Abstract
A nearly complete skull of Parapithecus grangeri from the early Oligocene of Egypt is described. The specimen is relatively undistorted and is undoubtedly the most complete higher primate skull yet found in the African Oligocene, which also makes it the most complete Oligocene primate cranium worldwide. Belonging in superfamily Parapithecoidea, a group regarded by some as the sister group to all other Anthropoidea, this skull reveals important information about the radiation of stem anthropoideans. This cranium is about 15% larger than size estimates based on a fragmentary cranium of its contemporary and close relative Apidium phiomense. It is about the same size as that of the gray gentle lemur, Hapalemur griseus, or of platyrrhines such as the owl monkey, Aotus trivirgatus, or the titi monkey, Callicebus torquatus. Comparatively small orbits and size differences in jaws and teeth show it was both diurnal and dimorphic. This is the only specimen of the species that shows (from sockets) that there were four small upper incisors. Several mandibular specimens of the species establish that there were no permanent lower incisors and that the symphysis was fused. Like other early anthropoideans this species possessed a lower encephalization quotient and less-developed orbital frontality than later anthropoideans. There is full postorbital closure and fusion of the metopic suture, and the ectotympanic forms a rim to the auditory aperture. A probable frontal/alisphenoid contact is a potentially derived resemblance to Catarrhini. A proposed separate genus for the species P. grangeri is not sustained.
Eocene and Oligocene continental beds exposed in badlands lying north of Lake Qarun, Fayum Province, Egypt have produced a diverse vertebrate fauna as well as a broad series of fossil primates that include the world's earliest undoubted and well documented anthropoideans (see refs. 1 and 2 and references therein). This specimen is of Oligocene age, being about 33 million years old (3). The skull was discovered in the central part of Fayum Quarry I, a site that lies ≈200 m above the base of the upper sequence of the Jebel Qatrani Formation, Fayum Province, Egypt (4). This quarry was discovered in December 1962 and has been collected from almost continuously since that time until the present. But over this long period, during which thousands of vertebrate fossils, including hundreds of primate maxillae and mandibles, were collected, no essentially complete primate cranium was ever found there. The closest approximation came in 1989 when a partial cranium of Apidium phiomense [Duke University Primate Center (DPC) 9867] was located in Quarry I. That specimen is dorsventrally crushed and lacks most of the basicranium and part of the facial region (5). In contrast, the cranial specimen described here, DPC 18651, is undistorted and has lost only the upper canines and two pairs of incisors (sockets only) and the left zygomatic arch. The specimen is nearly unique in another respect in that it was almost completely enclosed in a rock-hard sandstone concretion that accounted for its relative lack of distortion.
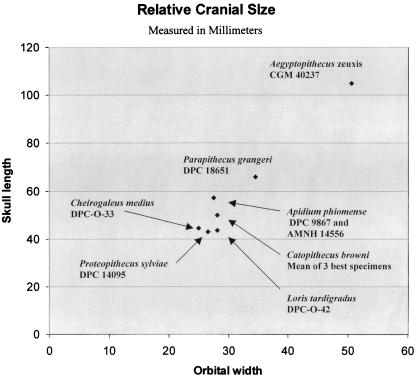
This cranium of Parapithecus is distinctly larger than well preserved skulls of Fayum late Eocene Proteopithecus and Catopithecus, which are only about 70% as long as Parapithecus. In turn, the length of this Parapithecus skull is only about 60% that of the best-preserved Aegyptopithecus skull (Fig. 1). In this species there are very few attributable postcranial bones from which absolute size could be estimated. Because of the quality of preservation almost every detail of taxonomic or phyletic significance about the cranium of Parapithecus can be determined such as the arrangement, structure and dental formula of the upper teeth, shape of the premaxilla, location of the lacrimal bone and foramen, extent of postorbital closure, exposure of the frontal/alisphenoid contact, size of the infraorbital and zygomaticofacial (malar) foramina, metopic sutural closure, extent of the lateral pterygoid alae, proportions of the palate and nares, and precise structure of the auditory region and basicranium. Most of the details of cranial structure described here can be seen more clearly than in almost all of the previously discovered skulls of Fayum primates.
Figure 1.
Relative cranial size of five species of Fayum Eocene and Oligocene primates, plotting orbital width against cranial length, and compared with a dwarf lemur, Cheirogaleus medius, and a slender loris, Loris tardigradus.
Systematics
Order Primates Linnaeus, 1758: Suborder Anthropoidea Mivart, 1864; Superfamily Parapithecoidea, Kälin, 1961; Family Parapithecidae, Schlosser, 1911.
