Abstract
Background and Purpose
X-linked bulbospinal muscular atrophy (X-BSMA) is characterized by bulbar and spinal muscular weakness and fasciculations. Although X-BSMA is a motor neuronopathy, there are several reports of myasthenic symptoms or decremental responses to repetitive nerve stimulation (RNS). We report the results of applying the RNS test to 15 patients among 41 with genetically confirmed X-BSMA; these 15 patients complained of fatigue, ease of becoming tired, or early muscular exhaustion.
Methods
The 3-Hz RNS test was performed on the trapezius, nasalis, orbicularis oculi, flexor carpi ulnaris, and abductor digiti quinti muscles. A decrement greater than 10% was considered abnormal. Additionally, a pharmacologic response to neostigmine was identified in three patients.
Results
A significant decrement was observed in 67% of patients, and was most common in the trapezius muscle (nine cases). The decrement of the trapezius muscle response ranged from 15.9% to 36.9%. The decrement was inversely correlated with the amplitude of compound muscle action potentials at rest. Neostigmine injection markedly improved the decrement in three patients, who showed noticeable decremental responses to 3-Hz RNS.
Conclusions
This study shows that myasthenic symptoms and abnormal decremental responses to low-rate RNS are common in X-BSMA.
Keywords: bulbospinal, muscular atrophy, myasthenia gravis, motor neuron disease, neuromuscular junction
Introduction
X-linked bulbospinal muscular atrophy (X-BSMA) is a slowly progressive motor neuronopathy characterized by adult-onset weakness and atrophy of the proximal limbs and bulbar muscles. Although X-BSMA differs pathogenetically from myasthenia gravis (MG), X-BSMA can be occasionally misdiagnosed as MG due to their similar clinical manifestations, including muscular weakness without sensory symptoms, ease of becoming tired, fatigue, and bulbar weakness. Although the repetitive nerve stimulation (RNS) test is used to evaluate the function of the neuromuscular junctions (NMJ), significant decremental responses to low-rate RNS (LRS) are frequently seen in motor neuron diseases (MND) such as amyotrophic lateral sclerosis (ALS).1-5 It is assumed that such decrements may be related to disease activity or the degree of muscle atrophy.2,3 Unlike ALS, myasthenic symptoms or an abnormal decrement to RNS in patients with X-BSMA has been mentioned in only a few reports.6-11
We report the results of RNS testing of 15 patients with X-BSMA who complained of myasthenic symptoms including fatigue, ease of becoming tired, or early muscular exhaustion.
Methods
Clinical and electrophysiologic data of 41 patients (37 families) with genetically confirmed X-BSMA were analyzed retrospectively. Fifteen of the 41 patients complained of muscular fatigability or diurnal fluctuation, and 4 of these 15 patients experienced transient ptosis or diplopia. The RNS test was subsequently performed because their symptoms were similar to those of MG and hence made it difficult to differentiate X-BSMA from MG. The serum titer of acetylcholine-receptor-binding antibody was measured in 3 of the 15 patients.
The peak-to-peak amplitudes of compound muscle action potentials (CMAPs) were recorded from five muscles during 3-Hz stimulation of the following three nerves according to a belly-tendon method: orbicularis oculi and nasalis muscles for the facial nerve, abductor digiti quinti and flexor carpi ulnaris muscles for the ulnar nerve, and the trapezius (TR) muscle for the spinal accessory nerve (Oh's technique).12 The first and fifth CMAP amplitudes were compared, and their difference expressed as a percentage. A decrement greater than 10% was considered abnormal, in accordance with the suggestion by the AAEM Quality Assurance Committee.13 The skin temperature at the recording site was maintained at or above 32℃. In a few patients, clinical and electrophysiologic responses were observed 30 minutes after the intramuscular injection of 1.5 mg of neostigmine.
The Spearman correlation coefficient was calculated using SPSS 12.0 for comparing CMAP amplitudes recorded from the TR muscle at rest and responses of the spinal accessory nerve to 3-Hz LRS. A correlation coefficient was considered as statistically significant if the p value was less than 0.05.
Results
The demographic characteristics are listed in Table 1. The 15 patients were aged 55.9±11.6 years (mean±SD), and all of them complained of fatigability and/or diurnal fluctuation. All of the patients had leg weakness, 14 had arm weakness, and 8 had facial weakness. In addition, two patients (Cases 2 and 6) presented with diplopia, and another two (Cases 1 and 14) had clinical histories of transient ptosis. Four patients (Cases 2, 6, 7 and 8) had been diagnosed with MG and received treatment in other hospitals.
Table 1.
Clinical manifestations and results of the repetitive nerve stimulation (RNS) test in 15 patients with X-linked bulbospinal muscular atrophy
*Case with history of treatment for myasthenia gravis, †Case showing improvement of decrement after intramuscular injection of an acetylcholinesterase inhibitor, ‡Character indicates abnormal values.
