Abstract
Background
Mitofusin 2 (MFN2) is a mitochondrial membrane protein mediating mitochondrial fusion and function. Mutated MFN2 is responsible for Charcot-Marie-Tooth type 2A2. In small kindreds, specific MFN2 mutations have been reported to associate with severity of axonal neuropathy, optic atrophy, and involvement of the central nervous system. The results of the nerve biopsy specimens suggested that the mitochondria are structurally abnormal in patients with MFN2 mutations.
Objective
To study a newly identified MFN2 mutation, Leu146Phe, and the associated phenotypes in a large kindred.
Patients
An American kindred of Northern European and Cherokee American Indian descent.
Results
Genetic analysis revealed a novel GTPase domain MFN2 mutation Leu146Phe that associated with clinical status of 15 studied persons (10 affected and 5 unaffected) and not found in 800 control persons. Clinical manifestations were markedly different. In 1 affected person, optic atrophy and brain magnetic resonance imaging abnormalities led to multiple sclerosis diagnosis and interferon β-1a treatment when neuropathy was initially unrecognized. Age of onset ranged from 1 to 45 years. In some affected family members, severe and rapid-onset motor sensory neuropathy led to early loss of ambulation, whereas other family members experienced minimal neuropathic sensory symptoms. Despite histologically significant loss of nerve fibers, the mitochondria were not distinguishable from diseased sural nerve biopsy specimens and healthy controls.
Conclusions
Novel MFN2 mutation Leu146Phe causes Charcot-Marie-Tooth type 2A2. Intrafamilial clinical phenotype variability is emphasized and has important implications in genetic counseling. The clinical phenotype may mimic multiple sclerosis when optic atrophy and the characteristic brain lesions of MFN2 on magnetic resonance imaging are present and neuropathy is mild or unrecognized. The predicted molecular pathogenesis may occur without evident histological abnormalities of mitochondria in nerve.
Mitofusin 2 (MFN2) mutations are responsible for the most common known axonal form of Charcot-Marie-Tooth(CMT) disease, CMT2A2.1–5 Mutation in Kinesin 1B (KIF1B) was first identified and designated as CMT2A1. Kinesin 1b co-localizes with MFN2 at chromosome position 1p36.2, but KIF1B mutation has been identified in only 1 rare family.6 In contrast, more than 80 MFN2 mutations have been reported that cause CMT2A2, and in 1 study,4 the frequency of MFN2 mutations was as high as 33% in tested patients with inherited axonal CMT. Mitofusin 2 is a large transmembrane mitochondrial GTPase protein located on outer mitochondrial membranes. Axonal mitochondria morphology is thought to be maintained by MFN2, which promotes mitochondria membrane fusion.7 Mutations have been identified throughout the MFN2 protein, but the majority of them occur in the hydrophobic coiled-coil (HR1) and GTPase domains, both of which are essential for mitochondrial fusion.8 Mutations of MFN2 are theorized to have complex pathogenic effects. One predicted mechanism of disease is that energy production required for axonal mitochondrial transport may be reduced by defective MFN2.9 Mitochondrial tethering to the endoplasmic reticulum is also thought to be defective, with MFN2 mutations triggering neuronal apoptosis by calcium uptake dysregulation.10 These molecular pathogenic mechanisms possibly change mitochondria histologic appearance. A limited number ofstudies4,11,12 on nerves from persons with mutated MFN2 have suggested misshaped and aggregated mitochondria, but the specificity of those findings is not clear.
With expanding clinical experience, we increasingly recognize that the patients affected by MFN2 mutations have diverse inheritance patterns including (1) dominant heterozygotes (most common), (2) recessive homozygotes, and (3) compound dominant heterozygotes. Previous reports13 suggested that the patients with compound heterozygosity had more severe neuropathy, and their phenotypes are consistent with additive dominant negative effect of MFN2 mutation. Axonal motor sensory neuropathy with more severe lower-extremity than upper-extremity involvement provides the predominant clinical phenotype of CMT2A2. However, observations of patients with MFN2 mutations with peripheral neuropathy and myelopathy (hereditary motor sensory neuropathy type V),14 or peripheral neuropathy and optic neuropathy (hereditary motor sensory neuropathy type VI),15 expand the clinical presentations. In addition, clinical involvement of the central nervous system (CNS) is supported by magnetic resonance imaging (MRI) of brain abnormalities in some affected persons.3,16
Two relatively distinct, temporal onset severity groups appear to distinguish different MFN2 mutations: (1) adult or adolescent onset, typically mild and insidious and (2) early-severe onset, often before age 5 years, with rapid progression.3 These 2 distinct phenotypes have tended to run in specific families, with optic atrophy occurring in more severely affected persons. However, 1 detailed kindred evaluation17 suggested that, within individual families, clinically affected status may be very mild and may require detailed neurologic and electrophysiologic examination to identify affected status. Nonetheless, most reports have highlighted the variability in expression between and not within CMT2A2 families.
