Abstract
Background and Purpose
Sleep-disordered breathing (SDB) is suggested to be strongly associated with ischemic strokes. Risk factors, stroke subtypes, stroke lesion distribution, and the outcome of SDB in stroke patients remain unclear in Korea.
Methods
We prospectively studied 293 patients (159 men, 134 women; age 68.4±10.5) with acute ischemic stroke. Cardiovascular risk factors, stroke severity, sleep-related stroke onset, distribution of stroke lesions, and 3-month score on the modified Rankin Scale (mRS) were assessed. Stroke severity was assessed by the US National Institutes of Health Stroke Scale (NIHSS) and the mRS. The apnea-hypopnea index (AHI) was determined 6.3±2.2 days after stroke onset with the Apnea Link portable sleep apnea monitoring device.
Results
The prevalence of SDB (defined as an AHI of ≥10) was 63.1% (111 men, 74 women). Those in the SDB group were older, had higher NIHSS and mRS scores, greater bulbar weakness, and a higher incidence of sleep-associated stroke onset. Among risk-factor profiles, alcohol consumption and atrial fibrillation were significantly related to SDB. The stroke outcome was worse in patients with SDB than in those without SDB. The lesion location and specific stroke syndrome were not correlated with SDB.
Conclusions
SDB is very common in acute cerebral infarction. Different risk-factor profiles and sleep-related stroke onsets suggest SDB as a cause of ischemic stroke. The higher NIHSS score and greater bulbar involvement in the SDB group seem to show the influence of ischemic stroke on the increased SDB prevalence.
Keywords: sleep-disordered breathing, acute cerebral infarction, prevalence
Introduction
Sleep-disordered breathing (SDB) is a well-known disorder characterized by recurrent episodes of nocturnal hypoxemia and resultant sympathetic activation and cardiovascular distress. Untreated obstructive sleep apnea syndrome (OSAS), the most prominent manifestation of SDB, is associated with many disease states, including arterial hypertension, diabetes, obesity, coronary heart disease, cardiac arrhythmia, and even sudden death.1 These diseases are also risk factors ischemic stroke. There have been many observatory reports of a high prevalence of SDB in acute stroke with 6080% of the patients with stroke and transient ischemic attack (TIA) reportedly showing sleep apnea.221
The mechanisms linking SDB with stroke are complex, and include hemodynamic, neural, metabolic, endothelial, coagulatory, and inflammatory changes secondary to respiratory events and recurrent hypoxemia.4 However, the very high prevalence of SDB suggests that stroke also aggravates or even causes SDB "de novo".
This study evaluated the influence of SDB on acute ischemic stroke and also the effect of stroke-related neurologic deficits on SDB. The influence of SDB may be reflected as a difference of risk-factor profiles in stroke patients with SDB and as sleep-related stroke onset (SRSO) and its effect on stroke recovery. The effect of stroke on SDB can be addressed by considering three questions: 1) is any specific brain region involved in stro-ke patients with SDB, 2) does pharyngeal muscle weakness contribute to the aggravation or provocation of SDB, 3) is stroke severity or infarct size proportional to the degree of SDB?
Methods
Subjects
Consecutive patients (aged 40 to 90 years) admitted to the Department of Neurology, Chosun University Hospital for acute cerebral infarction within 72 hours of stroke onset were enrolled in the present study. Patients whose neurologic deficits disappeared within 24 hours and those without an MRI-confirmed lesion were not included. Patients with any of the following were also excluded: 1) a decreased level of consciousness on admission, 2) a baseline oxygen saturation value of <90%, 3) any acute or chronic cardiopulmonary diseases that disturb pulmonary function, 4) a neuromuscular-junction disorder (e.g., myasthenia gravis), or 5) a neurodegenerative disorder, such as Parkinson's disease, spinocerebellar degeneration, Alzheimer's disease, or motor neuron disease.
