Abstract
Purpose
To assess independent prognostic factors for lymph node-negative metastatic gastric cancer patients following curative resection is valuable for more effective follow-up strategies.
Materials and Methods
Among 1,874 gastric cancer patients who received curative resection, 967 patients were lymph node-negative. Independent prognostic factors for overall survival in lymph node-negative gastric cancer patients grouped by tumor invasion depth (early gastric cancer versus advanced gastric cancer) were explored with univariate and multivariate analyses.
Results
There was a significant difference in the distribution of recurrence pattern between lymph node-negative and lymph node-positive group. In the lymph node-negative group, the recurrence pattern differed by the depth of tumor invasion. In univariate analysis for overall survival of the early gastric cancer group, age, macroscopic appearance, histologic type, venous invasion, lymphatic invasion, and carcinoembryonic antigen level were significant prognostic factors. Multivariate analysis for these factors showed that venous invasion (hazard ratio, 6.695), age (≥59, hazard ratio, 2.882), and carcinoembryonic antigen level (≥5 ng/dl, hazard ratio, 3.938) were significant prognostic factors. Multivariate analysis of advanced gastric cancer group showed that depth of tumor invasion (T2 versus T3, hazard ratio, 2.809), and age (hazard ratio, 2.319) were prognostic factors on overall survival.
Conclusions
Based on our results, independent prognostic factors such as venous permeation, carcinoembryonic antigen level, and age, depth of tumor invasion on overall survival were different between early gastric cancer and advanced gastric cancer group in lymph node-negative gastric cancer patients. Therefore, we are confident that our results will contribute to planning follow-up strategies.
Keywords: Gastric cancer, Lymph node negative, Prognostic factor, Overall survival
Introduction
The depth of tumor invasion and lymph node metastasis are the most important prognosis factors in early or advanced gastric cancer. These factors are already known to be closely related to cancer recurrence or prognosis of the patients after surgery.(1,2) As the diagnostic methods for gastric cancer have developed and gastric cancers are increasingly screened, the ratio of early gastric cancer and 5-year survival rate tend to increase steadily. Since the 5-year survival rate in early gastric cancer exceeds 90%, improving and maintaining the quality of life has gained attention with regards to the treatment of gastric cancer.(2-4)
On the other hand, many patients who underwent curative gastrectomy experience recurrence and die. For such reasons, many researchers make efforts to investigate the prognostic factors related to recurrence and survival.(2-6) Especially, since many patients experience recurrence and die even though the prognosis is favorable in cases where there is no lymph nodes metastasis in gastric cancer, additional research into prognostic factors is required.
The objective of this study was to investigate prognosis factors relevant to clinical pathologic characteristics, metastasis risks, and long-term survival rates of patients who had no lymph nodes metastasis after extensive lymph nodes dissection of more than D2. Therefore, this study would help establishing specialized tailored strategies with regards to follow-up observations and prognosis predictions of the patients without lymph nodes metastasis after surgery.
Materials and Methods
The study is a retrospective, record based. Total 1,874 patients who were diagnosed with gastric cancer and had curative gastrectomy including lymph node dissection of more than D2 at the Department of General Surgery in Hanyang University Medical Center from June 1992 to December 2010. Among 1,874 patients, 967 patients who didn't have lymph nodes metastasis based upon the result of histopathologic examination were assigned to the experimental group. To investigate the differences in clinical pathologic characteristics and long-term survival rates depending upon whether lymph nodes are metastasized, 907 patients who had the lymph nodes metastasis were selected as a control group.
Both groups were divided into early gastric cancer and advanced gastric cancer depending upon the depth of tumor invasion, followed by analysis of the prognostic factors in patients with gastric cancer who had negative lymph nodes metastasis versus positve lymph nodes metastasis.
The prognostic factors affecting 5-year survival rates were used as analytic variables, including demographic traits of patients (age and sex) and tumor factors (the stomach walls invasion degree, lymph nodes metastasis degree, tumor size, tumor position, blood vessels, lymphatic vessels, whether or not invading around nerves, histological classification, visual classification, and tumor marker value before surgery (carcinoembryonic antigen, CA19-9).
Whether or not the lymph nodes were metastasized was observed after dividing into half from the center of lymph nodes section and staining. Conventional H&E staining was used to stain the lymph nodes, but additional immunohistochemistry staining was carried out in case of difficulties with the observation. After the surgery, chemotherapy on entire body was performed for clinical stage III and IV and ruled not to be performed for stage I and II. Complex chemotherapy was carried out with the main medications including fluorourasil substance as oral anti-cancer drugs, and fluorourasil, cisplatin, and oxaliplatin given as intravenous injections.