METHODS
KINDRED EVALUATION
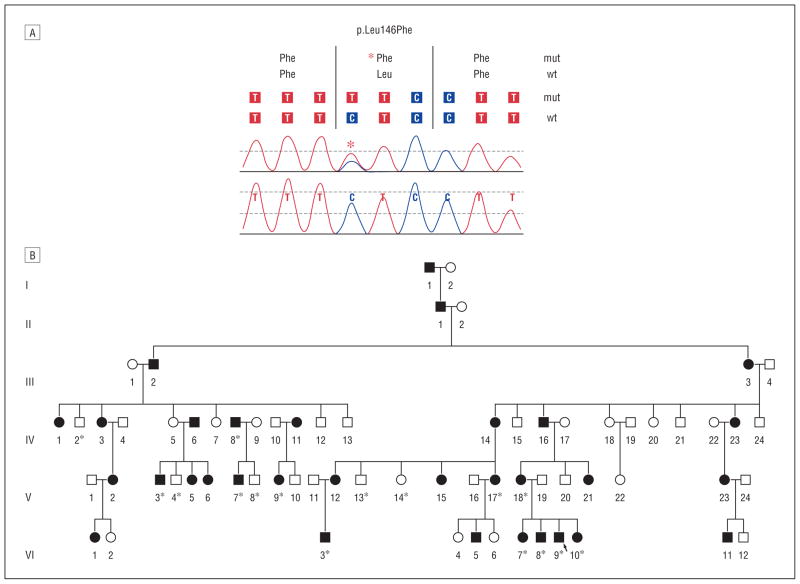
With institutional research board approval and informed patient consent, we undertook kindred evaluation and genetic analysis in a large American kindred of Northern European and Cherokee American Indian descent. Clinical examinations, clinical history evaluation, and MFN2 exon sequencing were performed within this large family. We identified an MFN2 DNA base alteration 436 C→T, changing amino acid sequence of leucine to phenylalanine at codon position 146 CTC→TTC (Figure 1). This base alteration had not been previously reported, to our knowledge, and 800 controls were sequenced to exclude the possibility of rare polymorphism. To examine whether this compound heterozygous status may cause the observed clinical variable expression, the entire MFN2 open reading frame was sequenced. The kindred and clinical features were extracted and medical records reviewed; the earliest known affected person (designated as I-1) had a Cherokee American Indian mother and father coming from England in the 1600s, but their affected status is unknown.
Figure 1.
Identified new MFN2 mutation and affected kindred. A, DNA sequencing results showing the identified missense alteration within MFN2 at nucleotide base 436 C→T with resultant amino acid change of leucine (Leu) to phenylalanine (Phe) at codon position 146 CTC→TTC: Leu146Phe. B, The examined American kindred of Northern European and Cherokee Indian descent with genetic testing (indicated by asterisk) revealing the affected status associated with the demonstrated base alteration. In addition to the shown persons, 800 evaluable healthy controls underwent DNA sequencing without MFN2 436 C→T alteration identified and not reported at Web-based Human Gene Mutation Database Biobase and National Center for Biotechnology Information dbSNP. mut Indicates mutation; wt, wild type. The arrow signifies the proband; shaded squares (males) and circles (females), clinically affected by examination and history.
NERVE HISTOLOGIC EVALUATION
A whole sural nerve biopsy of the proband (VI-9) was examined. Teased nerve fibers and paraffin- and epoxy-embedded semithin sections were studied by techniques previously described,18 including nerve morphometric and electron microscopy. Qualitative mitochondrial structural features were reviewed in the patient and in comparison with 21 healthy examined persons and 100 consecutively obtained diseased sural nerve biopsy specimens in longitudinal and transverse section at varied magnification under electron microscopy. The diseased and healthy control electron micrographs were collected as part of an earlier study.