Clinical evaluation of stroke
All subjects were questioned regarding their history of vascular risk factors, such as hypertension, diabetes, alcohol consumption, and cigarette smoking. Their admission blood pressures, complete blood counts, fasting glucose level, HbA1c level, and lipid profile were determined. The body mass index was calculated as weight (kilograms)/[height (meters)]2 for all subjects. All subjects were subject to EKG, blood pressure, and peripheral oxygen saturation monitoring within the stroke unit for more than 3 days. SRSO was diagnosed in patients who first noticed neurological symptoms upon awakening. Stroke subtypes were classified according to TOAST criteria.22 The location of stroke lesions was classified as left, right, or bilateral, and hemispheric, cortical, subcortical, brainstem, or cerebellar. On admission, neurological impairment was evaluated using the US National Institutes of Health Stroke Scale (NIHSS), and functional disability was assessed using the modified Rankin Scale (mRS). Dysphagia was considered in the occurrence of any of the following conditions after stroke: mani-fest incapacity to swallow solids or liquids oral dribbling after administering 10 mL of water or repetitive coughing or an absence of laryngeal movement on swallowing. Short-term stroke recovery was assessed by the 3-month mRS score.23
Evaluation of sleep-disordered breathing
SDB was measured around the 7th day after stroke onset using the Apnea Link device (Resmed, Australia). This time delay was used since the patients were then more stable and less disturbed by medical management than during the acute period. Apnea Link is a three-channel, portable device that use a nasal pressure transducer to measure the apnea-hypopnea index (AHI), flow limitations, and snoring, in addition to monitoring oxygen saturation during sleep and the pulse rate. Patients with a recorded sleep time of less than 120 minutes were excluded from the study. Apnea and hypopnea were defined as decreases in airflow by 80% and 50% relative to baseline for at least 10 seconds, respectively. The AHI values used for the analysis were automatically calculated by Apnea Link software. SDB was defined as an AHI of ≥10.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA). Categorical and continuous data were analyzed using the χ2 test and the independent t test, respectively. Multiple logistic regression analysis was used to identify variables that were independently correlated with SDB. Results are presented as mean±SD values or adjusted odds ratios and corresponding 95% confidence intervals. Probability values of p<0.05 were taken to indicate statistical significance.
Results
Two hundred and ninety-three patients were finally enrolled. They comprised 159 men and 134 women aged 68.4±10.5 years (mean±SD, range 4090 years). Subtypes of stroke were large-artery atherosclerosis in 97 patients, lacune in 46, cardio-embolism in 82, stroke of other determined etiology in 2, and stroke of undetermined etiology in 66. The time between sleep recording and sleep onset was 6.3±2.2 days. The overall prevalence of SDB was 63.1% (185 of 293; 111 men, 74 women). The prevalence of moderate to severe SDB (AHI ≥20) was 34.5%.