Genus Parapithecus Schlosser, 1911.
Emended generic diagnosis.
Original descriptions of the genus and its distinctiveness were based on the single individual lower dentition of the type specimen of the genus and species, Parapithecus fraasi. This specimen, Stutgart Natural History Museum (SNM) 12639a, contained lower teeth in a partly preserved mandible that lacked only the right P2, showed symphyseal fusion, and also showed a unique feature among primates in that it appears to have lacked adult lower incisors and had retained into young adulthood only a single pair of milk incisors. Almost every observation that could or can be made about this specimen has been discussed and challenged in a long series of papers (see refs. 5–14 and references therein). In the species to which the cranium described here belongs, Parapithecus grangeri, permanent lower incisors are lost, lower canines contact and wear against each other, and the mandibular symphysis is solidly fused. Dentally, Parapithecus differs from Serapia in having P2 smaller, not larger than P3. It is unlike Apidium in having premolars and molars that are less cuspidate, with relatively smaller hypocone and M3/3 as well as showing the P2–4 central cusp comparatively smaller than in the latter genus. Also unlike Apidium, where M1–3 distinctly increase in size posteriorly, M1–3 of Parapithecus are subequal in length or M3 < M2. Parapithecus shows a shorter rostrum, and comparatively smaller upper incisors (alveolae) as well as relatively larger canines (both upper and lower) than Apidium. It differs from Apidium in that the four main upper molar cusps are at the corners of a square, rather than having the hypocone much more lingually situated. Also, it differs from Qatrania in lacking the large M1 trigonid that is open lingually. Qatrania has relatively larger hypoconulids that are closer to the entoconid. It differs from Serapia in exhibiting almost no expression of the paraconid. P. grangeri has a comparatively larger zygomatic foramen than does A. phiomense. In my view (see refs. 11 and 13) no characters exist to validate a separate genus, Simonsius, used by some authors (8, 14) for the species P. grangeri.
Type species.
P. fraasi.
Hypodigm.
A single known specimen in the collection of the SNM, SNM 12639a, is discussed but see below under species distribution.
Species diagnosis.
P. fraasi possesses larger and more bulbous hypoconids on P3–4 than seen in P. grangeri. It retains into young adulthood a single pair of lower (?)milk incisors, which appears to be lost earlier in individual life in P. grangeri. Comparable measurements on teeth and mandible range from 10% to 30% smaller than in P. grangeri. Horizontal ramus of mandible maintains a similar depth from front to back whereas that of P. grangeri usually deepens posteriorly.
Distribution.
The type of P. fraasi was recovered by Markgraf at an unrecorded site, probably in the lower sequence Jebel Qatrani Formation (below Barite sandstone, see ref. 4). Nevertheless, two specimens in the DPC collections, DPC 2374, from Quarry M, and DPC 3135, from Quarry I are unusually small for P. grangeri and could well represent P. fraasi—being in the same size range in those comparable measurements that can be taken. However, both specimens are damaged, and DPC 3135 is heavily worn so that development of the premolar hypoconid cannot be observed. What is much more important is that, should the latter be referable to P. fraasi, it unequivocally shows that the symphysis is solidly fused and that, as an older individual, the single pair of incisors are lost.
Referred species.
P. grangeri.
Distribution.
P. grangeri occurs only in the Fayum Depression, Egypt, upper sequence Quarries I and M.
Species diagnosis.
P. grangeri differs from type in being about 30% larger and in having lost by early adulthood all lower incisors. The mandible is larger and more robust than in P. fraasi, compared with the relative size of teeth.
Hypodigm.
The hypodigm includes: the type left mandible with P2-M3, Geological Museum, Cairo (CGM) 26912; cranium, DPC 18651; left maxilla DPC 2385; associated left maxilla and frontal DPC 6641; left maxilla DPC 1090; left maxilla DPC 2385; right maxillae DPC 2372 and 8793; attached parietal bones DPC 1098; a series of mandibular fragments particularly Peabody Museum, Yale University (YPM) 21010; YPM 21019a, YPM 23796, YPM 26918;YPM 23953; YPM 23954; YPM 23973; DPC 1009; DPC 1049; DPC 1053; DPC 1091; DPC 2376; DPC 2399; DPC 2807; DPC 2944;DPC 3110; DPC 3135; DPC 3876; DPC 3899; DPC 5263; DPC 5527; DPC 6326; DPC 6313; DPC 7252; DPC 8796; DPC 9857; DPC 10692; DPC 12303; DPC 13584; DPC 13589; DPC 13590; DPC 14304; DPC 20580; numerous isolated teeth and less complete YPM and DPC jaw fragments, and numerous specimens at the CGM including CGM 26918.