RESULTS
All examined patients clinically confirmed with neuropathy (n=10) were shown to have the Leu146Phe mutation (Figure 1). None of the clinically unaffected persons (n=5) had the mutation, nor did the healthy screened controls. Compound heterozygote status was not found. The clinical symptoms, including ages of clinical onset, involvement of the CNS, and optic atrophy, are summarized in the Table. The clinical features among affected persons were demonstrated with illustrative cases highlighting largely 3 categories of clinical presentations: (1) optic atrophy and CNS disease with mild neuropathy; (2) early-onset, rapid-progression severe neuropathy; and (3) late-onset, slow-progression mild neuropathy. Hearing loss, scoliosis, tremor, and parkinsonism did not occur.
Table.
Clinical Characteristics of Genetically Confirmed MFN2 Leu146Phe Neuropathy
| Pedigree | Sex/Age, y | Clinical Summary | Age at Onset of Symptoms, y | Optic Atrophy | Involvement of Central Nervous System | Wheelchair Required; Age, y |
|---|---|---|---|---|---|---|
| VI-3 | M/64 | Early-onset, rapid, severe motor and sensory | 3 | No | No | Yes; 30 |
| VI-7 | F/52 | Late-onset, mild, sensory predominant | 45 | No | No | No |
| VI-8 | M/50 | Late-onset, mild, sensory predominant | 40 | No | No | No |
| VI-9 | M/45 | Vision loss, mild, sensory motor neuropathy | 38 | Yes | Yesa | No |
| VI-10 | F/43 | Vision loss, mild, sensory motor neuropathy; initially diagnosed as multiple sclerosisb | 27 | Yes | Yes | No |
| V-3 | M/41 | Late-onset sensory motor loss in feet | 25 | No | No | No |
| V-7 | M/42 | Early-onset, rapid, severe motor and sensory | 1 | No | No | Yes; 40 |
| V-17 | F/70 | Early-onset, gradually progressive | 7 | No | No | Yes; 43 |
| V-18 | F/72 | Late-onset, motor and sensory | 40 | No | No | Yes; 60 |
| IV-8 | M/69 | Late-onset, motor and sensory | 20 | No | No | No |
Tibial somatosensory–evoked potentials demonstrated conduction slowing in the spinal cord (see the “VI-10” subsection in the “Results” section).
Magnetic resonance image of brain shown in Figure 2.
CLINICAL VARIABILITY
Examples of Optic Atrophy and CNS Disease With Mild Neuropathy
VI-9
The proband was evaluated at our institution at age 45 years in consideration of multiple sclerosis, which was diagnosed in his sister V1-10 (see next subsection). His mother had an onset of neuropathy in childhood with severe motor involvements, no optic atrophy, and loss of ambulation by age 60 years. At age 38 years, he developed painless loss of vision in the left eye during a period of 1 to 2 months, documented 20/700. One year later, he developed an attack of vision loss of similar severity in his right eye. At age 43 years, he had loss of feeling in his feet and erectile dysfunction. On our clinical examination, he had improved visual acuity from the original attack at 20/200 OU with optic nerve pallor. Gait, station, and direct manual muscle testing showed trace toe dorsiflexion weakness; otherwise, strength was normal. There was loss of sensation to light touch, vibration, and temperature in the feet. Deep tendon reflexes were absent at the ankles and reduced at the knees without Babinski or Chaddock signs.
Magnetic resonance imaging of the head, cervical spine, and thoracic spine showed no abnormalities. Cerebro-spinal fluid examination showed mildly elevated protein of 65 mg/dL but was otherwise normal, including no oligoclonal bands. Nerve conductions and electro-myography studies were consistent with a mild sensorimotor length-dependent axonal peripheral neuropathy. The sural sensory amplitude when stimulated 7 cm above the ankle was 4 uV (normal, >6 uV), and the median antidromic sensory amplitude stimulated at the wrist was 13 uV (normal, >15 uV), with normal distal latencies and conduction velocities in both. Findings from the motor nerve conduction studies were normal (peroneal, tibial, and ulnar), and electromyography was with large reduced recruited motor unit potentials in distal ankle and intrinsic hand muscles. Visual evoked potentials had prolonged P100 responses bilaterally 123.2 ms (normal, >120 ms), consistent with optic nerve neuropathy. The tibial sensory evoked potentials suggested conduction slowing in the spinal cord (N22-P38=23 ms [normal, 12.2–20 ms]).
