Abstract
Hilar cholangiocarcinoma has an extremely poor prognosis and is usually diagnosed at an advanced stage. Palliative management plays an important role in the treatment of patients with inoperable hilar cholangiocarcinoma. Surgical, percutaneous, and endoscopic biliary drainage are three modalities available to resolve obstructive jaundice. Plastic stents were widely used in the past; however, self-expanding metal stents (SEMS) have become popular recently due to their long patency and reduced risk of side branch obstruction, and SEMS are now the accepted treatment of choice for hilar cholangiocarcinoma. Bilateral drainage provides more normal and physiological biliary flow through the biliary ductal system than that of unilateral drainage. Unilateral drainage was preferred until recently because of its technical simplicity. But, with advancements in technology, bilateral drainage now achieves a high success rate and is the preferred treatment modality in many centers. However, the choice of unilateral or bilateral drainage is still controversial, and more studies are needed. This review focuses on the endoscopic method and discusses stent materials and types of procedures for patients with a hilar cholangiocarcinoma.
Keywords: Hilar cholangiocarcinoma, Inoperable, Endoscopic, Drainage
INTRODUCTION
Cholangiocarcinoma is a cancer that originates from the bile duct epithelium and is classified as intrahepatic, hilar, or extrahepatic depending on its location. Hilar cholangiocarcinoma was first described by Altemeier et al. [1] in 1957. However, it was only after a study of a series of 13 patients in 1965 that this tumor was recognized as a distinct clinical entity and was named for Dr. Gerald Klatskin [2].
Resection with negative margins is the only available curative treatment for hilar cholangiocarcinoma. However, most patients with hilar cholangiocarcinoma display advanced disease, metastasis, and co-morbidity at the time of presentation and have an extremely poor prognosis, with less than 10% of patients surviving 5 years after diagnosis. Palliative management should be considered in these patients. The aims of palliation in patients with unresectable hilar cholangiocarcinoma according to the British Association for the Study of Liver are to improve the quality of life by relieving obstructive jaundice, pruritus, cholangitis, or pain, and secondarily, to prolong survival. Currently available modalities for palliation of unresectable hilar cholangiocarcinoma are surgical, percutaneous, and endoscopic drainage. An ideal palliative procedure should be simple and effective to relieve obstructive cholestasis, have low procedure-related morbidity and mortality, and offer durable palliation. Until now, there has been no clear consensus concerning which is the best procedure for biliary drainage of hilar cholangiocarcinoma. Endoscopic biliary drainage (EBD) has assumed an increasingly important role in the palliation of patients with unresectable hilar cholangiocarcinoma because of its efficacy, patient acceptance, and relatively low morbidity and mortality.
The two types of EBD are endoscopic nasobiliary drainage (ENBD) and endoscopic retrograde biliary drainage (ERBD). ENBD is an endoscopic procedure that uses a 250-cm long, preformed polyethylene tube placed above the stenosis in an obstructed biliary tract to provide flow out of the bile [3]. The presence of an external tube through the nose is uncomfortable and, thus, is temporarily used in patients awaiting surgery or evaluation. ERBD is the preferred choice for palliation of patients with hilar cholangiocarcinoma because it offers the advantages of physiological bile drainage and increased patient comfort.
Although EBD has been widely used with either plastic or metal stents, the choice of optimal treatment modality in unresectable hilar cholangiocarcinoma is complicated by the requirement of a higher degree of expertise and the considerably higher morbidity and mortality rates. The use of plastic versus metal stents and a single versus multiple stents for endoscopic palliation is controversial.
This review will focus on the endoscopic method and discuss stent materials and types of procedures.
PLASTIC VERSUS METAL STENTS
Plastic stents
The plastic stent has been used for EBD for more than three decades. Several studies have suggested that stents of 10- and 12-Fr diameters are superior in terms of patency compared to those with a 7-Fr diameter [4,5]. The average duration of patency is 3 to 4 months [6]. Therefore, a plastic stent diameter of at least 10 Fr is recommended for effective drainage for a particular period of time [5].
Plastic stents have advantages that include low cost, simple insertion, and easy removal. Plastic stents have been used to achieve biliary drainage in patients with a malignant hilar obstruction. However, the use of plastic stents for hilar cholangiocarcinoma has revealed poor results in some series [7-9]. Liu et al. [10] studied the usefulness of endoscopic insertion of plastic stents in 55 patients with hilar cholangiocarcinoma, and endoscopic stenting was attempted in 49 patients. Technical success rate, clinical success rate, and early complication rate were 73%, 41%, and 25%, respectively. The median patency of the first stent was just 1 week (range, 0 to 8). Stent patency was also relatively short (49 days) in another study [11].
The main disadvantage of plastic stents is their ease of clogging. Stent occlusion frequently results in cholangitis with rates as high as 20% to 40% for unilateral or bilateral plastic stents. Clogging is caused by a relatively narrow lumen (10 to 12 Fr) and is proportional to stent length [7]. The mechanism of stent clogging is multi-factorial and is associated mainly with bacterial contamination of undrained bile ducts [8,9]. Another disadvantage of plastic stents is the technical limitation of inserting a large diameter plastic stent. It is technically difficult to place more than one 10-Fr stent at the initial setting. Moreover, plastic stents in the hilum are prone to distal migration.