Description and Comparison
Cranial Size and Braincase.
DPC 18651 is the least distorted primate skull so far found in the Fayum Paleogene badlands and there seems to have been little dorsoventral compression of the braincase. The orbital margins have been somewhat deformed but are clearly smaller relative to overall size than in skulls of the South American monkey Callicebus torquatus. This cranium is 65.8 mm long, which is similar to the skull length of Aotus trivirgatus or C. torquatus, whereas the occlusal area of the upper premolars and molars of P. grangeri is clearly much greater and the brain volume distinctly smaller than in the latter two platyrrhines. The surface area of these upper teeth in Aotus is approximately one-third and that of C. torquatus is 60% of that of P. grangeri. Kay and I (15) estimated the body weight of P. grangeri as about 1,800 g (range 1632–1990). Such a body weight is more than twice the weight of these two monkeys and falls rather in the range of modern Cebus monkeys. Clearly, like Aegyptopithecus, Parapithecus had a relatively much smaller brain in relation to body size than does any living anthropoidean. Parapithecus is a medium-sized Fayum primate (Fig. 1). In comparison, Proteopithecus has a skull length of about two-thirds that of Parapithecus, DPC 18651, and it in turn is about 60% the length of crania of Aegyptopithecus, see Fig. 1.
I (5) figured and discussed facial (DPC 2385), frontal (DPC 6641), and parietal (DPC 1098) fragments that I assigned to P. grangeri. The newly discovered cranium establishes that these assignments were correct. The facial fragment shares with DPC 18651 the relatively large zygomaticofacial foramen, compared with that of A. phiomense, and the frontal and parietals show a similar slight development of the sagittal crest as was noted for DPC 6641 and 1098. A comparatively large zygomaticofacial foramen of this relative size characterizes several platyrrhines such as Callicebus, Alouatta, and Lagothrix.
The Brain.
Because this braincase is composed of egg shell-thin bone it has been possible to make a brain model of clay that is approximately the same absolute size as the volume contained in the cranium. The brain model has an approximate volume of 14 cm2. Taking the estimated body mass of 1,800 g (15) for P. grangeri this would give a brain-to-body ratio of about 1:13. The encephalization quotient also can be calculated by the method of Jerison (16) and is 0.77. This puts DPC 18651 between quotients calculated by the same author (16) for Eocene prosimians such as Tetonius (0.71) and Necrolemur (0.94) but well above his estimates for the Eocene adapids Notharctus and Adapis which he calculated both to have a quotient of 0.53. Jerison's encephalization quotient for Aegyptopithecus is 0.97. A number of other methods for determining encephalization quotients have been proposed (17) but whatever the method used, the basic implication is the same, which is that these earliest anthropoidean crania fall in with prosimians, and not with later anthropoids. Looking at the skull in profile, from the outside, the position of the olfactory bulbs is directed more anteriorly than in Aegyptopithecus and the breadth of the interorbital region suggests that the bulbs were also relatively larger. Although Aegyptopithecus also has a broad space between the orbits the best-preserved endocast shows that the olfactory bulbs are small proportionately and downward directed (18) unlike what they seem to be in P. grangeri.
Orbital, Frontal, and Jugal Region.
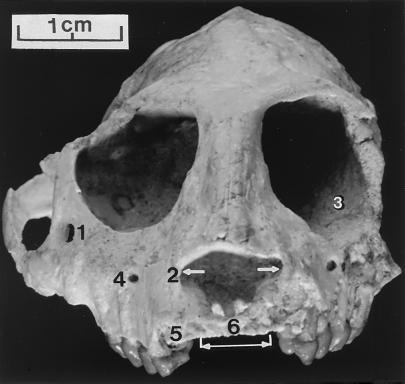
The proportionately relatively small size of the orbits indicates that P. grangeri was diurnal, as is the case with all of the other Fayum anthropoideans. Both eye sockets of DPC 18651 are preserved but they have been slightly distorted (Fig. 2). This is because the lower face has been rotated downward somewhat on the left side and a part of the dorsolateral margin of the eye socket on the right side has been pushed inward. Otherwise, the right orbital opening seems undistorted. As has been noted before (5, 18) the zygomaticofacial foramen is very large and is larger relatively than in Proteopithecus, Apidium, or Aegyptopithecus where this feature can be observed (Fig. 2). The zygomatic bone is not known in most Fayum primate species. This foramen also can be comparatively large in some of the small-bodied platyrrhines (19). The inferior orbital fissure on both sides is somewhat occluded with sand grains but appears to be small. The postorbital plate of the jugal is very large and expanded, establishing complete postorbital closure, possibly as much or more complete than in Aegyptopithecus, Catopithecus, and Proteopithecus-–the only other Fayum anthropoideans where the postorbital region is preserved (Fig. 3). It would appear that the frontals and alisphenoids contact as in catarrhines. Particularly on the left side the suture between these two bones, running horizontally, can be made out and the jugal is excluded from the parietal. This may be a derived catarrhine condition. I (20) described an isolated primate frontal bone that we now believe is probably of the parapithecid Apidium (5). On the medial orbital wall of that frontal is, dorsally, a venous foramen and below it are a pair of ethmoidal canals. These openings do not appear to be present in either orbit of DPC 18651.