VI-10
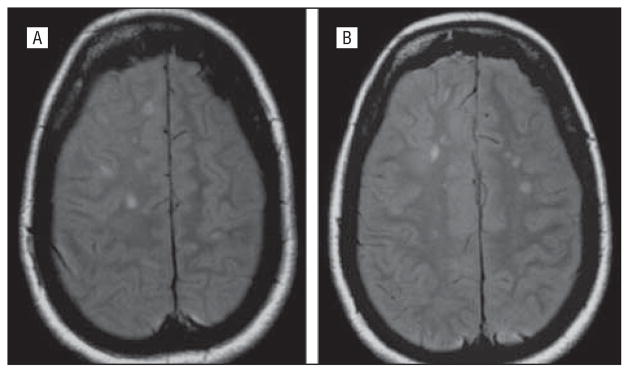
The proband’s sister at age 27 years developed painless loss of vision in her right eye and optic neuropathy without polyneuropathy initially recognized. There was insidious progression of that vision loss over years. She was diagnosed as having multiple sclerosis when her brain MRI showed increased T2 signal in paraventricular white matter (Figure 2). These regions did not enhance with gadolinium, and results of the MRIs of the spinal cord were normal. Serial brain MRI did not worsen despite, at age 40 years, the development of insidious left-eye vision loss with documented optic atrophy that also slowly worsened during 2 years (20/40 then 20/70 then 20/100). Treatment with interferon β-1a was initiated. Subsequently, she was recognized with distal mild toe dorsiflexor weakness, muscle cramps with reduced or absent reflexes, and no upper motor signs. She had high arches and hammer toes. At age 46 years, she began having complex partial seizures with abdominal aura and was given phenytoin. No attacks of extremity incoordination or asymmetric motor sensory transient deficits characteristic of relapsing remitting multiple sclerosis were reported.
Figure 2.
Magnetic resonance imaging of the brain of patient VI-10, who at age 27 years developed progressive optic atrophy in her right eye without polyneuropathy recognized. She was subsequently diagnosed as having multiple sclerosis when increased T2 signal changes in bilateral white matter paraventricular centrum semiovale regions were found that did not enhance by gadolinium. These changes did not progress, despite new left-eye progressive optic atrophy, characteristic of earlier reported brain imaging in Charcot-Marie-Tooth type 2A2.3,16 Axonal sensory motor polyneuropathy was subsequently diagnosed by clinical and electrophysiologic testing, and genetic testing confirmed her mitofusin 2 Leu146Phe mutation.
Cerebrospinal examination findings were normal, including no oligoclonal bands. Nerve conduction studies at age 45 years showed absent medial plantar sensory response with peroneal motor amplitude of 3.0 mV (>2 mV) and tibial motor amplitude of 5.5 mV (>4 mV) with conduction velocities of 37 m/s (>40 m/s). On needle electromyography, distal tibialis anterior and gastrocnemius muscles showed only rare polyphasic motor units and normal recruitment.
Examples of Early-Onset, Rapidly Progressive Severe Neuropathy
V-7
At age 1 year, patient V-7 had prominent ankle weakness with foot deformity that led to orthotic footwear. His father, IV-8, was also affected with neuropathy but not until young adulthood and without loss of ambulation at age 69 years. Weakness progressed during his toddler years. At age 9 years, he underwent ankle fusion surgery, with loss of ambulation in his 30s, with associated hand involvements such that he had to give up playing piano in high school. He had flail ankles with proximal weakness extending to the thigh and arms with sensory loss in the feet and hands. Electrophysiologic evaluation showed severe axonal motor sensory polyneuropathy. No optic atrophy was identified by dilated funduscopic eye examination.
VI-3
In patient VI-3, with rapidity at age 3 years, symmetric leg, thigh, and hand weakness developed. The speed of onset weakness raised concern of possible poliomyelitis, but that was excluded when prominent coexisting progressive sensory loss was also noted. He could not walk without assistance in his 30s, and direct muscle testing showed proximal involvements extending to the thighs and arms with associated loss of sensation. Nerve conductions and needle electromyography showed severe length-dependent axonal motor and sensory poly-neuropathy. No optic atrophy was seen on funduscopic examination.
