically relevant outcomes, as opposed to long-term outcomes such as healthspan or lifespan, and developing biomarkers and outcome measures acceptable to regulatory agencies will be important. Research funding is a major roadblock, as is lack of investigators with combined expertise in the basic biology of aging, clinical geriatrics, and conducting investigational new drug clinical trials. Options are reviewed for developing a path from the bench to the bedside for interventions that target fundamental aging processes.
Keywords: Aging, Cellular Senescence, Translation
1. Introduction1
The elderly are the fastest growing segment of the population. With advancing age, chronic diseases become increasingly prevalent. Aging is the single largest risk factor for stroke, heart attacks, cancers, diabetes, and most other chronic diseases (Research 2012). Numbers of chronic diseases per individual increase with aging, causing loss of independence, frailty, and increased risk of death.
Rather than extending lifespan at all costs, the elderly appear to be more interested in an increased healthspan, the portion of the life-span during which function is sufficient to maintain autonomy, control, independence, productivity, and well-being, although few hard data are available about this important issue. Loss of autonomy and control predict mortality (Fry and Debats 2006). Furthermore, the elderly are not fatalistic: they are not resigned to an old age of frailty (Fry 2000). Although much more study is needed about what the elderly hope to gain from biomedical research, it seems there is public interest in supporting research to enhance healthspan and compress the period of morbidity near the end of life. Prompted in part by an NIH conference in 2008, the basic biology of aging field has started to shift into placing increased emphasis on studying healthspan in animal models, rather than focusing almost exclusively on lifespan (Kirkland and Peterson 2009; Tatar 2009).
2. Healthspan
Limits to healthspan include disability, frailty, chronic diseases, and of course lifespan. Disability refers to functional deficits that are sequelae of diseases earlier in life, accidents, or developmental disorders. Frailty is an age-related clinical syndrome that entails loss of resilience and failure to recover from acute problems, such as pneumonia, stroke, influenza, heart attacks, dehydration, or fractures (Bandeen-Roche and others 2009; Bandeen-Roche and others 2006; Fried and others 2001; Kanapuru and Ershler 2009; Leng and others 2007; Qu and others 2009; Rockwood and others 2006; Walston and others 2006; Walston and others 2002; Walston and others 2009). Frailty can be diagnosed through clinically validated scales that are reasonably, but not completely, sensitive and specific. These scales involve combinations of assessments of weakness, fatigue, weight loss, low activity, and chronic disease and disability burden (Bandeen-Roche and others 2006; Fried and others 2001; Lucicesare and others 2010; Rockwood and Mitnitski 2011). Scales and biomarkers of frailty need to be developed for use in experimental animal models. This should be feasible in mammals, particularly since it has even been possible to develop parameters for evaluating frailty in an invertebrate, C. elegans (Iwasa and others 2010).
The prevalence of frailty increases with aging (Bandeen-Roche and others 2009; Bandeen-Roche and others 2006; Fried and others 2001; Kanapuru and Ershler 2009; Leng and others 2007; Lucicesare and others; Qu and others 2009; Rockwood and Mitnitski; Rockwood and others 2006; Walston and others 2006; Walston and others 2002; Walston and others 2009). It predisposes to chronic diseases, loss of independence, and high mortality. Frailty is linked to the “geriatric syndromes” of sarcopenia, immobility, falling, cachexia, depression, and confusion, as well as the chronic inflammation implicated in the genesis of chronic disease. Age-related chronic diseases and frailty combine to result in poor response to treatments, such as chemotherapy, surgery, organ or stem cell transplantation, and rehabilitation. They can initiate a downward spiral of dysfunction that progresses rapidly to loss of independence, institutionalization, and death. Intriguingly, chronic inflammation is closely associated with frailty, most age-related chronic diseases, including dementias, depression, atherosclerosis, cancers, and diabetes, as well as advanced old age and cellular senescence. Whether and how chronic inflammation causally links these processes remains to be determined (Brown and others 2001; Bruunsgaard and others 2003; Bruunsgaard and Pedersen 2003; Cesari and others 2003; Ferrucci and others 1999; Harris and others 1999; Howren and others 2009; Hu and others 2004; Kanapuru and Ershler 2009; Leng and others 2007; Margolis and others 2005; O’Connor and others; Pai and others 2004; Pradhan and others 2001; Schetter and others; Spranger and others 2003; Srikrishna and Freeze 2009; Tuomisto and others 2006; Walston and others 2002).