Due to the plastic stent clogging problem, stent exchange is required in one third of patients [12]. It is necessary to exchange plastic stents regularly every 3 to 4 months or when obstructive symptoms appear [13]. Many trials to prevent stent clogging, including increasing stent diameter, prophylactic administration of antibiotics, administration of drugs such as ursodeoxycholic acid that alter bile composition, and using newly modified stents have been tried, but most results have been disappointing [14-18]. Only increased stent diameter has prolonged stent patency [19,20]. Although no randomized controlled studies have compared plastic and metal stents, plastic stent use in patients with hilar cholangiocarcinoma has decreased because of its relatively short patency, difficulty of multiple stenting, and obstructive risk of side branches.
Metal stents
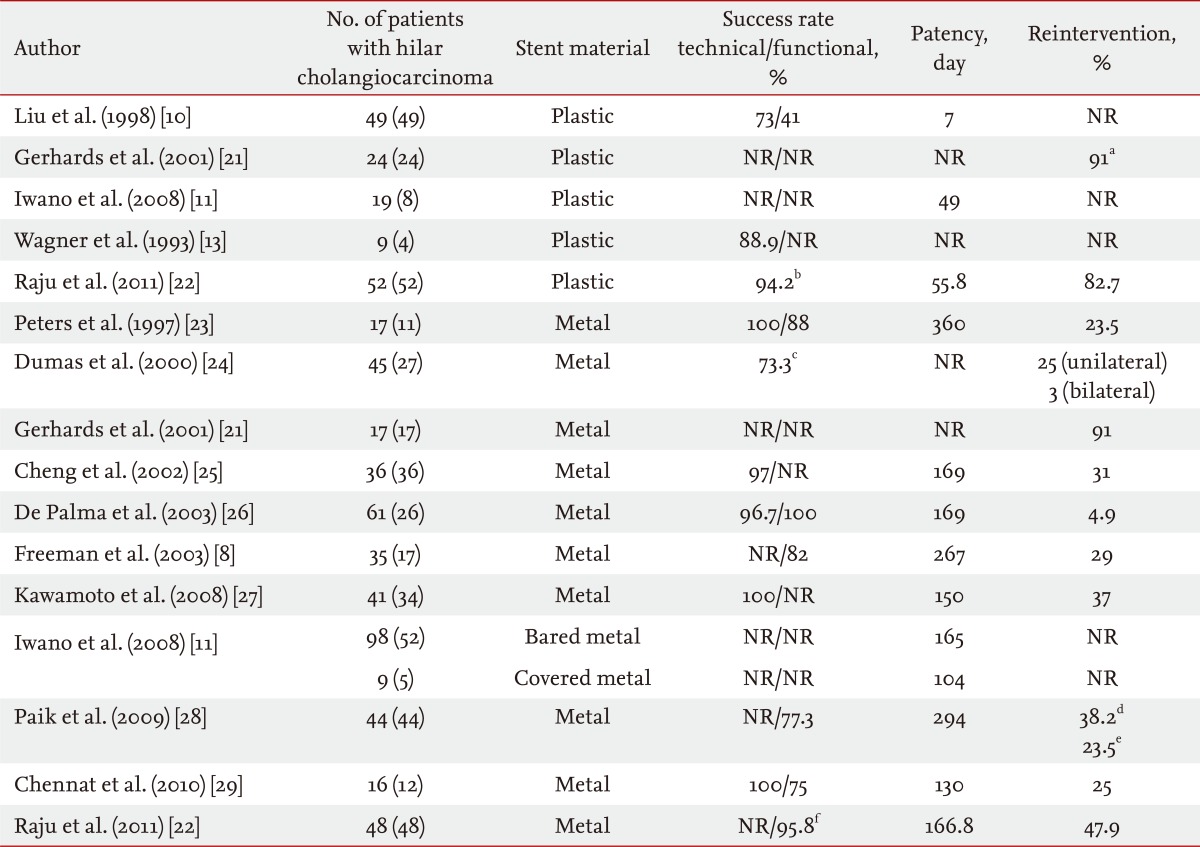
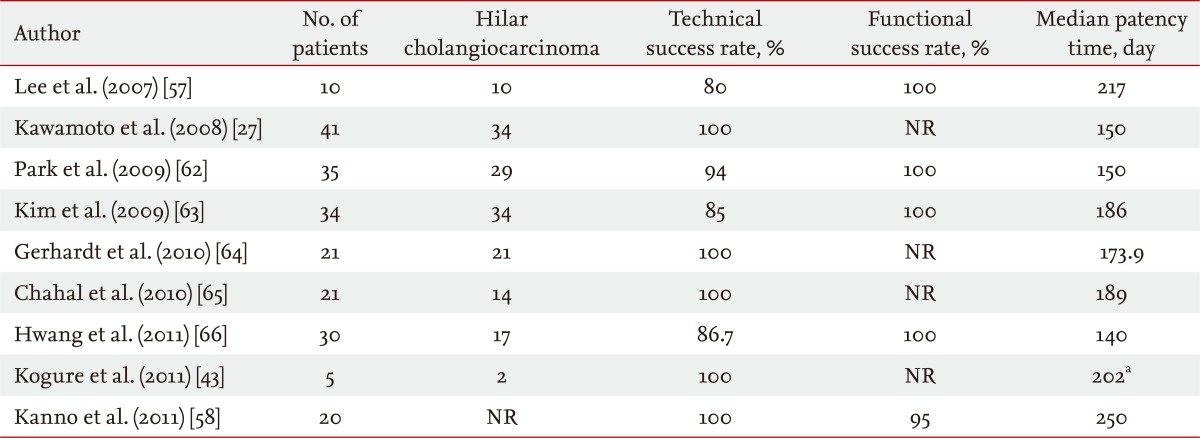
Self-expanding metal stents (SEMS) were developed to overcome the limitations of the plastic stent. The main advantage of SEMS is their large internal diameter (generally 10 mm; i.e., 30 Fr), and they provide patency duration of about 10 months, reducing the necessity for reintervention (Table 1) [8,10,11,13,21-29]. SEMS also allows drainage of secondary branches through the open side mesh of the stent. In a study comparing plastic and metal stents in 20 patients with hilar cholangiocarcinoma (Bismuth II to IV), SEMS showed higher patency rates, a lower rate of cholangitis (33.3% vs. 9.1%), and required fewer reinterventions (2.4 ± 2.6 vs. 0.4 ± 0.5). Median patency was not described accurately, but stent malfunction was observed in 50% of patients in the plastic group and 18.2% in the metal group after long-term follow-up (> 30 days) [13].