Example of Late-Onset, Slowly Progressive Mild Sensory Neuropathy
VI-7
Another sister of the proband developed paresthesias in the distal feet at age 45 years. The sensory symptoms correlated with mild sensory loss on bedside examination. Mild ankle and toe dorsiflexion weakness became apparent by age 50 years. Hands were not affected. The results of the nerve conduction studies at age 47 years were consistent with a mild length-dependent sensory predominant axonal neuropathy, with peroneal amplitude at the ankle being 1.3 mV (>2 mV) and tibial being 9.9 mV (>4 mV) with absent sural and peroneal sensory responses, without needle electromyography abnormalities of tibialis anterior and gastrocnemius muscles. No visual symptoms of optic atrophy were appreciated.
Histologic Results
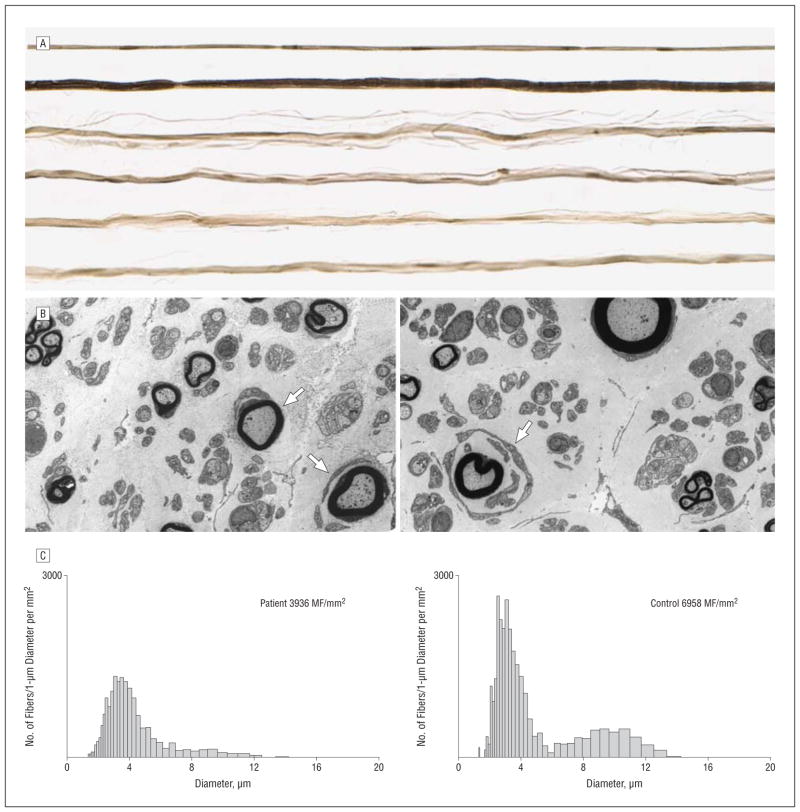
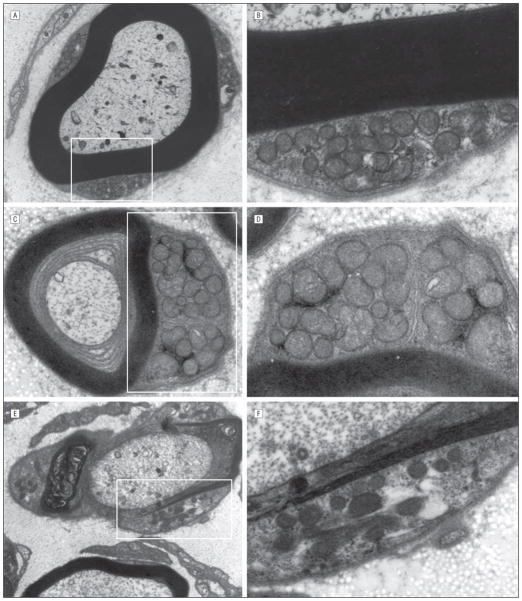
Sural nerve biopsy of patient VI-9 illustrated that the density of the large and small myelinated fibers decreased in a diffuse pattern with rudimentary myelin lamellar thickening without interstitial infiltrative abnormalities (Figure 3). Mitochondrial axonal and Schwann cell abnormalities were not observed compared with healthy controls and patients with diseased sural nerve biopsy specimens (Figure 4 and Figure 5).
Figure 3.
Selected pathologic abnormalities of the sural nerve of patient V1-9. A, Systematically sampled teased fibers. Of 109 evaluated nerve strands, 66 were devoid of myelinated features or their breakdown products, 1 showed segmental remyelination, and 1 showed reduplication/unequivocal abnormality.