3. Do We Have Translatable Interventions?
Although aging has long been recognized as the leading risk factor for chronic diseases and frailty, it has only recently become widely viewed as a potentially modifiable risk factor. Supporting this view are findings that: 1) maximum lifespan is extended and age-related diseases are delayed across species by a number of single gene mutations (Bartke 2011), suggesting the pathways affected by these mutations could be therapeutic targets. 2) Humans who live beyond age 100, a partly heritable trait, frequently have delayed onset of age-related diseases and disabilities (Lipton and others 2010), leading to compression of morbidity and enhanced healthspan. 3) Rapamycin increases lifespan in mouse models (Harrison and others 2009) and appears to delay cancers and age-related cognitive decline (Majumder and others 2012). 4) Caloric restriction, which increases maximum lifespan, is associated with delayed onset of multiple chronic diseases in animal models (Anderson and Weindruch 2012). 5) Factors produced by stem cells from young individuals ameliorate dysfunction in older individuals (Conboy and others 2005; Lavasani and others 2012). 6) Senescent cell accumulation is associated with chronic inflammation, which in turn promotes many age-related chronic diseases and frailty (Laberge and others 2012). Importantly, senescent cell elimination enhances healthspan in mice, at least in progeroid mice (Baker and others 2011). A pipeline is developing of yet more interventions that appear to enhance lifespan in rodent models. Many of these are promising, but not yet published.
Since interventions that increase lifespan and healthspan in mammals now exist, it appears plausible that, by targeting fundamental mechanisms of aging, clinical interventions might be developed that could delay or prevent age-related diseases and disabilities as a group, rather than one at a time. Even if a major chronic disease such as atherosclerotic heart disease were eradicated, as transformative as such an advance would be, it would only add 2 or 3 years to life expectancy (Fried and others 2009; Olshansky and others 1990). However, attacking the intersection between fundamental aging mechanisms and processes that lead to chronic diseases could delay age-related diseases and disabilities. This would have a substantially larger impact on healthspan and health costs than curing any one major chronic disease.
Targeting the intersection between aging and predisposition to chronic diseases would circumvent a problem encountered in studying the pathogenesis of many of these diseases in humans. Many chronic diseases, such as Alzheimer’s or atherosclerosis, are restricted to humans or a very limited number of species. Many human age-related chronic diseases only become manifest clinically after the disease has advanced considerably at the molecular and cellular levels. Both these points make delineation of initiating or upstream etiological mechanisms very challenging because of difficulty in obtaining appropriate tissue samples for analysis sufficiently early during disease development. Targeting upstream, fundamental aging processes that predispose to these diseases in humans could circumvent these difficulties. Despite the huge potential promise of this approach, the financial, infrastructure, and personnel resources needed for basic and translational aging research are insufficient. A strategy to optimize resource development is needed to accelerate progress and avoid duplication.
Promising interventions that could enhance healthspan and delay age-related chronic diseases as a group appear to be at or close to the point of being ready for initial translational studies. More solid data testing the hypothesis that these interventions do, in fact, delay multiple age-related diseases in mouse models are needed. Experimental animal models of human chronic diseases need to be developed more fully with respect to application in the context of aging. Particularly in the case of genetically-modified animal models, it is important to conduct studies in old animals, rather than in young animals with accelerated onset of conditions modeling human age-related diseases. Use of old animals is more likely to phenocopy the systemic aging context in which chronic disease occurs in humans.
Since multiple, potentially effective interventions are emerging, there will be an opportunity to select those that are more readily translatable. Some, such as lifestyle interventions, are particularly challenging (e.g., caloric restriction in the context of an obesity epidemic). Desirable characteristics of interventions to be suitable for translation include: 1) low toxicity and few side effects, 2) effectiveness by oral as opposed to parenteral administration, 3) low dosing frequency (i.e., relatively long half-life), 4) stability, 5) scalability and low manufacturing cost, 6) detectability in blood, and 7) very importantly, effectiveness of interventions if initiated in later life or once symptoms have started to develop. Interventions that need to be applied in childhood or early adulthood, when subjects are still asymptomatic, in order to affect health much later in life would be very difficult to translate into humans. To be acceptable to regulatory bodies, such interventions would need to have virtually no side effects. Furthermore, it would take decades to demonstrate efficacy. Such interventions would be of little or no interest to the pharmaceutical industry because of time and expense required for clinical trials and expiration of patents during the time it takes to take drugs through clinical trials and regulatory approval processes. Similarly, lifestyle interventions that need to be initiated in early life would be difficult to implement.