Table 1.
Studies of plastic and metal stents
NR, not reported.
aThese data include both plastic and metal stents.
bTechnical and functional success rates combined.
cSuccess rate defined by stent placement and contrast removal.
dMedian follow-up time, reintervention needed in patients.
eCumulative reintervention needed in patients.
fData were collected retrospectively; therefore, technical success rate could not be calculated.
Raju et al. [22] compared plastic and metal stents in 100 patients with hilar cholangiocarcinoma. Metal and plastic stents were placed in 52 and 48 patients, respectively. Successful drainage was achieved in 46 (95.8%) in the SEMS group and 49 (94.2%) in the plastic group. Median patency was 5.56 months in the SEMS group and 1.86 months in the plastic group. Median survival was 9.08 and 8.22 months in the SEMS and plastic groups, respectively. More reintervention was needed in the plastic group [22].
Moss et al. [5] reported that metal stents are associated with a significantly lower relative risk (0.44) of stent occlusion than that of plastic stents. The median increase in the cost effectiveness ratio of metal stents is US $1,820 per endoscopic retrograde cholangiopancreatography (ERCP) prevented [6]. Another study reported that even in countries where the cost of the ERCP procedure are lower than those of SEMS, the average total cost is lower for the SEMS group compared with that in the plastic group [30].
Although their initial cost is higher, SEMS have been increasingly used based on the above results. Compared with plastic stents, a larger diameter SEMS results in improved stent patency and fewer repeat procedures [9,31,32]. In addition, the open-mesh design allows drainage of secondary branches through the side of the stent and less likelihood for obstructive segmental cholangitis. Insertion of an uncovered SEMS is preferred when unilateral drainage is planned due to prolonged patency and prevention of branch duct obstruction. Although few data are available on hilar cholangiocarcinoma, current evidence favors placement of SEMS as they provide superior palliation in comparison to plastic stents in terms of early and late complications [23,33].
The main problem with uncovered SEMS is tumor in growth into the stent lumen, which results in stent obstruction. Polyurethane or silicone covered SEMS have been developed to prevent tumor in-growth. The results of some studies support this hypothesis, but contradictory results have also been reported [34-37]. Another study performed with 65 patients with hilar cholangiocarcinoma compared 19 plastic stents, 98 bare metal stents, and nine covered metal stents. The patencies of the plastic stent, bare metal stent, and covered metal stent were 49, 165, and 104 days, respectively. The median survival time and median event-free survival time were also longer in the bare metal stent group than those in the plastic stent group. Although that study was limited by the heterogeneity of the patient groups and types of stents, the patency of the covered metal stent seemed not to be superior to that of the bare metal stent. A covered stent is not usually used in patients with hilar cholangiocarcinoma due to unintentional obstruction of contralateral ducts or side branch ducts and lack of improved stent patency. Thus, uncovered SEMS is the treatment of choice in patients with hilar cholangiocarcinoma.
UNILATERAL VERSUS BILATERAL STENTS
Adequate palliation of obstructive cholestasis is achieved by draining only 25% of the liver volume [38]. Inserting a single biliary stent into one functional liver lobe for unilateral drainage can provide adequate palliation in the majority portion of patients with hilar tumors [39].
Some clinicians agree that placing a unilateral stent is sufficient to provide adequate drainage and has lower morbidity due to the ease of the procedure and fewer complications compared to those of bilateral stents [23,40-44]. De Palma et al. [44] reported that inserting a unilateral stent is associated with a significantly higher stent insertion success rate, higher rate of successful drainage, and lower incidence of early complications including cholangitis. No differences were observed with regard to late complications and survival. Although very limited data are available comparing unilateral with bilateral SEMS in unresectable hilar obstructions, those available indicate that unilateral biliary stenting is sufficient for Bismuth type I to IV cases [26,44-46]. Five major studies compared unilateral and bilateral endoscopic stenting in patients with malignant hilar obstruction, and the results were conflicting [33,44-47].
A higher incidence of cholangitis, 30-day mortality, and short survival duration have been reported in patients treated with unilateral drainage [47]. Reintervention is frequently necessary due to stent occlusion caused by debris and food occlusion, tumor in growth, tumor over growth, and stent migration [48].