B, Low-power transverse electron micrographs showing decreased myelinated and probably also unmyelinated fibers and small onion bulb formation (arrows).
C, Myelinated diameter histograms of myelinated fibers (MF) show reduced fiber density of the patient’s nerve and decreased number of both small and large MFs. The patient, left, was a 45-year-old man, and the control, right, was a 54-year-old man.
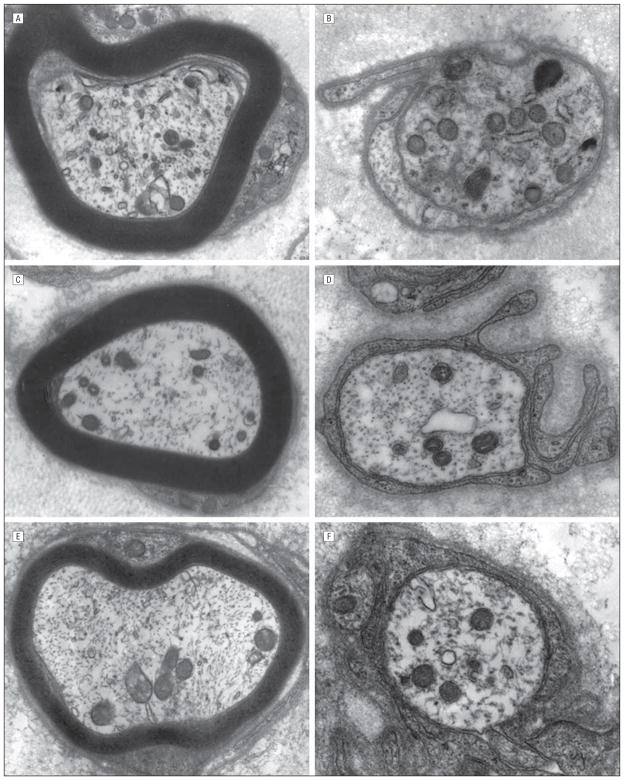
Figure 4.
Mitochondrial qualitative axonal evaluation in myelinated large and small unmyelinated fibers of patient VI-9 (A–B), and 100 consecutively examined controls with disease (C and D), and sural nerves of healthy controls (E and F). Unequivocal difference in number, distribution, and size of mitochondria was not found.
Figure 5.
Mitochondrial qualitative evaluation by electronic microscopic evaluation of the Schwann cell cytoplasms was performed in the nerve of patient VI-9 (A and B, high magnification), and 100 consecutively examined controls with disease (C and D, high magnification), and sural nerves of healthy controls (E and F, high magnification). Unequivocal difference in number, distribution, and size of mitochondria was not found.
COMMENT
We describe a new MFN2 amino acid alteration Leu146Phe within the GTPase domain of the MFN2 protein. This mutation appears pathogenic for a large CMT2A2 American kindred with markedly varied clinical phenotypes. To our knowledge, to date, this mutation has not been described among the many identified European-origin CMT2 families, raising the question of a unique American mutation arising from this kindred with Cherokee American Indian ancestry. The broad phenotypic spectrum seen included (1) optic atrophy and CNS involvement with mild neuropathy; (2) early-onset, rapidly progressive severe neuropathy; and (3) late-onset, slowly progressive mild sensory neuropathy. Compound heterozygous mutations were not found and therefore did not account for the most severely affected persons with toddler onset, as has been described previously.13 The study supports the belief that MFN2 mutations do not necessarily associate with a particular discrete phenotype, as previously observed.3 Small kindred size from earlier studies did not emphasize that there could be a significant clinical variability within 1 family with all affected carrying the same mutation. In counseling families with MFN2 gene mutations (OMIM *608507), it is important to provide understanding that a relatively mildly affected parent may give rise to a severely affected child; conversely, a severely affected parent with motor neuropathy may give rise to a child with mild neuropathy. Both situations were observed here; mildly affected parent IV-8 had severely affected child V-7. Parent V-18 had severe neuropathy, whereas her children, VI-9 and VI-10, had mild neuropathy but with blindness from optic atrophy.