Figure 2.
Facial aspect of the cranium of P. grangeri, DPC 18651. Note especially 1) the comparatively large zygomaticofacial foramen, 2) broad nasal aperture, 3) complete postorbital closure, 4) infraorbital foramen, 5) large canine socket and 6) small incisor sockets (white bracket).
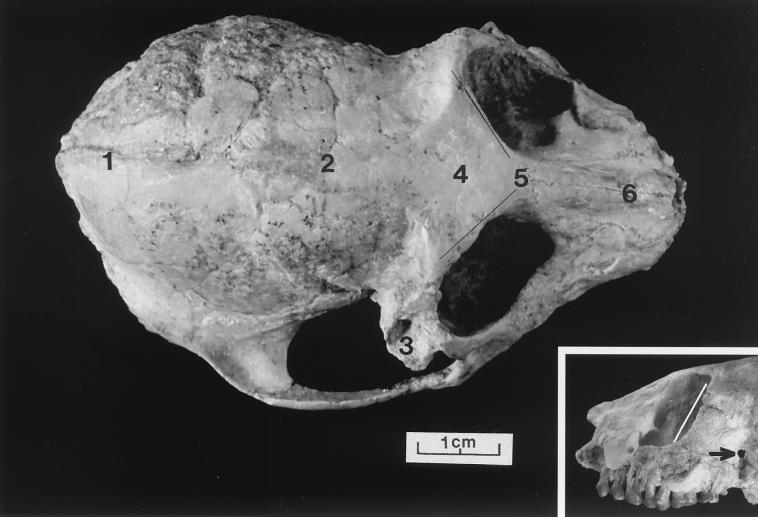
Figure 3.
Dorsal view of the skull of P. grangeri, DPC 18651. Note: 1) low sagittal crest, 2) posterior convergence of the temporal lines, 3) displacement of right postorbital region, 4) closure of the metopic suture, 5) lines indicate lower angle of orbital convergence than in most later anthropoideans, 6) relatively short face. (Inset) Lateral view of orbit shows less frontation than in Aegyptopithecus and later anthropoideans. Arrow indicates very small postorbital fissure.
Rasmussen and I (13) measured angles of orbital convergence for Catopithecus as falling within 120° to 132°. Here, in DPC 18651, the angle is lower, about 105° whereas in the Fayum frontal, AMNH 14556, of Apidium, this angle is about 115o. In Aegyptopithecus, the only other Fayum primate with undistorted frontals the same angle is around 135o to 140o in subadults but the angle increases to around 180o in full adults. This evidence is scanty but it appears that—among parapithecids—the orbits are slightly less convergent than in other Fayum anthropoideans and also less frontated (Fig. 3).
Rostral Region.
The rostrum of DPC 18651 is completely preserved. There is an interorbital septum and, dorsally, the interorbital region is reasonably broad, although not as broad as in the best preserved facial region of Aegyptopithecus zeuxis, DPC 8794, where this breadth is about 70% of orbital height. In the skull of P. grangeri, DPC 18651, breadth between the eye sockets is ≈50% of orbital height. Posteroventrally between the eyeballs the septum is much thinner than in front but the interorbital partition remains substantial and there is no fenestra between the eyeballs as there is in Tarsius or Saimiri. In both A. zeuxis and P. grangeri the lacrimal bone and foramen lie within the orbit, and although DPC 18651 is slightly damaged in the region, both specimens show a maxillary component of the ventral orbital margin between the jugal and lacrimal. Both Parapithecus, DPC 18651, and Aegyptopithecus, DPC 8794, have comparatively long nasals in relation to most Miocene-Recent anthropoids. The nasal bones are completely preserved only in these two specimens. Orbital height in Aegyptopithecus is relatively greater in relation to nasal length, about 70%, whereas in DPC 18651 this ratio is 63%, and hence, the nasals are relatively a little longer in Parapithecus. The upper surface of the nasals is concave ventrally in Aegyptopithecus whereas in Parapithecus this surface in profile is flat with a slight upturn distally. Because of these relationships the rostrum of Parapithecus rides higher between the orbits than in Aegyptopithecus so that the orbital opening is directed somewhat more upward i.e., is less frontated. Hence, in this species, P. grangeri, effective stereoscopic vision would have been enhanced by dipping the snout slightly, but not to the degree seen in lorisoids. No complete nasals have been found for Catopithecus or Proteopithecus, but in the latter two genera they seem to have been at least as long as in Aegyptopithecus. Posteriorly the two nasals of Parapithecus come together in a central point as in Aegyptopithecus, DPC 8794 whereas distally the apex of each is a rounded central point, so that, together their margins describe a lowercase, rounded w.