4. Recent Changes in the Biology of Aging Field
The aging field has moved from description of effects of aging through hypothesis-driven mechanistic studies into development of interventions in experimental animals. A phase of translating these interventions from the bench to the bedside and, eventually, clinical application, is about to begin. In making these transitions, it is important to sustain or increase funding for important descriptive, mechanistic, and animal intervention work in aging, since the discovery pipeline must be maintained and even expanded.
Although “descriptive” is sometimes used as a pejorative term by scientific review committees, descriptive or discovery research leading to hypothesis generation has become highly sophisticated and of great relevance to the aging field. New descriptive approaches include sequencing, genome-wide association studies, epigenetic, microRNA, transcriptional, proteomic, and metabolomic profiling, organism-, cell-, and target molecule-based high throughput screens, bioinformatics, and systems biological and epidemiological techniques. Multiple drug discoveries have come from descriptive approaches, especially high-throughput screening. There is a need in the aging field to continue or increase support for descriptive research, not reduce it, in order to maintain the intervention discovery pipeline.
The mechanism-based, hypothesis-driven approach has been the mainstay of basic aging research for the past couple of decades. Frequently, hypothesis-driven research is not focused solely on potential application or relevance. Many transformative discoveries leading to interventions did not originate from goal-directed research. Mechanism-based, hypothesis-driven, non-application-directed research has been productive and needs to be supported, not reduced. Although a degree of consideration about relevance for hypothesis-driven research is perhaps reasonable, the current tendency by many funding agencies in many countries to force hypothesis-driven aging research to become highly application-directed could be counterproductive.
The aging field has moved into developing interventions that enhance healthspan in experimental animals, particularly over the past 4 years. Novel pharmacologic interventions are being explored that have potential to extend lifespan, delay cancers, dementias, and possibly other age-related diseases, and promote resilience in experimental animals, in addition to any effects on lifespan. These include rapamycin, JAK1/2 inhibitors, senescence-associated secretory phenotype and protein aggregation inhibitors, and other, as yet unpublished approaches (Harrison and others 2009; Laberge and others 2012; Lucanic and others 2011; Verstovsek and others 2010). Based on the finding that eliminating senescent cells increases healthspan in progeroid mice, screens to discover small molecules and biologicals that target senescent cells are underway. Discovery efforts to characterize factors released by stem cells from young individuals that potentially restore function in older individuals are also underway.
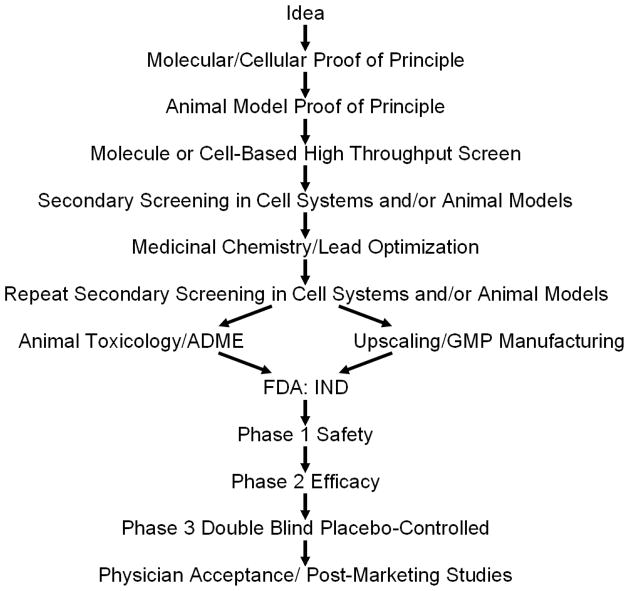
While many of these findings are very recent and in some cases not yet published, it is likely that the aging field will soon find itself at the point of beginning to translate interventions from lower mammals to humans. Successfully translating interventions effective in experimental animals into clinical application is very difficult, time-consuming, and expensive (Figure 1). It can take over 17 years to complete translation, even in fields with an established translational tradition, such as infectious diseases or oncology. The process involves pre-clinical basic studies demonstrating efficacy and safety as well as definition of pharmacokinetics in mammals, generally in at least two species and using good laboratory practices (GLP) conditions, as specified by regulatory agencies (Steinmetz and Spack 2009). It is important to select clinical outcome measures that have been validated, are reproducible, can be measured in a short time-frame, are as non-invasive as possible, and are accepted by regulatory agencies. Iterative bench-to-bedside coupled to “reverse translational” bedside-to-bench developmental phases are likely to be necessary, especially for investigational new drugs (IND’s). This requires an equal partnership between basic biologists and clinicians with a strong basic biology background. Intellectual property protection at a very early stage is necessary to motivate the commercial collaborations necessary to bring agents into the clinic. Without early attention to protecting intellectual property, the chances of getting a treatment to patients are greatly reduced. Finally, early attention to marketing and potential interest of payers for treatments can help to motivate commercialization and getting treatments into the clinic.