This study demonstrates that persons with MFN2 mutations having optic atrophy and paraventricular white matter T2 signal changes may be considered to have multiple sclerosis when the neuropathy symptom is mild or unrecognized. Our proband’s sister, VI-10, had bilateral optic atrophy and paraventricular MRI abnormalities that predated identification of her neuropathy. This led to initiation of interferon β-1a for presumed multiple sclerosis. In her case, the absence of CSF oligoclonal bands, no gadolinium enhancement on MRI brain lesion, insidious progression of optic atrophy without MRI brain progression, and absence of upper motor neuron signs would all suggest against the diagnosis of multiple sclerosis. The identification of her MFN2 mutation and electrophysiologic confirmation of her neuropathy led to the diagnosis of her CMT2A2. Of additional note is that she developed complex partial seizures years into her disease. This may be incidental because seizures have not been reported in other MFN2 mutation cases; however, alteration of consciousness with fatal encephalopathy is described.19 Evaluation of her brother (VI-9), who also has optic neuropathy and peripheral neuropathy, assisted in identification of the pathogenic cause in her case. The brother (VI-9) had no clinical stigmata of CNS disease with normal brain and spinal cord imaging, but his evoked potentials were consistent with spinal cord involvement. Because MFN2 is ubiquitously expressed, including in the mitochondria of brain and spinal cord, the pathologic involvements of these tissues is predicted and has been previously reported.3,4,20 In the evaluation of a patient with optic atrophy, a family history of axonal length-dependent neuropathy should not be considered an incidental occurrence, and MFN2 mutations need to be considered.
A limited number of electron microscopy reports4,12 have suggested abnormal shape, size, aggregation, and cristae of the mitochondria in patients with MFN2 mutation. However, we did not observe abnormal shape, size, aggregation, and cristae of the mitochondria in our studied case, despite the loss of large myelinated fibers. The mitochondria appeared evenly distributed in the axons in our case, and the cristae and other mitochondrial features in Schwann cell and axonal cytoplasms were indistinguishable from equivalently prepared healthy controls and patients with diseased sural nerve biopsy specimens4 (Figures 4 and 5).
This study expands the genetic causes of CMT2A2. The optic neuropathy and brain imaging findings may lead to major consideration of multiple sclerosis when neuropathy is overlooked or mild. The fact that there is a range of severity and presentation in a single large family is important. Specifically, in genetic counseling, a parent with mild disease may give rise to a child with severe early-onset of disease or a person with blindness. Last, mutations of MFN2 may exert their molecular pathogenic effect without clear structural mitochondrial abnormalities compared with normal and diseased biopsy specimens.
Acknowledgments
Funding/Support: This work was funded by grant K08 NS065007 from the National Institutes of Health and a Mayo Foundation Neuroscience Development award (Dr Klein).
Footnotes
Financial Disclosure: Dr Pittock and Mayo Clinic have a financial interest in the technology titled “Aquaporin-4 Autoantibody as a Cancer Marker,” which has been licensed to a commercial entity, but no royalties have been received. In addition, he is an inventor of technology titled “Aquaporin-4 Binding Autoantibodies in Patients With Neuromyelitis Optic Impair Glutamate Transport by Down-Regulating EAAT2.” Mayo Clinic has filed a nonprovisional patent application for this technology. Also, he received a research grant from Alexion Pharmaceuticals for his investigator-initiated study titled “An Open Label Study of Eculizumab in NMO.” Dr Pittock is a consultant in the Department of Laboratory Medicine and Pathology. In his role as codirector of the Neuroimmunology Laboratory, he has no additional intellectual property related to any tests performed on a service basis in the laboratory. He receives no royalties from the sale of these tests when used for patients. Mayo Collaborative Services, Inc, does receive revenue for conducting these tests.
Author Contributions: Study concept and design: Klein and Dyck. Acquisition of data: Klein, Engelstad, and Cunningham. Analysis and interpretation of data: Klein, Kimmel, Pittock, and Wu. Drafting of the manuscript: Klein and Kimmel. Critical revision of the manuscript for important intellectual content: Klein, Pittock, Engelstad, Cunningham, Wu, and Dyck. Statistical analysis: Klein. Obtained funding: Klein. Administrative, technical, and material support: Klein and Kimmel. Study supervision: Klein and Dyck.
Additional Contributions: We thank the family for their participation and Mr Larry Witt for coordinating the DNA library used in this work.