AChR-Ab: anti-acetylcholine-receptor-binding antibody, ADQ: abductor digiti quinti, FCU: flexor carpi ulnaris, NA: nasalis, OO: orbicularis oculi, TR: trapezius.
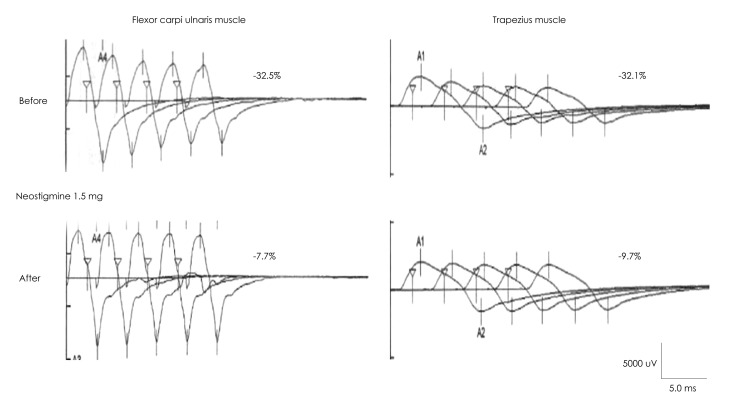
Abnormal decremental responses to the 3-Hz RNS test were noted in 10 of the 15 patients (67%), and were most common for the TR (9 cases) followed by the nasalis or orbicularis oculi (4 cases), flexor carpi ulnaris (3 cases), and abductor digiti quinti (2 cases) muscles. Response decrements of up to 36.9% were recorded in the TR (Table 1). The magnitude of the response decrement in the TR muscle was inversely correlated with the baseline CMAP amplitude (r=-0.55, p<0.05) (Fig. 1). In three patients (Cases 6, 8 and 10) who showed a noticeable decrement to LRS, neostigmine injection elicited a significant improvement in the decremental response (Fig. 2).
Fig. 1.
Spearman-correlation-coefficient-based comparison between amplitudes of compound muscle action potentials (CMAPs) at rest and decremental responses to 3-Hz repetitive stimulation recorded for the trapezius muscle.
Fig. 2.
Changes in responses to 3-Hz repetitive nerve stimulation of case 8 before and after injecting 1.5 mg of neostigmine.
Discussion
The principal features of X-BSMA are proximal and bulbar muscular weakness. However, fatigability or early muscular exhaustion, which is the main characteristic of NMJ disorders such as MG, has been reported infrequently.6-11 In our data, 15 (37%) of a total of 41 X-BSMA patients exhibited these myasthenic symptoms, making the differential diagnosis difficult. In fact, 4 of the 15 patients had been misdiagnosed as MG and had previously received treatment for the wrong condition.
A significant decremental response to LRS, which implies an impairment of neuromuscular transmission, was found in 10 (67%) of the 15 patients who underwent the RNS test. In 9 of these 10 cases, the abnormal decremental response was observed in the TR muscle. Inoue et al. recently also reported a decremental response in the TR muscle in 90% of their patients with X-BSMA.11 On the other hand, previous studies involving ALS patients have mainly found decremental responses in the distal hand muscles.2-4 Some of the authors insisted that the decrement was more pronounced in patients with rapidly developing disease, whereas others found that the magnitude of the decrement was correlated with the degree of muscle atrophy.2,3 Decrements in the TR muscle of up to 36.9% were recorded in the present study, while rates within the range of 10-20% have been found in most studies of ALS patients.1-4 We found a negative correlation between the CMAP amplitudes at rest and the magnitude of the decremental response to LRS in the TR muscle. However, no correlation was found between the disease duration and the magnitude of the decrement. Therefore, a decremental response may be more strongly correlated with the number of activated muscle fibers than with the rate of disease progression in X-BSMA.
Observation of clinical and electrophysiologic improvement after administration of an acetylcholinesterase inhibitor is important for the diagnosis of MG. However, 3 of our 15 X-BSMA patients exhibited a significant improvement in the decremental response after neostigmine injection. Myasthenic symptoms and a positive response to acetylcholinesterase inhibitor have also been described in some case reports of X-BSMA.6-8 Therefore, it is possible that RNS and pharmacological tests are not helpful for the differential diagnosis of MG and X-BSMA.