Premaxilla and Maxilla.
The premaxilla is comparatively small and the ascending premaxillary wing, as in Proteopithecus, relatively smaller than in Catopithecus or Aegyptopithecus. Although the incisors and canines have fallen out, the alveolae of the latter are extremely large, especially in relation to the upper incisor alveolae which are much reduced in correlation with the loss of the lower incisors. The canine root thus establishes slightly raised canine pillars. The upper central incisor alveolae are closely approximated demonstrating that, as in other higher primates, there was no frenulum or rhinarium. The frontal process of the maxilla, between the lacrimal and the posterior nasals bears two small foramina on each side. The anteriormost margin of the orbit is directly above a vertical line passing between P4 and M1, which puts the orbital position slightly further forward, at least ventrally on the maxilla, than in an arrangement which correlates with the more upward orientation of orbital frontation across the rostrum. As mentioned already, the maxilla contributes a portion to the lower orbital rim. Well below this ventral orbital margin on both sides lies a single, small infraorbital foramen. The maxilla is distinctly less high dorsoventrally than in Aegyptopithecus, but may be a little deeper relatively than in Catopithecus or Proteopithecus. The palatal aspect of the maxillae resembles that of other anthropoideans.
Upper Dentition and Palate.
Alveolar size indicates that the central incisors were slightly larger than the lateral and that the canines were large and robust in DPC 18651 as would be expected for a male. A left maxilla DPC 2385 holds a comparatively very large canine, elongated at the base in the line of the tooth row. It is blunt or truncated and heavily worn on the apex. There is no groove along the vertical face of the upper canine as in Apidium where this groove also runs dorsally into the root as occurs also in many cercopithecoids. Another right maxilla with upper canine, DPC 8793, is similar to the former. The upper premolars increase in size posteriorly whereas the molars decrease from front to back. As in Apidium there are three premolars and the P3 and P4 both show the distinct central cusp, between the inner and outer cusps, that is unique to parapithecids. This cusp can be called a paraconule or less appropriately a centrocone. The lingual third of right M2, the canines and the incisors have been lost in DPC 18651. DPC 18651 is a relatively old individual, judging from deep wear excavations into the protocone-hypocone areas of all molars. This has led to premortem removal by wear of much of the inside of left M2 and breaking away of the entire inner part of right M2 with erosion of alveolar bone at its base apparently due to caries. Wear has removed most of the occlusal structure but it would seem that the M3 had hardly any expression of a metacone and the protocone-hypocone may have been coalesced. The small relative size of M3 also is seen in Proteopithecus, Catopithecus, and primitive platyrrhines (18). The palate is slightly arched but mesially there are no ventrally extended alveolar processes holding the teeth as in Aegyptopithecus where the palate is much more arched above the dental occlusal surfaces. The incisive foramina and posterior palatine foramina of DPC 18651 are well defined. The posterior nares open slightly forward of a line drawn across the back of the third molars. As in anthropoideans there is no posterior palatine torus of the sort usually seen in omomyids and in Tarsius.
Posterior Dorsal Braincase.
The braincase of this specimen is preserved with little distortion. It is long and slightly flattened, much as in Aegyptopithecus or even Callithrix. The temporal lines converge posteriorly to form a slightly developed saggital crest joining lambdoid or nuchal crests at the inion (Fig. 3). Unlike the condition in Proteopithecus there is no strongly developed medial occipital crest, but only a slightly rounded ridge in this position much as in Aegyptopithecus. Curiously, in Catopithecus, DPC 11388, this area seems to be flat and entirely lacks the central crest or ridge.
Basicranium and Basioccipital.