Several studies have attempted to address whether unilateral or bilateral drainage is effective for palliative treatment of obstructive jaundice in patients with hilar cholangiocarcinoma [33,44,47,49]. Inserting bilateral stents is associated with an increased survival rate and reduced risk of cholangitis, compared to that of unilateral drainage for Bismuth types II and III hilar tumors [47]. The highest survival rate is noted in patients with hilar tumors who have bilateral drainage, whereas the lowest survival rate is evident in those who show cholangiographic opacification of both lobes but only unilateral drainage was available [33]. However, an unsuccessful attempt at bilateral drainage can lead to increased incidence of postprocedural cholangitis and lower survival rates [42,44]. Therefore, palliation of hilar obstructions should be undertaken only in highly regarded institutions with high success rates for drainage of hilar obstructions.
Although there are advantages to bilateral drainage and disadvantages to unilateral drainage, endoscopists hesitate to place bilateral biliary stents because of the inherent technical difficulties that result in lower success rates and higher complication rates compared with those of unilateral stent insertion [26]. The necessity to drain both liver lobes is still questioned and controversial in Bismuth type II to IV strictures due to a paucity of data from randomized controlled trials of the usefulness of bilateral stenting.
Bilateral drainage theoretically provides more normal and physiological biliary flow through both ductal systems into the common bile duct than that of unilateral drainage [45-47,50]. In addition, a recent study of the effective volume of liver lobe drainage (hepatic volume assessed by cross-sectional imaging) supported the use of bilateral or multiple drainage. The main factor associated with drainage effectiveness is a drained liver volume > 50%, which frequently requires bilateral stent placement and which is associated with a longer median survival (119 days vs. 59 days) in patients with hilar cholangiocarcinoma [51]. Bulajic et al. [52] also assumed that ≥ 50% drainage of liver volume leads to sufficient drainage. Both these studies favored bilateral drainage.
Many centers now prefer bilateral drainage with advancements in technology because of the potentially increased risks of cholangitis and complications associated with unilateral drainage [33,38,47,53,54]. Bilateral drainage may provide increased functional liver volume and prevent possible complications during future chemotherapy. In addition, bilateral biliary drainage is necessary when unilateral drainage cannot relieve jaundice or when initial unilateral drainage is complicated by contralateral cholangitis.
Two recent studies reported results comparing bilateral and unilateral drainage. Naitoh et al. [54] retrospectively reviewed 46 patients including 22 with hilar cholangiocarcinoma and a malignant hilar obstruction. The median stent patency rate was significantly higher in the bilateral SEMS stenting group (n = 17; 448 days) compared with that in the unilateral group (n = 29; 210 days). No significant difference was observed in successful stent insertions, successful drainage, early or late complications, or survival between the unilateral and bilateral groups [54]. Bulajic et al. [52] comcompared unilateral versus bilateral drainage in patients with hilar malignant strictures (n = 49, including 32 hilar cholangiocarcinomas). The unilateral group (n = 21) and the bilateral group (n = 18) were not different in terms of early complication rates or survival time. However, clinical success and late complications were significantly different, and favored the bilateral group [52].
TECHNICAL ASPECTS OF BILIARY STENTING
Unilateral stenting
Unilateral stenting is simple and similar to the conventional endoscopic stenting technique. An unresolved issue concerns which duct should be selected and drained when placing a unilateral stent. A developing approach to manage malignant hilar strictures is the use of magnetic resonance cholangiopancreatography (MRCP) or computed tomography (CT)-guided unilateral endoscopic drainage [42]. This technique involves obtaining an MRCP and/or CT image before the endoscopic intervention. MRCP/CT images are useful to determine the main hepatic duct that drains the largest number of viable segments, which avoids draining atrophied lobes [41,42,49]. The postprocedural cholangitis risk can be reduced by avoiding drainage of atrophied lobes. However, it is very difficult to select the intended duct endoscopically compared to the percutaneous route. Other authors have suggested no difference whether the right or left hepatic duct is drained, and that the most easily accessible duct should be used [45]. However, the right hepatic duct is frequently and more easily accessed anatomically, regardless of obstruction severity. A mildly obstructed duct that drains well and has less necessity for drainage is frequently selected and drained, instead of an obstructed duct or a duct causing cholangitis.