The pathomechanism of the decremental response to LRS in MND has been attributed to the instability of NMJ induced by the repetitive process of denervation and reinnervation.10 However, the decremental response in X-BSMA might be due to the negative effect of a mutant androgen receptor (AR) gene on neuromuscular transmission. The serum level of muscle enzyme is elevated in most X-BSMA patients, sug-gesting muscle involvement.14,15 Doyu et al.14 identified that mutant AR genes are widely expressed in various tissues including the testis, liver, and skeletal muscles. Soraù et al.15 described the myopathic changes in X-BSMA through muscle biopsies of patients and carriers. Moreover, Monks et al.16 demonstrated that AR expression was enhanced in myonuclei located at or in the vicinity of NMJ. These results suggest that the AR gene influences muscles or NMJ.
Our study shows that clinical and electrophysiological features similar to those of MG can be commonly observed in X-BSMA patients. Clinicians should therefore understand the limitations of the RNS test and take care not to overinterpret the results of electrophysiologic studies. The retrospective design of this study made it difficult to establish the pathomechanism underlying the decremental response on LRS in X-BSMA, and so extensive prospective studies of the RNS test and the role of the AR gene are required to elucidate the mechanism underlying the instability of NMJ.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kim JY, Park KD, Kim SM, Sunwoo IN. Repetitive nerve stimulation test in amyotrophic lateral sclerosis with predominant oropharyngeal manifestations. J Clin Neurol. 2011;7:31–33. doi: 10.3988/jcn.2011.7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denys EH, Norris FH., Jr Amyotrophic lateral sclerosis. Impairment of neuromuscular transmission. Arch Neurol. 1979;36:202–205. doi: 10.1001/archneur.1979.00500400056008. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein LP, Antel JP. Motor neuron disease: decremental responses to repetitive nerve stimulation. Neurology. 1981;31:204–207. doi: 10.1212/wnl.31.2.202. [DOI] [PubMed] [Google Scholar]
- 4.Wang FC, De Pasqua V, Gérard P, Delwaide PJ. Prognostic value of decremental responses to repetitive nerve stimulation in ALS patients. Neurology. 2001;57:897–899. doi: 10.1212/wnl.57.5.897. [DOI] [PubMed] [Google Scholar]
- 5.Killian JM, Wilfong AA, Burnett L, Appel SH, Boland D. Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve. 1994;17:747–754. doi: 10.1002/mus.880170708. [DOI] [PubMed] [Google Scholar]
- 6.Yamada M, Inaba A, Shiojiri T. X-linked spinal and bulbar muscular atrophy with myasthenic symptoms. J Neurol Sci. 1997;146:183–185. doi: 10.1016/s0022-510x(96)00303-6. [DOI] [PubMed] [Google Scholar]
- 7.Ertekin C, Sirin H. X-linked bulbospinal muscular atrophy (Kennedy's syndrome): a report of three cases. Acta Neurol Scand. 1993;87:56–61. doi: 10.1111/j.1600-0404.1993.tb04076.x. [DOI] [PubMed] [Google Scholar]
- 8.Boz C, Kalay E, Sahin N, Velioglu S, Ozmenoglu M, Karagüzel A. Ocular myasthenia gravis associated with x-linked recessive spinal and bulbar muscular atrophy. J Clin Neuromuscul Dis. 2004;5:115–118. doi: 10.1097/00131402-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lee BJ, Kwon KH, Kim SM, Na HR, Lee BC, Ryu SH, et al. A case of Kennedy syndrome mimicking myasthenia gravis. J Korean Neurol Assoc. 2001;19:544–546. [Google Scholar]
- 10.Meriggioli MN, Rowin J. Fatigue and abnormal neuromuscular transmission in Kennedy's disease. Muscle Nerve. 2003;27:249–251. doi: 10.1002/mus.10295. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Hemmi S, Miyaishi M, Kutoku Y, Murakami T, Kurokawa K, et al. Muscular fatigue and decremental response to repetitive nerve stimulation in X-linked spinobulbar muscular atrophy. Eur J Neurol. 2009;16:76–80. doi: 10.1111/j.1468-1331.2008.02349.x. [DOI] [PubMed] [Google Scholar]
- 12.Oh SJ. Repetitive nerve stimulation test. In: Oh SJ, editor. Principles of Clinical Electromyography: Case Studies. Baltimore: Williams & Wilkins; 1998. pp. 59–75. [Google Scholar]
- 13.AAEM Quality Assurance Committee. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2001;24:1239–1247. doi: 10.1002/mus.1140. [DOI] [PubMed] [Google Scholar]
- 14.Doyu M, Sobue G, Kimata K, Yamamoto K, Mitsuma T. Androgen receptor mRNA with increased size of tandem CAG repeat is widely expressed in the neural and nonneural tissues of X-linked recessive bulbospinal neuronopathy. J Neurol Sci. 1994;127:43–47. doi: 10.1016/0022-510x(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.Sorarù G, D'Ascenzo C, Polo A, Palmieri A, Baggio L, Vergani L, et al. Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J Neurol Sci. 2008;264:100–105. doi: 10.1016/j.jns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Monks DA, O'Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]