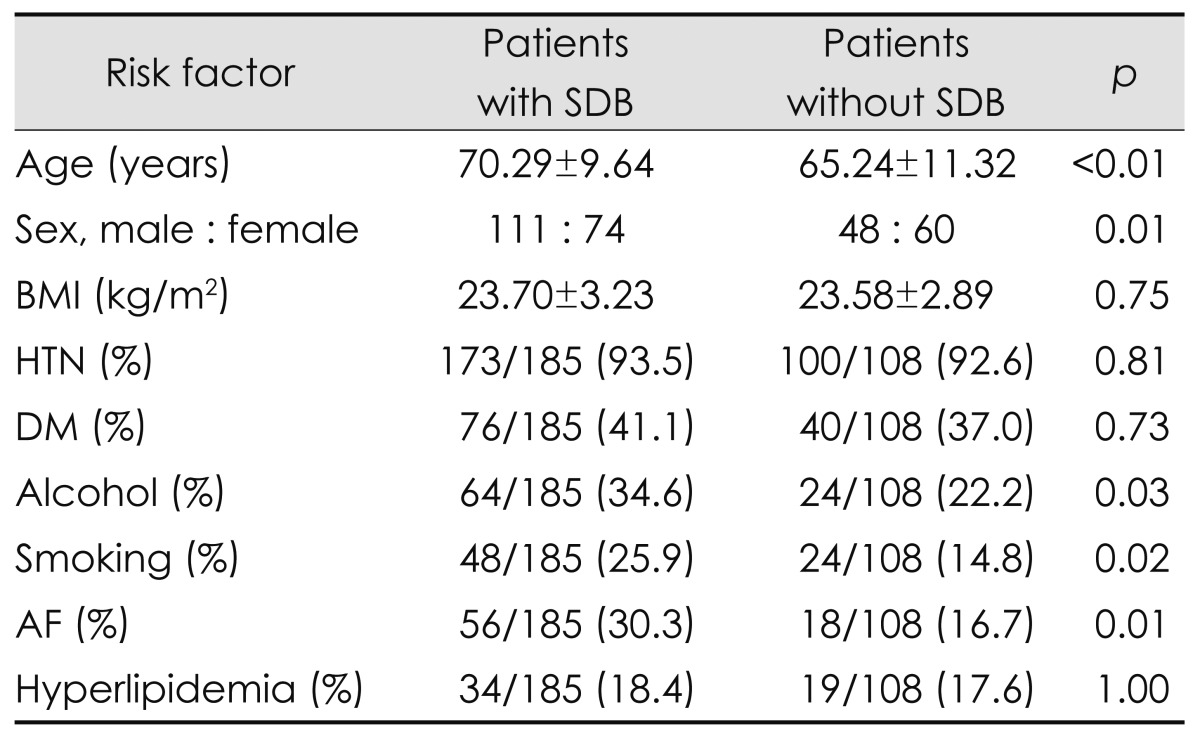
The following demographic and risk factor findings differed significantly between patients with and without SDB: age, sex, alcohol consumption, habitual smoking, and atrial fibrillation (AF). Table 1 indicates that the patients with SDB were older and tended to be male, but there were no differences in body mass index, hypertension, diabetes, or hyperlipidemia.
Table 1.
Risk factor profiles in patients with and without sleep-disordered breathing (SDB)
AF: atrial fibrillation, BMI: body mass index, DM: diabetes mellitus, HTN: hypertension.
The average blood oxygen saturation value did not differ significantly between those without SDB (95.07±2.01 mg/L) and those with SDB (94.90±2.12 mg/L): the corresponding minimum blood oxygen saturation values were 88.56±3.94 and 85.55±4.74 mg/L, respectively (p<0.001). There was no significant correlation between minimum blood oxygen saturation value and NIHSS score (R=0.070).