The basicranium of this specimen is better preserved and less distorted than of any other of the three Fayum species with known basicrania: Aegyptopithecus zeuxis, Catopithecus browni, and Proteopithecus sylviae. Because of completeness of DPC 18651 it can be seen, on the right side, that the lateral pterygoid wing extends far back to overlap the lateral bullar wall. The situation of this wing in Tarsius is that the wing also rides onto the bulla, but because the bulla is extended so far forward the bulla and body of the pterygoids are closely approximated, rather than having, as in Parapithecus, the wing extended very far backward. A similar contact of the lateral pterygoid occurs in certain omomyids, Rooneyia, Mahgarita, and perhaps Pronycticebus and hence, may simply be a primitive condition but it is a resemblance to both tarsiids and omomyids.
Auditory Region.
The ear region of Parapithecus is clearly the derived anthropoidean condition showing a large foramen for the internal carotid artery. On the left side the bullar wall has been damaged so that some of the internal structure of the petrosal can be seen, but it is difficult to interpret. However, Cartmill et al. (21) discussed several isolated petrosals found in quarry I in the Fayum and one of these, YPM 25973, is closely comparable to the same region of the cranium of P. grangeri and it is figured and discussed in detail. This and other specimens described (21) indicate that parapithecids possess a trabeculated anterior accessory cavity of the petrosal and also show a well developed internal carotid, evidently lacking a stapedial branch. DPC 18651 has an annular ectotympanic bone that is co-ossified around the lateral opening of the petrosal bulla, situated much as it is in Aegyptopithecus, where the tubular ectotympanic characteristic of later catarrhines was yet to develop. The bullae are more inflated and somewhat more laterally situated than in Aegyptopithecus and the whole basicranial area is similar to that of various small bodied platyrrhines. The carotid foramen is located forward, much as it is in Cebus.
Temporo-Mandibular Joint.
The glenoid fossa extends outward laterally somewhat farther here than it appears to in Aegyptopithecus or Proteopithecus and is less anteroposteriorly broad. This difference in proportion may have something to do with the odd dental adaptation of Parapithecus, where lower incisors are entirely missing in adults, the only primate to exhibit this condition. Particularly the middle of the upper molars of DPC 18651 have a deep anteroposterior trough worn into them, indicating heavy chewing stress in the region. This wear can also be seen on lower molars of specimens such as in DPC 8796, 1113, or 12303. Differences in chewing stress and spacing of the tooth rows are likely to affect mandibular articulation as well. This region of DPC 18651 shows that the mandibular articular condyle rides in a transverse notch in front of a broad, mesiolaterally extended postglenoid process. A rather different articular region can be seen in Proteopithecus (18) where there is no trough and a mesiolaterally narrower process. Catopithecus and Aegyptopithecus are more similar to each other here than to Proteopithecus.
Mandibular Form and Lower Dentition.
Because of the reduction and loss of incisors Parapithecus has a more bell-shaped outline of the dentition, when viewed occlusally, above and below, than do other Fayum anthropoideans (Fig. 4). Especially when looking at the crown view of the lower dental arcade this lateral bowing out can be seen. This bowing is more pronounced in P. grangeri and is only slightly developed in P. fraasi. It does not occur in A. phiomense. The mandibular articular condyles are situated slightly above the long axis of the dentition, as in Apidium, but the coronoid process is lower and anteroposteriorly longer than in the latter. In both species the angle is smoothly rounded. When P. grangeri was described (10) it was stated that this species differs from the type in having a mandibular ramus that deepened posteriorly whereas that of P. fraasi did not. With more specimens of P. grangeri it is now clear that some mandibles do not deepen posteriorly. Variation in P. fraasi cannot be determined. As stated, lower incisor loss in P. grangeri is unique among primates, and also the lower canine pair of this species wear flat apically against the upper incisors, also a unique feature. In P. grangeri the large lower canines (left and right), when fully erupted, form a interstitial contact wear facet between themselves. As these permanent lower canines erupt they resorb the roots above them and thus eliminate the lower milk incisors. There is no room left for permanent lower incisors even to develop. If DPC 3135 is correctly referred to P. fraasi then the same is true for mature individuals of both species of Parapithecus. Kay and Williams (14) state: “Of course, if P. fraasi could be shown to have had incisor reduction to one tooth [sic] forshadowing the complete loss of lower incisors in Simonsius this would be an important synapomorphy lending support to a hypothesis of sister-group relationship between the two species.” But I believe that the two species do share this unusual synapomorphy because the type of P. fraasi has only one lower incisor pair, and, being of very light color this pair are almost certainly milk incisors. In any case, my examination of the type at Stuttgart, SNM 12639a, showed that there is no room for additional incisors and no indication of additional alveoli or broken lateral alveolar margins, as is very common with specimens of A. phiomense where this region is preserved, even when teeth have fallen out. Hence, the P. fraasi type, SNM 12639a, shows incisor reduction to one tooth pair and moreover, that pair also might have been lost later in life as has happened with DPC 3135. Of the other distinctions cited between these two species (14) four are matters of only slight degrees of difference where presence or absence, degree of size expression or degree of inflation fall within the realm of individual judgement calls and simply cannot, with any degree of reliability, be given discrete character states for use in cladistic analyses. The degree of development of characters of the sort under consideration are usually gradational, not either/or, like the flipping of a coin which must come down on one side or the other. There is a chance with this methodology, as is the case here, that several rankings, which appear to be differential, are added together to give the impression that a real distinction has been drawn. For instance, I read the degree of development of the buccal molar cingulum and amount of molar inflation (two features cited in ref. 14) in the two Parapithecus species as being the same, and hence, not worthy of different numerical rankings. The one feature of tooth crown structure that does separate P. grangeri from P. fraasi is the more clearly developed premolar hypoconid cusp in the latter of these two species. Nevertheless, there is a lower crowned and less well defined premolar cusp in P. grangeri when unworn. This is a difference, that in my view, is insufficient to form the basis for a generic distinction.