Figure 1.
The path from an idea about an intervention to clinical application. ADME refers to pharmacokinetic studies of absorption, distribution, metabolism, and excretion. GMP refers to “good manufacturing practices”. FDA refers to Food and Drug Administration. IND refers to investigational new drug.
5. Initial Clinical Studies
While progress over the past few years in developing successful interventions that target basic aging processes in mice has been impressive, the way forward to translation into humans has not yet been fully mapped. We need innovative, new solutions to the limitations in experimental paradigms, infrastructure, strategies, and personnel that will be required for successful translation in the near future.
The field of translating the biology of aging into clinical interventions is in its infancy. Few drug intervention trials of any type have been conducted in elderly populations, as elderly subjects have generally been excluded from clinical trials, especially those involving completely new formulations. However, initial trials have commenced in humans using some of the approaches that have been shown to enhance life- or healthspan in rodents using drugs already approved for other indications. These drugs are being used to target aspects of age-related dysfunction or particular chronic diseases. For example, at least three clinical trials of rapamycin in Alzheimer’s disease and another for frailty are currently underway or about to begin. Resveratrol congeners are being developed to treat type 2 diabetes (Baur and others 2012). JAK1/2 inhibitors, which interfere with IL-6 signaling, appear to ameliorate frailty in elderly patients with myelofibrosis, a hematologic disease associated with inflammation marked by high circulating IL-6, without affecting the clonal hyperproliferation that is the underlying cause of the hematologic disorder (Geron and others 2008; Pardanani and others 2011; Tefferi and Pardanani 2011; Verstovsek and others 2010).
At least three potential drug development paradigms can be envisaged for whether interventions based on targeting fundamental aging processes improve function (Table 1). The first is to determine if these treatments ameliorate dysfunction due to chronic, age-related diseases using well-defined endpoints. For example, effects of interventions could be determined on HbA1C or fasting glucose in age-related type 2 diabetes. Other examples could be improvements in psychometric indices of cognitive function in dementias or primary tumor size or rate of metastatic spread in cancers. The second set of paradigms is to study effects on disease events more narrowly associated with the agent being tested. An example might be resolution of fracture non-union, which is associated with local increases in senescent cells, with senolytic agents (Bajada and others 2009). The third approach is to test if agents can reduce aspects of frailty. For example, effects of agents could be tested on strength, endurance, nausea, and appetite after chemotherapy, time needed for return of function and wound healing after elective surgery, or delay of imminent nursing home admission in pre-frail, at-risk elderly subjects. These approaches would be more feasible than using long-term, unproven, or problematic endpoints, such as lifespan in humans.
Table 1. Potential Paradigms for Translating Interventions that Extend Health- or Life-Span in Experimental Animals into Clinical Treatments.
| Amelioration of Age-Related Diseases |
| Cancer: growth or metastases |
| Cognitive or neurological dysfunction due to Alzheimer’s or other neurodegenerative diseases |
| Atherosclerosis: vessel flow or patency, calcification, acute coronary syndrome, cardiac function |
| Diabetes (particularly that associated with obesity or metabolic syndrome): HbA1C, HOMA index, glucose or insulin clamps |
| Niche Indications Specifically Targeted by the Intervention |
| Myelofibrosis with elevated IL-6 by JAK1/2 Inhibitors |
| Senolytics for fracture non-union |
| Amelioration of Frailty (in pre-frail, as opposed to very frail, subjects) |
| Short term: |
| Chemotherapy constitutional side effects |
| Recovery from elective surgery |
| Duration of rehabilitation required after myocardial infarction or hip fracture |
| Intermediate term: |
| Prevention of nursing home admission |
Outcomes that are acceptable to regulatory agencies will be needed for clinical trials of drugs that target fundamental aging mechanisms. Little work has been done on surrogate outcomes for frailty and age-related disability or on validating biomarkers from drug studies in young subjects for use in the elderly. Endpoint and pre-clinical testing paradigms need to be developed in aging animal models for initial pre-clinical studies. These need to be based on the clinical endpoints to be used in subsequent trials in elderly subjects. Ideally, already established endpoints that the FDA recognizes, such as measures of glucose tolerance, muscle strength, or cognitive function could be used. Work needs to continue on developing other, possibly more relevant biomarkers, such as indices of frailty, to the point that the FDA can accept them.