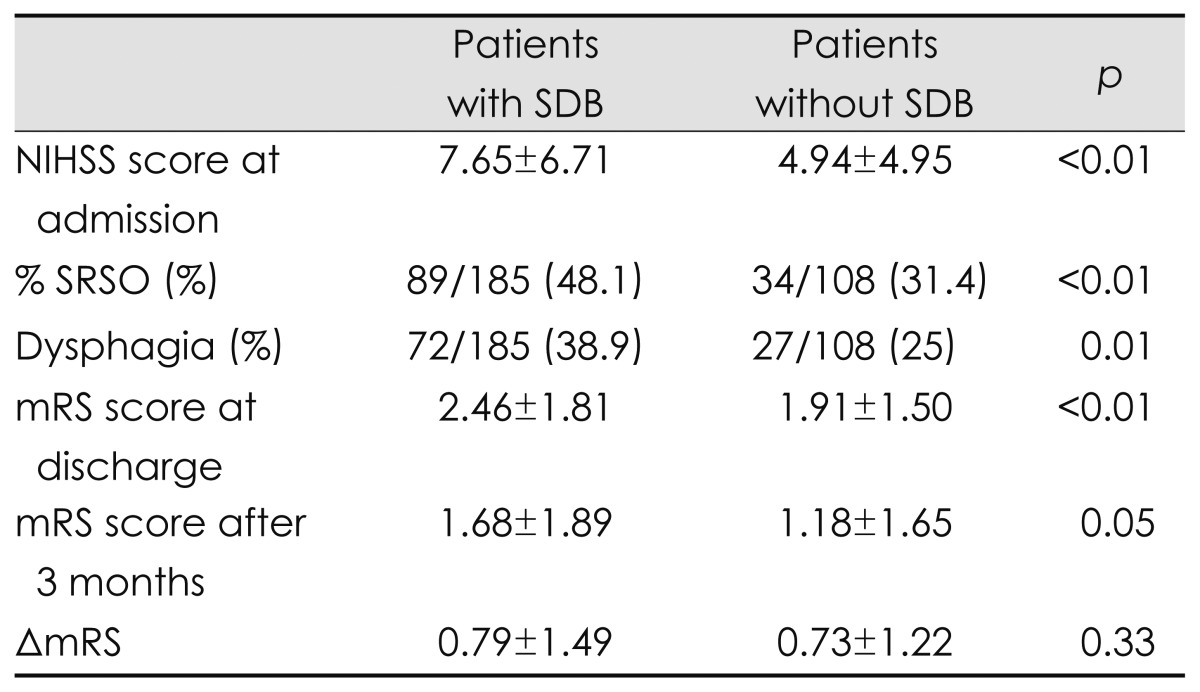
The clinical factors that significantly affected acute cerebral infarction with SDB were SRSO, stroke severity, functional disability, involvement of dysphagia, and short-term outcome (i.e., mRS after 3 months) (Table 2). Severe neurologic and functional deficits, especially involving dysphagia, seemed to predispose the patients to sleep apnea. To analyze the influence of SDB on functional recovery, we compared the difference in the mRS between at discharge and 3 months after stroke onset (ΔmRS: mRS at discharge minus mRS after 3 months). ΔmRS was not significantly associated with SDB (p=0.33) and also did not differ between patients without SDB and patients with severe SDB (AHI ≥30, p=0.37).
Table 2.
Neurologic severities and clinical outcomes in patients with and without SDB
mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, SDB: sleep-disordered breathing, SRSO: sleep-related stroke onset, ΔmRS: difference in mRS scores at discharge and after 3 months.
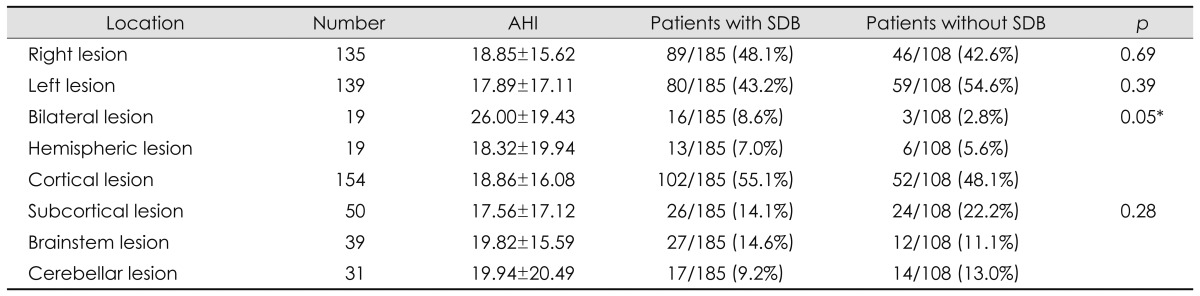
The location and size of the infarct were also examined. There were no differences between huge hemispheric and small subcortical infarctions. Bilateral involvement was the only significant positive predictor (Table 3). AHI did not differ between the groups.
Table 3.