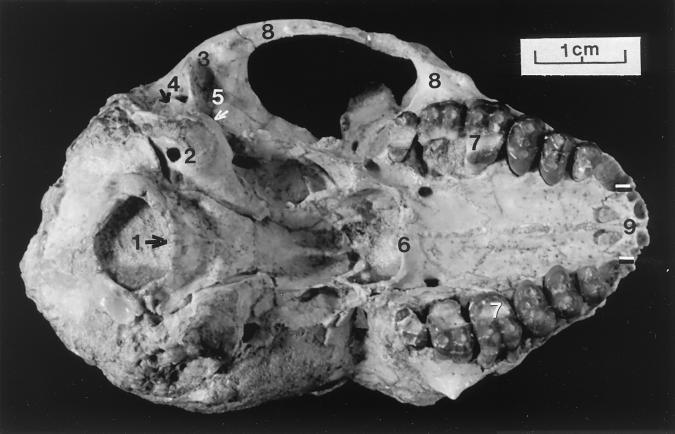
Figure 4.
Ventral view of the cranium of P. grangeri, DPC 18651. Note: 1) relatively anterior location of foramen magnum, 2) position of the carotid foramen, 3) trough for the articulation of the mandibular condyle, 4) relatively lateral location of auditory bullae, 5) posterior overlapping of auditory bulla by pterygoid wing, 6) back of palate in line with posterior margin of third molar, 7) wear trough on medial crown surface of upper molars, 8) flaring zygomatic arch, and 9) reduced breadth of the four upper incisor alveolae (between black/white lines).
Conclusions
The general cranial anatomy of Parapithecus shows very little similarity to that of Tarsiiformes or to strepsirrhines, other than in its comparatively small brain size. This newly recovered skull shows not only most distinctions that were pointed out earlier for Parapithecidae but also many features newly demonstrated for the family and superfamily. Taken by itself DPC 18651 seems not to have noticeable derived features that could be interpreted as compelling synapomorphies with other parapithecids or other clades of undoubted anthropoids. It seems reasonable to continue to consider the group as the sister of all other Anthropoidea a position followed by many authors (6, 14, 22–24). Many of the similarities to platyrrhines that can be observed are presumably shared primitive features, such as the absence here of a tubular ectotympanic that is known to be absent also in the early catarrhines Catopithecus and Aegyptopithecus. The backward extended and rounded mandibular angle with a well defined lunar notch above it seen in parapithecids and platyrrhines is certainly also a shared primitive feature. Interestingly, the lunar notch is only slightly expressed in Aegyptopithecus. The large zygomaticofacial or malar foramen resembles that of platyrrhines whereas the jugal and parietal bones being separated by an alisphenoid-frontal contact is seen in catarrhines. The cercopithecoid feature of having a vertical groove running up and down the anterior face, and into the root, of the upper canine that is seen in Apidium, but not in Parapithecus, is presumably a convergence between Apidium and the cercopithecoids. The new cranium also shows the lateral pterygoid plate extending far back to touch the bulla, as in tarsiers, omomyids and some extant catarrhines but which does not typically occur in platyrrhines or in Aegyptopithecus. Unlike tarsiers, omomyids and even Aegyptopithecus the basisphenoid and basioccipital of DPC 18651 are broad, more as in platyrrhines or even Rooneyia. Fleagle and Kay (6) presented plausible reasons for concluding that supposed dental similarities between parapithecids and catarrhines that had been put forward earlier (9, 25) are convergences. Similarly, the mosaic nature of the anatomy of this skull, combining as it does a mixture of primitive, platyrrhine and perhaps even catarrhine characteristics would indicate that cited postcranial resemblance to extant catarrhines (26) are also convergent.