After the surgery, follow-up examinations of pathologic stage I and II were carried out once every 6 months for 5 years and then every year. Follow-up examinations of pathologic stage III and IV were carried out once every 3 months for 3 years after the surgery, once every 6 months from 3 years to 5 years, and then annually after 5 years. For the follow-up examination, gastrofiberscopy and chest X-ray were performed once in a year and abdominal computed tomography and abdominal ultrasound were performed every 6 months alternatively. Other than that, serum tumor markers, blood, and biochemical examinations were carried out every 3 to 6 months. Recently, in advanced gastric cancer patients, positron emission tomography-computed tomography scanning was performed additionally. The follow-up duration was from 1 to 218 months and the median was 60 months based on July 31st in 2011. The follow-up rate was 98.3%.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (IBM Co., Armonk, NY, USA) and Chi-square test was used to compare several clinical pathologic characteristics between early gastric cancer and advanced gastric cancer. For survival rates, Kaplan-Meier method was used to obtain survival curves and the significance was verified utilizing Log-Rank test. Multi-variable analysis of the survival rates was performed using Cox regression. Null hypotheses of no difference were rejected if P-values were less than 0.05, or, equivalently, if the 95% confidence intervals of risk point estimates excluded 1.
Results
1. Clinical pathologic differences depending upon whether lymph nodes were metastasized
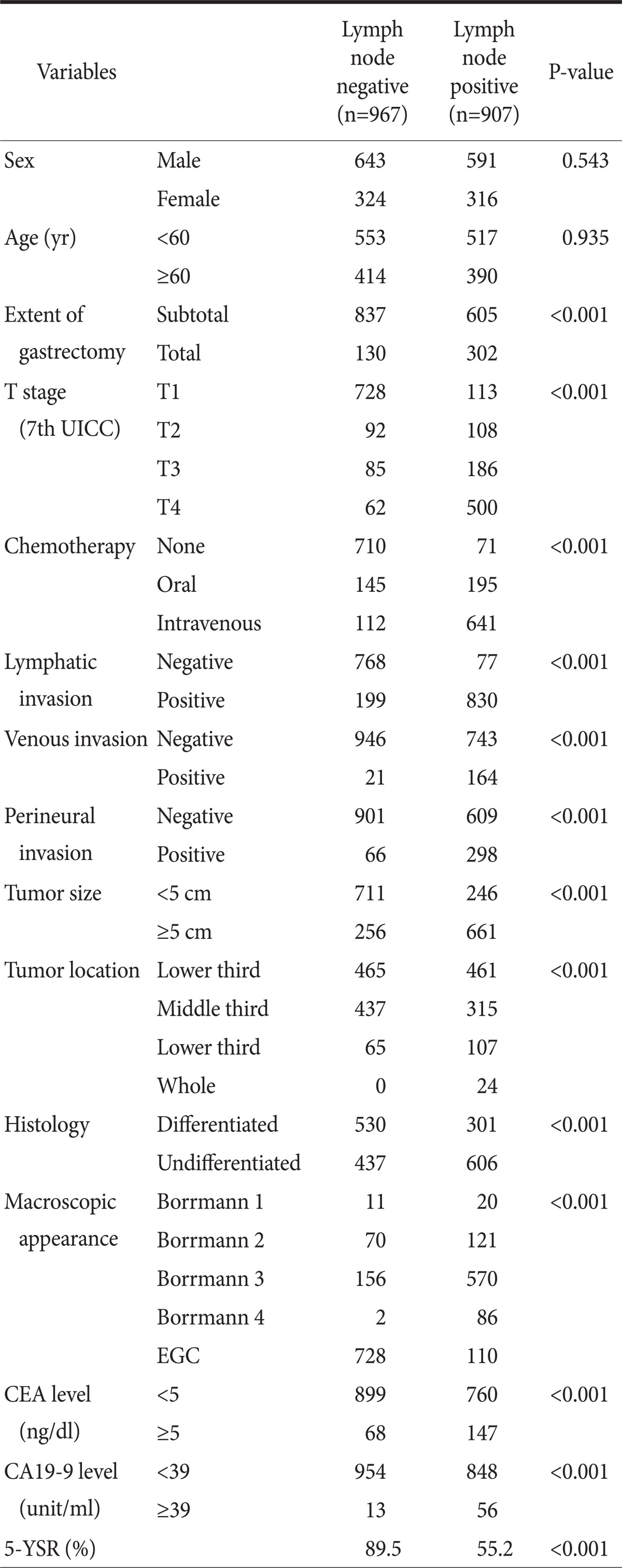
In the comparison analysis depending upon whether lymph nodes were metastasized, distributions of sex and elderly patients (P=0.935) did not show differences. However, statistically significant differences were seen in extent of gastrectomy, depth of invasion, chemotherapy, the presence of tumor invasion in lymphatic vessels/blood vessels/around nerves, large tumors, tumor location, degree of differentiation, Borrmann type, preoperative serum CEA and CA19-9 level (Table 1).