Stroke lesion locations in patients with and without SDB
*p value between bilateral and other lesions.
AHI: apnea-hypopnea index, SDB: sleep-disordered breathing.
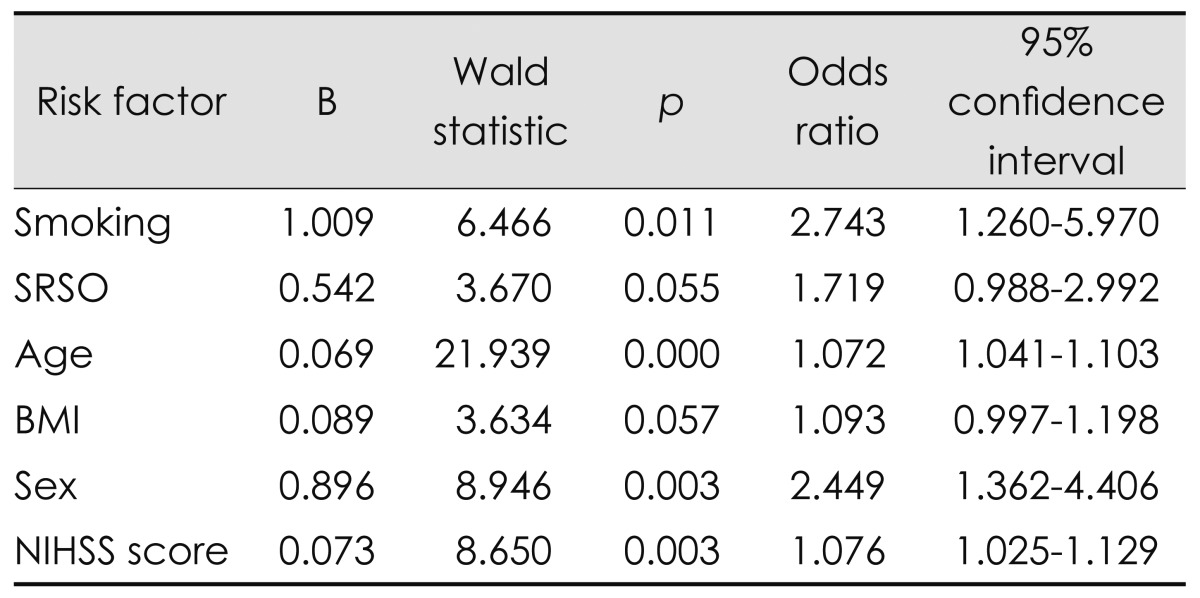
We used multiple linear regression analysis to identify the most plausible predictors of SDB. Smoking (OR=2.743, p=0.011), age (OR=1.072, p=0.000), sex (OR=2.449, p=0.003), and NIHSS score (OR=1.076, p=0.003) were found to independently predict SDB in patients (Table 4).
Table 4.
Multiple logistic regression analysis for prediction of SDB after cerebral infarction
BMI: body mass index, NIHSS: National Institutes of Health Stroke Scale, SDB: sleep-disordered breathing, SRSO: sleep-related stroke onset.