6. Investigators Needed for Translational Aging Research
In the US, there are 7,000 American Board of Internal Medicine-certified geriatricians (source: Association of Directors of Geriatric Academic Programs). Fewer than a dozen have R01 grants from the Division of Aging Biology at the National Institutes of Health. Indeed, worldwide there are few clinical geriatricians who are also basic aging researchers. This is very unlike many other biomedical fields, such as endocrinology, in which many academic clinicians also conduct basic laboratory research. Few geriatricians attend meetings in the basic biology of aging. Furthermore, basic biologists rarely attend clinical geriatrics meetings. In addition, unlike infectious disease specialists or oncologists, few geriatricians have experience with translating IND’s into clinical application. This means there is a critical shortage of investigators with the combination of basic biological and clinical skills necessary to design and conduct the pre-clinical and clinical studies and to navigate the regulatory framework necessary to translate recent advances.
We need to begin to train a new group of investigators in the basic biology of aging who have a thorough grasp of translational strategies and clinical geriatrics We need a group of geriatricians with sufficient understanding of the both basic biology of aging and clinical trials methodology to lead the process of taking IND’s through pre-clinical studies, clinical trials, and the regulatory approval process. It will take well over 5 years for these investigators to be ready, even if we can entice trainees into this area and begin intensive training programs right away. In the meantime, we need to devise collaborative strategies that bring trios of basic biologists, geriatricians, and clinical trials investigators together to start the process of translating new agents as they become available. We also need to consider developing clinical trials networks, perhaps emulating the successful approaches taken by the networks established in the cancer field.
A major barrier in the US to attracting trainees into careers combining clinical geriatrics with the biology of aging is the very poor reimbursement for geriatric clinical services, resulting in a general shortage of geriatricians (Committee on the Future Health Care Workforce for Older Americans 2008). Geriatricians actually earn less than general internists, even though American Board of Internal Medicine certified geriatricians need to complete training as general internists before they do additional training in geriatric medicine. Because of this, much of the energy of leaders in academic geriatric medicine has to be devoted to devising ways to improve practice efficiency and volume to compensate for poor reimbursement, rather than concentrating on other academic issues, such as developing methods to translate fundamental aging processes into the clinic.
Despite this, it is encouraging that trainees are beginning to embark on the training needed to conduct translational aging research. This seems to be related to the recent, high profile advances in basic aging biology, especially interventions that enhance health- and life-span in mammals. A number of steps need to be taken to help these trainees in their careers, including development of well-thought out curricula, securing funding for training programs, enhancing the reimbursement system for geriatrician/basic scientists, and developing a much fairer and supportive scientific review process for the grants for which these investigators will need to compete. Currently, many grant review committees are quite hostile to translational research, even if this is not intentional. The system is stacked against such applications, in part because basic scientists reviewing these grants do not understand the clinical components and the clinicians do not understand the basic science components. With page limitations, these applications have less room to describe the basic science component than purely basic grants being reviewed by the same group, with the same being true of the clinical component of the grant. Trainees are very aware of this issue, and considerable thought is needed to find ways to address this in order to attract trainees into the field and to get the research going.
7. Conclusions
We are on the verge of a new era in the basic biology of aging, an era in which we can finally contemplate beginning clinical translation for a range of applications. While interventions with potential to delay age-related dysfunction appear to be at hand, creation of new experimental paradigms with relevant, measureable outcomes, funding, and training for personnel with new skills are needed soon. All this has to be done without taking resources away from the current descriptive and mechanistic approaches in the aging biology field that led to these important advances.
Can every age-related change be reversed? Some involve progenitor depletion, some involve chronic inflammation, and others involve extracellular matrix and structural changes (e.g., cataracts, osteoporosis). Different mechanisms are implicated to different degrees in different organs and cell types. This suggests that several therapeutic strategies may need to be combined to be maximally effective: no one strategy is very likely to be a panacea. A process involving at least two steps may ultimately be needed, based on the longstanding surgical principle of first removing dead material and then replacing with good tissue. The first step may involve removing senescent cells, ameliorating the senescence-associated secretory phenotype and reducing inflammation, and removing cytotoxic lipids and lipofuscin, protein aggregates, damaged macromolecules, and advanced glycation endproducts. The second step may involve transplanting tissues, differentiated cells, or stem cells or restoring endogenous progenitor function to repopulate damaged tissues. Advances are being made in each of these areas, and highly effective combined approaches, while currently science fiction, may not be that far off.