Table 1.
Comparison of clinicopathologic characteristics between lymph node negative and positive gastric cancer patients
UICC = Union for International Cancer Control; EGC = early gastric cancer; CEA = carcinoembryonic antigen; 5-YSR = 5 year survival rate.
2. Univariate survival rate analysis of early gastric cancer patients who had negative lymph nodes metastasis
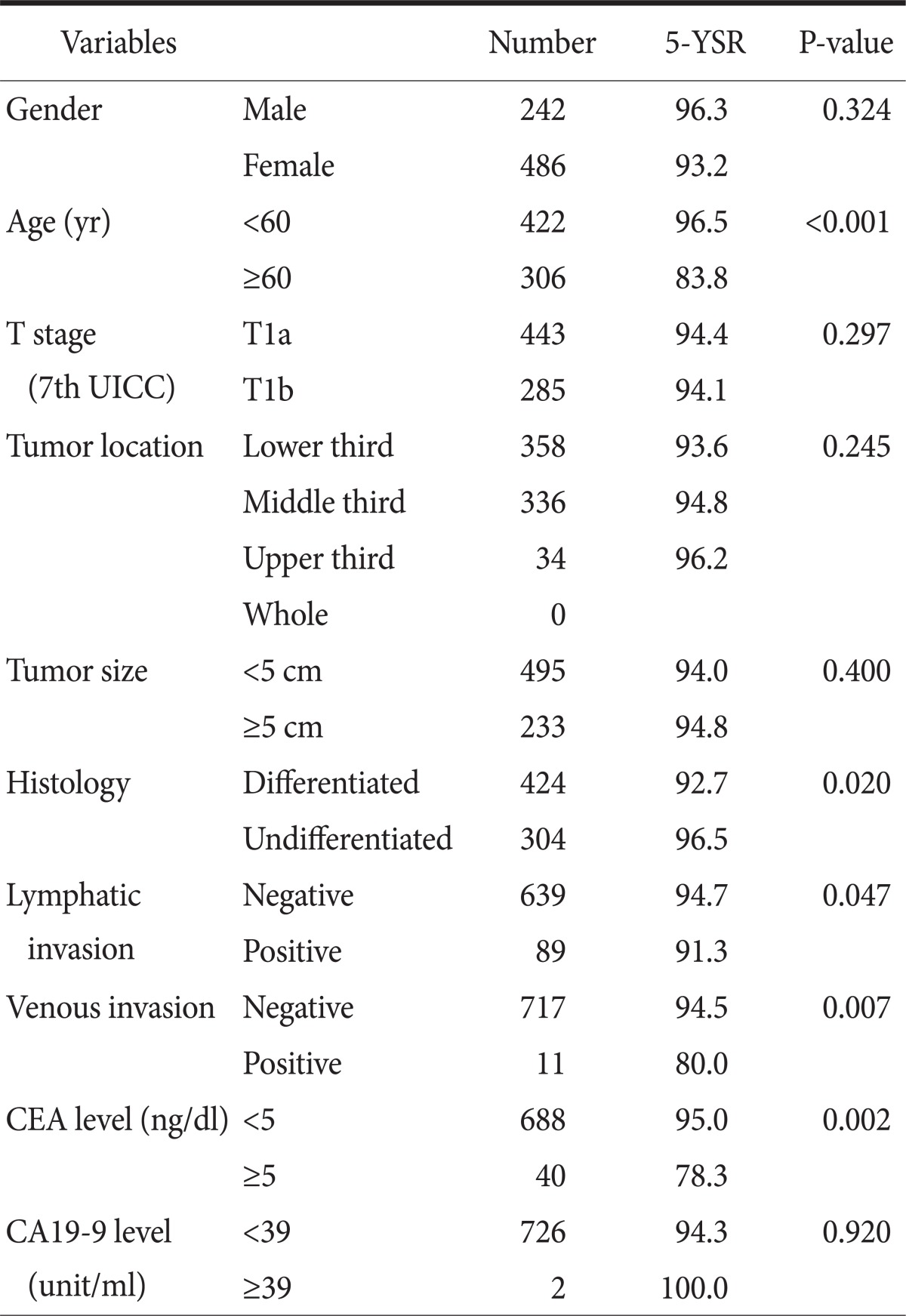
In the prognosis factor analysis of the 5 year survival rate, there were significant differences in age (≥60, P<0.001), histology (undifferentiated, P=0.020), state of lymphatic invasion (lymphatic invasion, P=0.047), state of venous invasion (venous invasion, P=0.007), and CEA level (≥5 ng/dl, P=0.002) (Table 2).