Discussion
Several prospective cohort studies have found OSAS to be an independent predictor of stroke, with the risk of stroke, TIA, and death typically being between two- and fourfold higher than in normal controls (OR=1.97-4.48).24-27 The prevalence of SDB is much higher after stroke than in the normal population. Dozens of articles report that the prevalence of SDB (AHI ≥10) combined with stroke is around 60% (range 44-77%), compared with about 20% in age-matched controls (12-32%).2-21 In our study the rate was 63.1. Factors that influence the prevalence include 1) when the measurement is made 2) whether or not patients with TIA are included 3) AHI criteria used to diagnose SDB, and 4) whether full nocturnal polysomnography or a simplified portable device is used for diagnosis. We performed our sleep study at 6.3±2.2 days after stroke onset using a simplified portable protocol that exclude TIA patients. We chose 17 articles reporting the prevalence of SDB,2-5,7-17,19-21 in which the sleep measurements were made within 24, 48, or 72 hours, within 1 or 2 weeks, and around 1 or 3 months after stroke onset. These can be grouped into hyperacute (≤3 days), acute (1-2 weeks), and subacute (1-3 months) periods, whose mean prevalence rates of SDB were 59% (range 51-71%), 57% (50-67%), and 59% (44-71%), respectively. However, Parra et al.3 reported that the prevalence of SDB was 71% in the acute stage and 62% in the subacute stage. Hui et al.11 and Bassetti et al.4 stated that the incidence of AHI was 20-40% lower in the subacute stage than in the acute stage, with no difference in the prevalence of SDB between stroke patients including and excluding TIA (58.9% vs. 59.2%). Over 80% of the reports related to the prevalence adopted an AHI of ≥10 as the diagnostic criterion for SDB.2-21 Only one-sixth of reports have used full nighttime polysomnography as a diagnostic tool, and their reported prevalence rates did not differ from those obtained using a portable device. We used the Apnea Link device, which recent studies have found to have a sensitivity of 82.1-100% and a specificity of 83.9-87.5% at AHI >10 in comparison with standard polysomnography.28,29
Our study found different risk-factor profiles in patients with SDB. Univariate analysis showed older age, male sex, alcohol consumption, cigarette smoking, and AF as meaningful factors for SDB. Gami et al.30 found that the proportion of patients with OSAS was significantly higher in their AF group than in their general cardiology group (49% vs. 32%, p=0.0004). We thought that a higher proportion of AF in patients with SDB would result from the combination of the underlying SDB and being older (mean age 70.29 vs. 65.24 years) than patients without OSAS. However, cardioembolic infarctions were not significantly increased in patients with SDB.
We wondered if any particular lesion in the brain would evoke or aggravate SDB. This has not been reported previously, but negative result could be due to the relatively small number of subjects included. Nearly 300 patients with sufficient lesion types were enrolled, but no locations specific to SDB were found. The size of the stroke lesion also appeared to be unrelated. The AHI did not differ between large hemispheric lesions (18.32±19.94) and small subcortical lesions (17.56±17.12) or brainstem lesions (19.82±15.59). One-way ANOVA analysis of these AHIs revealed no significant relationships (p=0.965). Clinical manifestations of ischemic stroke were found to be very important in our study. SRSO, severe neurologic deficits, functional disability at the acute and subacute stages, and involvement of dysphasia were all statistically significant. These findings suggest that poor neurologic function is responsible for the increased prevalence of SDB. Improvement in AHI at the subacute stage may be related to neurologic and functional recoveries insensitivity.
Our study did not find any negative influence of SDB on the functional recovery. This negative result may be due to of the mRS; more sensitive scales such as the Functional Independence Measure score introduced by Kaneko et al.31 would be more useful. There are several reports of the beneficial prognostic effects of nasal continuous-positive-airflow-pressure therapy.
Multivariate analysis revealed age, sex, and stroke severity (NIHSS score) as positive predictors of SDB. Excluding age and sex, which are universal contributors to SDB, leaves the NIHSS score as the only factor. This suggests that stroke has a role in the genesis of SDB.
Our study was subject to several limitations. The use of a portable monitoring device with a nasal pressure transducer tends to overestimate AHI. Since portable device without EEG recordings cannot differentiate between waking and sleep stages, it may underestimate respiratory events because the waking stage can be included in the total sleep time. Overall the results from a portable monitoring device are similar to but less accurate than those of routine polysomnography. The second main limitation of our study is that age and sex were not controlled. The prominence of both of these factors could mean that they obscure the effects of other factors.