Acknowledgments
I thank F. A. Ankel-Simons, T. M. Bown, E. R. Seiffert, and two unknown reviewers for comments and criticisms of the manuscript. Field operations were facilitated by P. S. Chatrath. Preparation of the intractable matrix encasing DPC 18651 was carried out by Chuck Schaff at the Museum of Comparative Zoology, Harvard University, Cambridge. Photographs were prepared at Duke University by R. Usery. Fig. 1 was prepared by Barker Fariss. The specimen was found by Issa Zekri of Kom Oshim, Egypt. Many staff of the Egyptian Geological Survey and Mining Authority and of the Cairo Geological Museum are thanked for assisting with and supporting my field work. The field research leading to this discovery was financed by a National Science Foundation Grant in Physical Anthropology (SBR98–07770). This is Duke Primate Center publication no 706.
Abbreviations
- CGM
Geological Museum, Cairo
- YPM
Peabody Museum, Yale University
- DPC
Duke University Primate Center
- SNM
Stutgart Natural History Museum
References
- 1.Simons E L. Proc Natl Acad Sci USA. 1992;89:10743–10747. doi: 10.1073/pnas.89.22.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons E L. Proc Natl Acad Sci USA. 1989;86:9956–9960. doi: 10.1073/pnas.86.24.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappleman J, Simons E L, Swisher C C., III J Geol. 1992;100:647–668. [Google Scholar]
- 4.Bown T M, Kraus M J. U.S. Geological Survey Professional Paper 1452. Washington, DC: United States Government Printing Office; 1988. [Google Scholar]
- 5.Simons E L. Am Mus Nov. 1995;31241:1–10. [Google Scholar]
- 6.Fleagle J G, Kay R F. J Hum Evol. 1987;16:483–532. [Google Scholar]
- 7.Schlosser M. Beitr Palaeontol Geol Oesterr-Ung. 1911;24:51–167. [Google Scholar]
- 8.Gingerich P D. Folia Primatol. 1973;19:329–337. doi: 10.1159/000155549. [DOI] [PubMed] [Google Scholar]
- 9.Simons E L. Coll Int Cent Nat Rech Sci Paris. 1967;63:597–602. [Google Scholar]
- 10.Simons E L. Postilla. 1974;166:1–12. [Google Scholar]
- 11.Simons E L. Yrbk Phys Anthropol. 1995;38:199–238. [Google Scholar]
- 12.Kay R F, Simons E L. Am J Phys Anthropol. 1983;63:353–375. doi: 10.1002/ajpa.1330620403. [DOI] [PubMed] [Google Scholar]
- 13.Simons E L, Rasmussen D T. Int J Primatol. 1991;12:163–178. [Google Scholar]
- 14.Kay R F, Williams B A. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 361–445. [Google Scholar]
- 15.Kay R F, Simons E L. Int J Primatol. 1980;1:21–37. [Google Scholar]
- 16.Jerison H J. J Hum Evol. 1979;8:615–635. [Google Scholar]
- 17.Simons E L, Rasmussen D T. Am J Phys Anthropol. 1996;100:261–292. doi: 10.1002/(SICI)1096-8644(199606)100:2<261::AID-AJPA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Simons E L. Am J Sci. 1993;293:383–390. [Google Scholar]
- 19.Simons E L. Proc Natl Acad Sci USA. 1997;94:14970–14975. doi: 10.1073/pnas.94.26.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons E L. Am Mus Nov. 1959;1976:1–16. [Google Scholar]
- 21.Cartmill M, MacPhee R D E, Simons E L. Am J Phys Anthropol. 1981;56:3–21. [Google Scholar]
- 22.Delson E. Coll Int Cent Nat Rech Sci Paris. 1975;281:839–850. [Google Scholar]
- 23.Harrison T. J Hum Evol. 1987;16:41–80. [Google Scholar]
- 24.Hoffstetter R. Bull Mem Soc Anthropol Paris. 1977;4:327–352. [Google Scholar]
- 25.Simons E L. In: Dental Morphology and Evolution. Dahlberg A A, editor. Chicago: Univ. Chicago Press; 1970. pp. 193–208. [Google Scholar]
- 26.Gebo D, L, Simons E L. Am J Phys Anthropol. 1987;74:83–101. doi: 10.1002/ajpa.1330740108. [DOI] [PubMed] [Google Scholar]