If any or all of this comes to fruition and is translated into clinical treatments successfully, we may be able to delay chronic age-related diseases as a group, rather than picking them off one-at-a-time, delay or reverse frailty, and even extend health- or lifespan. If these speculations come about, there will be myriad economic and social consequences, for which the public is not prepared. To quote Pliny the Elder, the only certainty is that there is nothing certain.
Highlights.
New interventions that enhance healthspan in animals may be translatable to humans
Pre-clinical and clinical trials paradigms need to be devised to do so
Research funding and lack of translational investigators are roadblocks
Options for translating interventions that target aging processes are reviewed
Acknowledgments
The authors are grateful for administrative and editing assistance from L. Wadum and J. Armstrong. This work was supported by NIH grants AG13925, AG41122, and the Noaber, Ellison, and Glenn Foundations.
Footnotes
Abbreviations and terms: Senolytic – an agent that targets senescent cells; ADME – absorption, distribution, metabolism, and excretion of a drug; SASP – senescence-associated secretory phenotype; mTOR – mammalian target of rapamycin; JAK—Janus-activated kinase; GLP – Good laboratory practices; GMP – Good manufacturing practices; FDA – Food and Drug Administration; IND – investigational new drug
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–735. doi: 10.1016/j.bone.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Giles WH, Croft JB. White blood cell count: an independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol. 2001;54:316–322. doi: 10.1016/s0895-4356(00)00296-1. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Committee on the Future Health Care Workforce for Older Americans, I.o.M. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry PS. Whose quality of life is it anyway? Why not ask seniors to tell us about it? Int J Aging Hum Dev. 2000;50:361–383. doi: 10.2190/DD41-W8F4-W6LK-FY8Q. [DOI] [PubMed] [Google Scholar]
- Fry PS, Debats DL. Sources of life strengths as predictors of late-life mortality and survivorship. Int J Aging Hum Dev. 2006;62:303–334. doi: 10.2190/3VAT-D77G-VCNQ-6T61. [DOI] [PubMed] [Google Scholar]
- Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, Durocher J, Mak CC, Noronha G, Soll RM, Tefferi A, Kaushansky K, Jamieson CH. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13 doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Yu S, Xue J, Driscoll M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell. 2010;9:490–505. doi: 10.1111/j.1474-9726.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, Desprez PY, Hughes RE, Campisi J. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11:569–578. doi: 10.1111/j.1474-9726.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, Huard J. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Hirsch J, Katz MJ, Wang C, Sanders AE, Verghese J, Barzilai N, Derby CA. Exceptional parental longevity associated with lower risk of Alzheimer’s disease and memory decline. J Am Geriatr Soc. 2010;58:1043–1049. doi: 10.1111/j.1532-5415.2010.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, Gill MS. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 2011;473:226–229. doi: 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucicesare A, Hubbard RE, Searle SD, Rockwood K. An index of self-rated health deficits in relation to frailty and adverse outcomes in older adults. Aging Clin Exp Res. 2010;22:255–260. doi: 10.1007/BF03324805. [DOI] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411–1420. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250:634–640. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Vannucchi AM, Passamonti F, Cervantes F, Barbui T, Tefferi A. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia. 2011;25:218–225. doi: 10.1038/leu.2010.269. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Amer Med Assoc. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, Ferrucci L, Rose NR, Leng SX. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130:161–166. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research, A.f.A. The Silver Book: Chronic Disease and Medical Innovation in an Aging Nation. Washington, DC: 2012. [Google Scholar]
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz KL, Spack EG. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009;9(Suppl 1):S2. doi: 10.1186/1471-2377-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Can we develop genetically tractable models to assess healthspan (rather than life span) in animal models? J Gerontol A Biol Sci Med Sci. 2009;64:161–163. doi: 10.1093/gerona/gln067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood Rev. 2011;25:229–237. doi: 10.1016/j.blre.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Hadley E, Ferrucci L, Guralnick JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Walston JD, Matteini AM, Nievergelt C, Lange LA, Fallin DM, Barzilai N, Ziv E, Pawlikowska L, Kwok P, Cummings SR, Kooperberg C, LaCroix A, Tracy RP, Atzmon G, Lange EM, Reiner AP. Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol. 2009;44:350–355. doi: 10.1016/j.exger.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]