Bilateral stenting
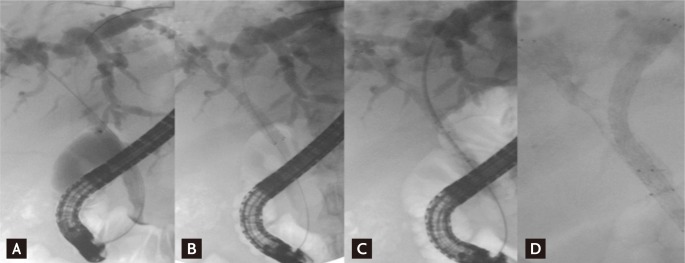
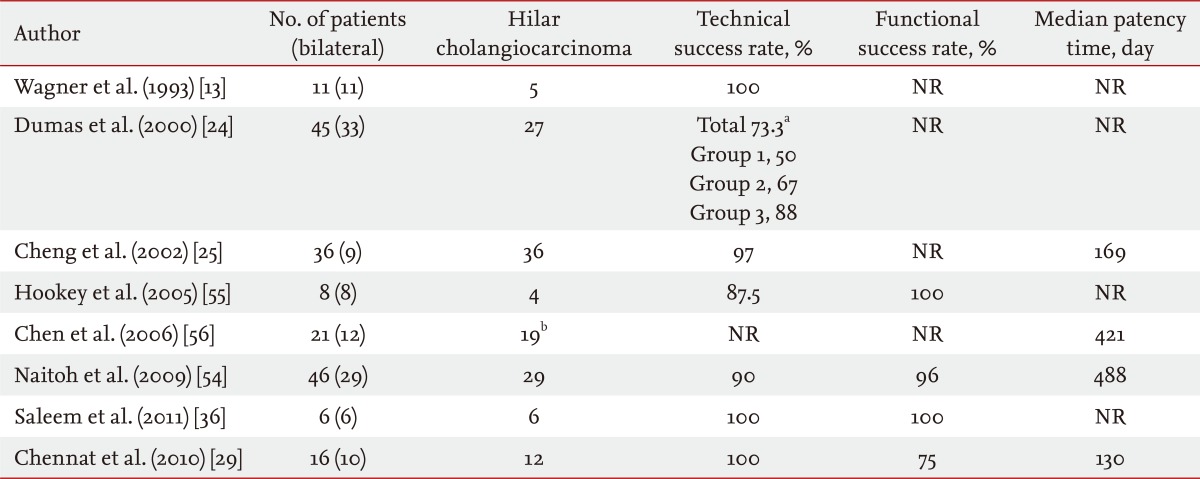
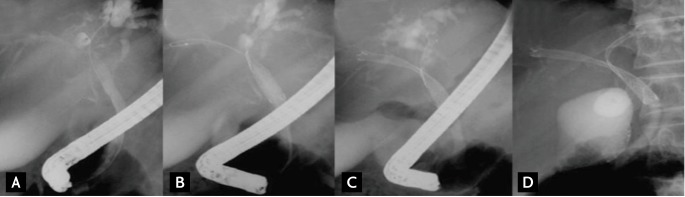
Bilateral drainage is technically difficult and challenging. Sphincterotomy is always performed with the use of prophylactic antibiotics. Care must be taken during the initial positioning of every guidewire to ensure that the left-sided guidewire is inserted first if possible because left stenting is more difficult to achieve than right stenting. When it is very difficult to initially place the guidewire into the left hepatic duct, "vice versa" is an alternative. "Stent by stent" and "stent in stent" are the two techniques available for endoscopic bilateral stenting. The "stent-by-stent" technique is a bilateral stenting procedure with parallel SEMS to drain both intrahepatic ducts. The technique is performed as follows: 1) initial insertion of bilateral semi-rigid hydrophilic guidewires with flexible tips, 2) ensuring that the guidewires are not entangled, 3) preliminary bilateral dilation of the stenosis using a hydrostatic balloon, and 4) initial insertion of the left-sided stent followed by insertion of the right-sided stent (Fig. 1). Several studies have reported endoscopic bilateral biliary drainage using this technique (Table 2) [13,24,25,29,36,54-56].
Figure 1.
"Stent-by-stent" method. (A) Guidewires are placed in both intraheptic ducts (IHDs). (B) The first stent is placed in the right IHD. (C) The undeployed stent is introduced into the left IHD. (D) Both stents are deployed in a parallel arrangement.
Table 2.
The "stent-by-stent" technique
NR, not reported.
aGroup 1, 1994-1995; Group 2, 1996; Group 3, 1997-1998; total, overall success rate.
bTwelve of the 19 patients with cholangiocarcinoma received bilateral stents.
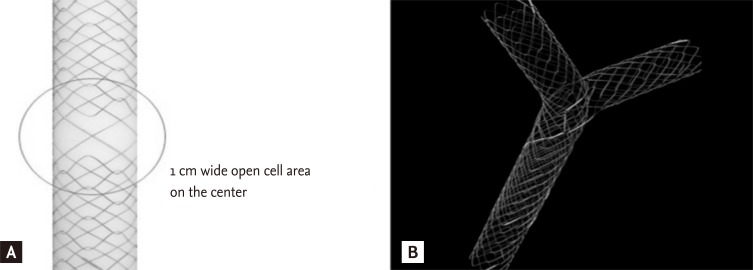
The "stent-by-stent" technique has some problems and difficulties that include potential entanglement of the two guidewires, fragility and dislodgement of the stents, and difficulty positioning both stents exactly. As an alternative, the "stent-in-stent" technique was developed to place bilateral SEMS in a Y configuration, in which the second stent traverses through the open-mesh wall of the first stent to enter the contralateral bile duct [57]. The transverse stent with the Y configuration, called a Y stent (Niti-S Biliary Y stent, Taewoong, Seoul, Korea), was developed as a hybrid of the spiral and Z components (Fig. 2).
Figure 2.
Configuration of the Y stent. (A) Central wide open-mesh portion. (B) Y configuration when placed bilaterally.
The mesh of the central part of the Y stent becomes bigger by omitting the Z component (forming a central area of relatively wide-open mesh), resulting in an open-weave portion that is 10 mm long in the middle of the stent (the present form is composed of a longer central wide portion, whole large cell type, or open mesh design). The Y stent is 6 to 8 cm in length and 10 mm in diameter, with gold markers indicating the central mesh. The first Y stent has two major disadvantages: the tendency for easy tumor in-growth in the wider-central mesh portion compared to that of the smaller mesh portions on both ends, and poor stent expansion due to weak radial force. Several improved SEMS versions have been developed to overcome the disadvantages of the first Y stent (M-Hilar stent, Standard Sci-Tech, Seoul, Korea and LCD type Y stent, Taewoong, respectively). Several types of second stents (Niti-S, Niti-D, LCD type stent, Taewoong; M-Hilar stent, Standard Sci-Tech; Zilver stent, Cook Endoscopy, Winston-Salem, NC, USA), introduced via the central mesh of the Y stent, have a length of 6 to 8 cm and a diameter of 10 mm. The stent placement technique is as follows. After the strictures are negotiated with the guidewire into the left hepatic duct, the Y stent is deployed in the left hepatic duct. The guidewire left in place across the Y stent is carefully withdrawn with an ERCP catheter, without pulling it back completely, and is then inserted under fluoroscopic guidance into the right hepatic duct through the central open mesh of the Y stent. Another uncovered second metal stent is then introduced over the guidewire through the central open mesh. This stent is deployed in the right hepatic duct (Fig. 3).