Acknowledgements
This study was supported (in part) by research funds from Chosun Unversity, 2010.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3:333–342. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti C, Aldrich MS, Chervin RD, Quint D. Sleep apnea in patients with transient ischemic attack and stroke: a prospective study of 59 patients. Neurology. 1996;47:1167–1173. doi: 10.1212/wnl.47.5.1167. [DOI] [PubMed] [Google Scholar]
- 3.Parra O, Arboix A, Bechich S, García-Eroles L, Montserrat JM, López JA, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 5.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 7.Dziewas R, Humpert M, Hopmann B, Kloska SP, Lüdemann P, Ritter M, et al. Increased prevalence of sleep apnea in patients with recurring ischemic stroke compared with first stroke victims. J Neurol. 2005;252:1394–1398. doi: 10.1007/s00415-005-0888-7. [DOI] [PubMed] [Google Scholar]
- 8.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 9.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–47. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 10.Turkington PM, Bamford J, Wanklyn P, Elliott MW. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke. 2002;33:2037–2042. doi: 10.1161/01.str.0000023576.94311.27. [DOI] [PubMed] [Google Scholar]
- 11.Hui DS, Choy DK, Wong LK, Ko FW, Li TS, Woo J, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in Chinese patients with first-ever ischemic stroke. Chest. 2002;122:852–860. doi: 10.1378/chest.122.3.852. [DOI] [PubMed] [Google Scholar]
- 12.Rola R, Wierzbicka A, Wichniak A, Jernajczyk W, Richter P, Ryglewicz D. Sleep related breathing disorders in patients with ischemic stroke and transient ischemic attacks: respiratory and clinical correlations. J Physiol Pharmacol. 2007;58(Suppl 5):575–582. [PubMed] [Google Scholar]
- 13.Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med. 2009;10:717–719. doi: 10.1016/j.sleep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Broadley SA, Jørgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 15.McArdle N, Riha RL, Vennelle M, Coleman EL, Dennis MS, Warlow CP, et al. Sleep-disordered breathing as a risk factor for cerebrovascular disease: a case-control study in patients with transient ischemic attacks. Stroke. 2003;34:2916–2921. doi: 10.1161/01.STR.0000103748.31609.4E. [DOI] [PubMed] [Google Scholar]
- 16.Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58:911–916. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 17.Siccoli MM, Valko PO, Hermann DM, Bassetti CL. Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. J Neurol. 2008;255:1687–1692. doi: 10.1007/s00415-008-0981-9. [DOI] [PubMed] [Google Scholar]
- 18.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171:1048–1052. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- 19.Harbison J, Ford GA, James OF, Gibson GJ. Sleep-disordered breathing following acute stroke. QJM. 2002;95:741–747. doi: 10.1093/qjmed/95.11.741. [DOI] [PubMed] [Google Scholar]
- 20.Szücs A, Vitrai J, Janszky J, Migléczi G, Bódizs R, Halász P, et al. Pathological sleep apnoea frequency remains permanent in ischaemic stroke and it is transient in haemorrhagic stroke. Eur Neurol. 2002;47:15–19. doi: 10.1159/000047941. [DOI] [PubMed] [Google Scholar]
- 21.Joo BE, Seok HY, Yu SW, Kim BJ, Park KW, Lee DH, et al. Prevalence of sleep-disordered breathing in acute ischemic stroke as determined using a portable sleep apnea monitoring device in Korean subjects. Sleep Breath. 2011;15:77–82. doi: 10.1007/s11325-009-0325-8. [DOI] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-García MA, Galiano-Blancart R, Soler-Cataluña JJ, Cabero-Salt L, Román-Sánchez P. Improvement in nocturnal disordered brea-thing after first-ever ischemic stroke: role of dysphagia. Chest. 2006;129:238–245. doi: 10.1378/chest.129.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 25.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz R, Duran-Cantolla J, Martínez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 27.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 28.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–392. [PMC free article] [PubMed] [Google Scholar]
- 29.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39:757–762. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 30.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]