References
- 1.Calvo J, Funalot B, Ouvrier RA, et al. Genotype-phenotype correlations in Charcot-Marie-Tooth disease type 2 caused by mitofusin 2 mutations. Arch Neurol. 2009;66(12):1511–1516. doi: 10.1001/archneurol.2009.284. [DOI] [PubMed] [Google Scholar]
- 2.Barisic N, Claeys KG, Sirotković-Skerlev M, et al. Charcot-Marie-Tooth disease: a clinicogenetic confrontation. Ann Hum Genet. 2008;72(pt 3):416–441. doi: 10.1111/j.1469-1809.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung KW, Kim SB, Park KD, et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain. 2006;129(pt 8):2103–2118. doi: 10.1093/brain/awl174. [DOI] [PubMed] [Google Scholar]
- 4.Verhoeven K, Claeys KG, Züchner S, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129(pt 8):2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 5.Züchner S, Mersiyanova IV, Muglia M, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A [published correction appears in Nat Genet. 2004;36(6):660] Nat Genet. 2004;36(5):449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Takita J, Tanaka Y, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bβ [published correction appears in Cell. 2001;13:106(1):127] Cell. 2001;105(5):587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 7.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305 (5685):858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 8.Cartoni R, Martinou JC. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Exp Neurol. 2009;218(2):268–273. doi: 10.1016/j.expneurol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Loiseau D, Chevrollier A, Verny C, et al. Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Ann Neurol. 2007;61(4):315–323. doi: 10.1002/ana.21086. [DOI] [PubMed] [Google Scholar]
- 10.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 11.Benedetti S, Previtali SC, Coviello S, et al. Analyzing histopathological features of rare Charcot-Marie-Tooth neuropathies to unravel their pathogenesis. Arch Neurol. 2010;67(12):1498–1505. doi: 10.1001/archneurol.2010.303. [DOI] [PubMed] [Google Scholar]
- 12.Vallat JM, Ouvrier RA, Pollard JD, et al. Histopathological findings in hereditary motor and sensory neuropathy of axonal type with onset in early childhood associated with mitofusin 2 mutations. J Neuropathol Exp Neurol. 2008;67(11):1097–1102. doi: 10.1097/NEN.0b013e31818b6cbc. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson GA, Magdelaine C, Zhu D, et al. Severe early-onset axonal neuropathy with homozygous and compound heterozygous MFN2 mutations. Neurology. 2008;70(19):1678–1681. doi: 10.1212/01.wnl.0000311275.89032.22. [DOI] [PubMed] [Google Scholar]
- 14.Zhu D, Kennerson ML, Walizada G, Züchner S, Vance JM, Nicholson GA. Charcot-Marie-Tooth with pyramidal signs is genetically heterogeneous: families with and without MFN2 mutations. Neurology. 2005;65(3):496–497. doi: 10.1212/01.wnl.0000171345.62270.29. [DOI] [PubMed] [Google Scholar]
- 15.Züchner S, De Jonghe P, Jordanova A, et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59(2):276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 16.Brockmann K, Dreha-Kulaczewski S, Dechent P, et al. Cerebral involvement in axonal Charcot-Marie-Tooth neuropathy caused by mitofusin2 mutations. J Neurol. 2008;255(7):1049–1058. doi: 10.1007/s00415-008-0847-1. [DOI] [PubMed] [Google Scholar]
- 17.Lawson VH, Graham BV, Flanigan KM. Clinical and electrophysiologic features of CMT2A with mutations in the mitofusin 2 gene. Neurology. 2005;65(2):197–204. doi: 10.1212/01.wnl.0000168898.76071.70. [DOI] [PubMed] [Google Scholar]
- 18.Dyck PJ, Giannini C, Lais A. Pathologic alterations of nerves. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo J, editors. Peripheral Neuropathy. 3. Vol. 1. Philadelphia, PA: WB Saunders; 1993. pp. 514–595. [Google Scholar]
- 19.Boaretto F, Vettori A, Casarin A, et al. Severe CMT type 2 with fatal encephalopathy associated with a novel MFN2 splicing mutation. Neurology. 2010;74(23):1919–1921. doi: 10.1212/WNL.0b013e3181e240f9. [DOI] [PubMed] [Google Scholar]
- 20.Del Bo R, Moggio M, Rango M, et al. Mutated mitofusin 2 presents with intra-familial variability and brain mitochondrial dysfunction. Neurology. 2008;71 (24):1959–1966. doi: 10.1212/01.wnl.0000327095.32005.a4. [DOI] [PubMed] [Google Scholar]