Figure 3.
"Stent-in-stent" method. (A) Guidewire is introduced into the left intrahepatic duct (IHD). (B) Y stent is placed in the left IHD. Guidewire is introduced into the right IHD through the central open-mesh of the Y stent. (C) The second stent is deployed in the right IHD. (D) Y configuration bilateral stenting accomplished.
When difficulties are encountered when inserting the guidewire into the intended hepatic duct, modified techniques involve using a more hydrophilic Terumo guidewire, a stiffer guidewire, a retrieval balloon catheter, a pull-type endoscopic sphincterotomy knife, or a triple-lumen catheter (Haber RAMP catheter, Cook Endoscopy) [24,29,42,54,58,59].
Passing a tapered-tip catheter or a stent through the stricture can be unsuccessful, even after obtaining guidewire access to the desired hepatic duct. In these cases, a 7-Fr Soehendra stent extractor (Cook Endoscopy), a 6 to 8 mm hydrostatic balloon (Hurricane CRE wire-guided esophageal/pyloric balloon dilation catheter, Boston Scientific, Cork, Ireland), or ENBD or guidewire left in place for several days can be used [60]. Few studies have compared the "stent-by-stent" and "stent-in-stent" bilateral stenting techniques. One retrospectively reviewed the results of these two techniques, but no statistical analysis was performed [61]. There is still no consensus as to which method is more useful to achieve bilateral stenting. However, the "stent-in-stent" method has become more widely used with recent improvements in technique and devices (Table 3) [27,43,57,58,62-66].
Table 3.
The "stent-in-stent" technique
NR, not reported.
aMedian stent patency period was 202 days in the pool of 12 patients as determined by Kaplan-Meier analysis.
CONCLUSIONS
The endoscopic approach has been considered the treatment of choice for palliation of hilar cholangiocarcinoma. Although many endoscopists perform bilateral drainage, a lack of clear consensus on unilateral versus bilateral drainage for hilar cholangiocarcinoma remains. The choice of the number or type of SEMS should be based on the biliary tree configuration, presence of an eventual atrophied lobe, and the need to obtain maximal decompression to eventually offer chemoradiation, and should not be based on operator preference. The decision should also be made based on the availability of expertise at a given institution.
In the near future, spy glass cholangioscope, drug eluting stents, and photodynamic therapy are expected to be used to improve treatment of hilar cholangiocarcinomas.
Acknowledgements
Supported by a 2-year Research Grant of Pusan National University and a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, South Korea, No. A091047.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Altemeier WA, Gall EA, Zinninger MM, Hoxworth PI. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg. 1957;75:450–460. doi: 10.1001/archsurg.1957.01280150140015. [DOI] [PubMed] [Google Scholar]
- 2.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 3.Classen M, Hagenmuller F. Endoscopic biliary drainage. Scand J Gastroenterol Suppl. 1984;102:76–83. [PubMed] [Google Scholar]
- 4.Siegel JH. Improved biliary decompression with large caliber endoscopic prostheses. Gastrointest Endosc. 1984;30:21–23. doi: 10.1016/s0016-5107(84)72288-7. [DOI] [PubMed] [Google Scholar]
- 5.Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119–1124. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JH, Pullano W, Wright G, Halpern G. The ultimate large caliber endoprosthesis-12F: poiseuille was right: bigger is better. Gastrointest Endosc. 1985;31:158. [Google Scholar]
- 7.Singhal D, van Gulik TM, Gouma DJ. Palliative management of hilar cholangiocarcinoma. Surg Oncol. 2005;14:59–74. doi: 10.1016/j.suronc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Freeman ML, Sielaff TD. A modern approach to malignant hilar biliary obstruction. Rev Gastroenterol Disord. 2003;3:187–201. [PubMed] [Google Scholar]
- 9.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43–57. doi: 10.1634/theoncologist.9-1-43. [DOI] [PubMed] [Google Scholar]
- 10.Liu CL, Lo CM, Lai EC, Fan ST. Endoscopic retrograde cholangiopancreatography and endoscopic endoprosthesis insertion in patients with Klatskin tumors. Arch Surg. 1998;133:293–296. doi: 10.1001/archsurg.133.3.293. [DOI] [PubMed] [Google Scholar]
- 11.Iwano H, Ryozawa S, Harano M, et al. Management of unresectable hilar biliary obstruction: plastic stents versus metallic stents and unilateral versus bilateral biliary drainage. Bull Yamaguchi Med Sch. 2008;55:9–14. [Google Scholar]
- 12.Kim YT. Endoscopic stent drainage for malignant biliary obstruction: the Korean experience. Dig Endosc. 2006;18:154–156. [Google Scholar]
- 13.Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction: a prospective and randomized trial. Endoscopy. 1993;25:213–218. doi: 10.1055/s-2007-1010295. [DOI] [PubMed] [Google Scholar]
- 14.Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. Am J Gastroenterol. 1996;91:1301–1308. [PubMed] [Google Scholar]
- 15.Galandi D, Schwarzer G, Bassler D, Allgaier HP. Ursodeoxycholic acid and/or antibiotics for prevention of biliary stent occlusion. Cochrane Database Syst Rev. 2002;(3):CD003043. doi: 10.1002/14651858.CD003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen B, Goodman LP, Ruiten D. Bacterial adherence to hydrophilic polymer-coated polyurethane stents. Gastrointest Endosc. 1993;39:670–673. doi: 10.1016/s0016-5107(93)70220-5. [DOI] [PubMed] [Google Scholar]
- 17.Rees EN, Tebbs SE, Elliott TS. Role of antimicrobial-impregnated polymer and Teflon in the prevention of biliary stent blockage. J Hosp Infect. 1998;39:323–329. doi: 10.1016/s0195-6701(98)90298-5. [DOI] [PubMed] [Google Scholar]
- 18.Sung JY, Shaffer EA, Lam K, Rususka I, Costerton JW. Hydrophobic bile salt inhibits bacterial adhesion on biliary stent material. Dig Dis Sci. 1994;39:999–1006. doi: 10.1007/BF02087551. [DOI] [PubMed] [Google Scholar]
- 19.Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34:412–417. doi: 10.1016/s0016-5107(88)71407-8. [DOI] [PubMed] [Google Scholar]
- 20.Siegel JH, Pullano W, Kodsi B, Cooperman A, Ramsey W. Optimal palliation of malignant bile duct obstruction: experience with endoscopic 12 French prostheses. Endoscopy. 1988;20:137–141. doi: 10.1055/s-2007-1018158. [DOI] [PubMed] [Google Scholar]
- 21.Gerhards MF, den Hartog D, Rauws EA, et al. Palliative treatment in patients with unresectable hilar cholangiocarcinoma: results of endoscopic drainage in patients with type III and IV hilar cholangiocarcinoma. Eur J Surg. 2001;167:274–280. doi: 10.1080/110241501300091444. [DOI] [PubMed] [Google Scholar]
- 22.Raju RP, Jaganmohan SR, Ross WA, et al. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557–1564. doi: 10.1007/s10620-010-1550-5. [DOI] [PubMed] [Google Scholar]
- 23.Peters RA, Williams SG, Lombard M, Karani J, Westaby D. The management of high-grade hilar strictures by endoscopic insertion of self-expanding metal endoprostheses. Endoscopy. 1997;29:10–16. doi: 10.1055/s-2007-1004054. [DOI] [PubMed] [Google Scholar]
- 24.Dumas R, Demuth N, Buckley M, et al. Endoscopic bilateral metal stent placement for malignant hilar stenoses: identification of optimal technique. Gastrointest Endosc. 2000;51:334–338. doi: 10.1016/s0016-5107(00)70364-6. [DOI] [PubMed] [Google Scholar]
- 25.Cheng JL, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002;56:33–39. doi: 10.1067/mge.2002.125364. [DOI] [PubMed] [Google Scholar]
- 26.De Palma GD, Pezzullo A, Rega M, et al. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50–53. doi: 10.1067/mge.2003.310. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto H, Tsutsumi K, Harada R, et al. Endoscopic deployment of multiple JOSTENT SelfX is effective and safe in treatment of malignant hilar biliary strictures. Clin Gastroenterol Hepatol. 2008;6:401–408. doi: 10.1016/j.cgh.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Chennat J, Waxman I. Initial performance profile of a new 6F self-expanding metal stent for palliation of malignant hilar biliary obstruction. Gastrointest Endosc. 2010;72:632–636. doi: 10.1016/j.gie.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 30.Yoon WJ, Ryu JK, Yang KY, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc. 2009;70:284–289. doi: 10.1016/j.gie.2008.12.241. [DOI] [PubMed] [Google Scholar]
- 31.Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178–182. doi: 10.1067/mge.2003.66. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Hamda EM, Baron TH. Endoscopic management of cholangiocarcinoma. Semin Liver Dis. 2004;24:165–175. doi: 10.1055/s-2004-828893. [DOI] [PubMed] [Google Scholar]
- 33.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 34.Li F, Wang F, Yang X, et al. Covered stents versus uncovered stents for the palliation of malignant extrahepatic biliary obstruction caused by direct tumor invasion: a cohort comparative study. Med Oncol. 2012;29:2762–2770. doi: 10.1007/s12032-012-0187-y. [DOI] [PubMed] [Google Scholar]
- 35.Isayama H, Nakai Y, Kawakubo K, et al. Covered metallic stenting for malignant distal biliary obstruction: clinical results according to stent type. J Hepatobiliary Pancreat Sci. 2011;18:673–677. doi: 10.1007/s00534-011-0411-8. [DOI] [PubMed] [Google Scholar]
- 36.Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321–327. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 37.Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996–1000. doi: 10.1016/j.gie.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 38.Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180–1186. doi: 10.1016/0016-5085(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 39.Baer HU, Rhyner M, Stain SC, et al. The effect of communication between the right and left liver on the outcome of surgical drainage for jaundice due to malignant obstruction at the hilus of the liver. HPB Surg. 1994;8:27–31. doi: 10.1155/1994/17262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parodi A, Fisher D, Giovannini M, Baron T, Conio M. Endoscopic management of hilar cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:105–112. doi: 10.1038/nrgastro.2011.271. [DOI] [PubMed] [Google Scholar]
- 41.Brauer BC. Endoscopic palliation of malignant biliary obstruction. Tech Gastrointest Endosc. 2009;11:26–34. doi: 10.4253/wjge.v14.i10.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41–49. doi: 10.1067/mge.2003.292. [DOI] [PubMed] [Google Scholar]
- 43.Kogure H, Isayama H, Nakai Y, et al. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011;25:463–467. doi: 10.1007/s00464-010-1194-8. [DOI] [PubMed] [Google Scholar]
- 44.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 45.Polydorou AA, Chisholm EM, Romanos AA, et al. A comparison of right versus left hepatic duct endoprosthesis insertion in malignant hilar biliary obstruction. Endoscopy. 1989;21:266–271. doi: 10.1055/s-2007-1012966. [DOI] [PubMed] [Google Scholar]
- 46.Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95–101. doi: 10.1016/s0016-5107(88)71271-7. [DOI] [PubMed] [Google Scholar]
- 48.Ridtitid W, Rerknimitr R, Janchai A, Kongkam P, Treeprasertsuk S, Kullavanijaya P. Outcome of second interventions for occluded metallic stents in patients with malignant biliary obstruction. Surg Endosc. 2010;24:2216–2220. doi: 10.1007/s00464-010-0931-3. [DOI] [PubMed] [Google Scholar]
- 49.Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40–46. doi: 10.1067/mge.2001.111388. [DOI] [PubMed] [Google Scholar]
- 50.Sherman S. Endoscopic drainage of malignant hilar obstruction: is one biliary stent enough or should we work to place two? Gastrointest Endosc. 2001;53:681–684. doi: 10.1067/mge.2001.114714. [DOI] [PubMed] [Google Scholar]
- 51.Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 52.Bulajic M, Panic N, Radunovic M, et al. Clinical outcome in patients with hilar malignant strictures type II Bismuth-Corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage. Hepatobiliary Pancreat Dis Int. 2012;11:209–214. doi: 10.1016/s1499-3872(12)60150-7. [DOI] [PubMed] [Google Scholar]
- 53.Motte S, Deviere J, Dumonceau JM, Serruys E, Thys JP, Cremer M. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology. 1991;101:1374–1381. doi: 10.1016/0016-5085(91)90091-x. [DOI] [PubMed] [Google Scholar]
- 54.Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 55.Hookey LC, Le Moine O, Deviere J. Use of a temporary plastic stent to facilitate the placement of multiple self-expanding metal stents in malignant biliary hilar strictures. Gastrointest Endosc. 2005;62:605–609. doi: 10.1016/j.gie.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 56.Chen JH, Sun CK, Liao CS, Chua CS. Self-expandable metallic stents for malignant biliary obstruction: efficacy on proximal and distal tumors. World J Gastroenterol. 2006;12:119–122. doi: 10.3748/wjg.v12.i1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JH, Kang DH, Kim JY, et al. Endoscopic bilateral metal stent placement for advanced hilar cholangiocarcinoma: a pilot study of a newly designed Y stent. Gastrointest Endosc. 2007;66:364–369. doi: 10.1016/j.gie.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 58.Kanno Y, Ito K, Fujita N, et al. Single-session endoscopic bilateral y-configured placement of metal stents for hilar malignant biliary obstruction. Dig Endosc. 2011;23:91–96. doi: 10.1111/j.1443-1661.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim JY, Kang DH, Choi CW, Kim HW, Park SB, Kim DU. Selective intrahepatic duct cannulation by using a triple-lumen catheter for endoscopic bilateral stenting in hilar cholangiocarcinoma. Gastrointest Endosc. 2010;72:192–198. doi: 10.1016/j.gie.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 60.Silverman W, Slivka A. New technique for bilateral metal mesh stent insertion to treat hilar cholangiocarcinoma. Gastrointest Endosc. 1996;43:61–63. doi: 10.1016/s0016-5107(96)70263-8. [DOI] [PubMed] [Google Scholar]
- 61.Mukai T, Yasuda I, Isayama H, et al. Comparison of axial force and cell width of self-expandable metallic stents: which type of stent is better suited for hilar biliary strictures? J Hepatobiliary Pancreat Sci. 2011;18:646–652. doi: 10.1007/s00534-011-0406-5. [DOI] [PubMed] [Google Scholar]
- 62.Park do H, Lee SS, Moon JH, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos) Gastrointest Endosc. 2009;69:1357–1360. doi: 10.1016/j.gie.2008.12.250. [DOI] [PubMed] [Google Scholar]
- 63.Kim JY, Kang DH, Kim HW, et al. Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video) Gastrointest Endosc. 2009;70:1109–1115. doi: 10.1016/j.gie.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Gerhardt T, Rings D, Hoblinger A, Heller J, Sauerbruch T, Schepke M. Combination of bilateral metal stenting and trans-stent photodynamic therapy for palliative treatment of hilar cholangiocarcinoma. Z Gastroenterol. 2010;48:28–32. doi: 10.1055/s-0028-1109983. [DOI] [PubMed] [Google Scholar]
- 65.Chahal P, Baron TH. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointest Endosc. 2010;71:195–199. doi: 10.1016/j.gie.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol. 2011;46:326–332. doi: 10.3109/00365521.2010.536253. [DOI] [PubMed] [Google Scholar]