Table 2.
Univariate survival analysis in lymph node negative early gastric cancer patients (n=728)
5-YSR = 5 year survival rate; UICC = Union for International Cancer Control; CEA = carcinoembryonic antigen.
3. Univariate survival rate analysis of advanced gastric cancer patients who had negative lymph nodes metastasis
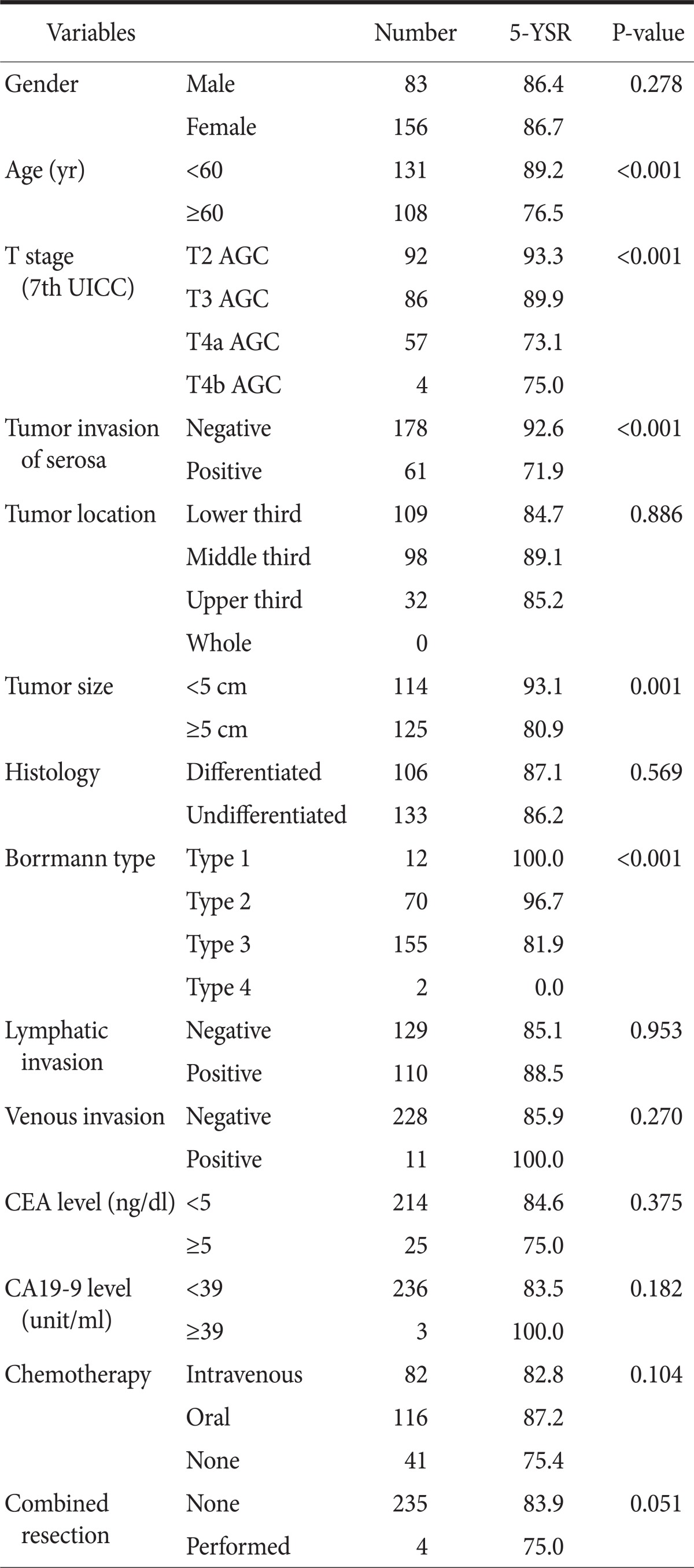
In advanced gastric cancer without lymph nodes metastasis, statistically significant differences were seen in age (≥60, P<0.001), depth of tumor invasion (7th Union for International Cancer Control [UICC] T classification or Serosa penetration negative vs. positive, P<0.001), a tumor size (≥5 cm, P=0.001), and Bormann types (P<0.001) (Table 3).
Table 3.
Univariate survival analysis in lymph node negative advanced gastric cancer patients (n=239)
5-YSR = 5 year survival rate; UICC = Union for International Cancer Control; AGC = Advanced Gastric Cancer; CEA = carcinoembryonic antigen.
4. Multi-variate survival rate analyses according to the nodal status in early and advanced gastric cancer patients
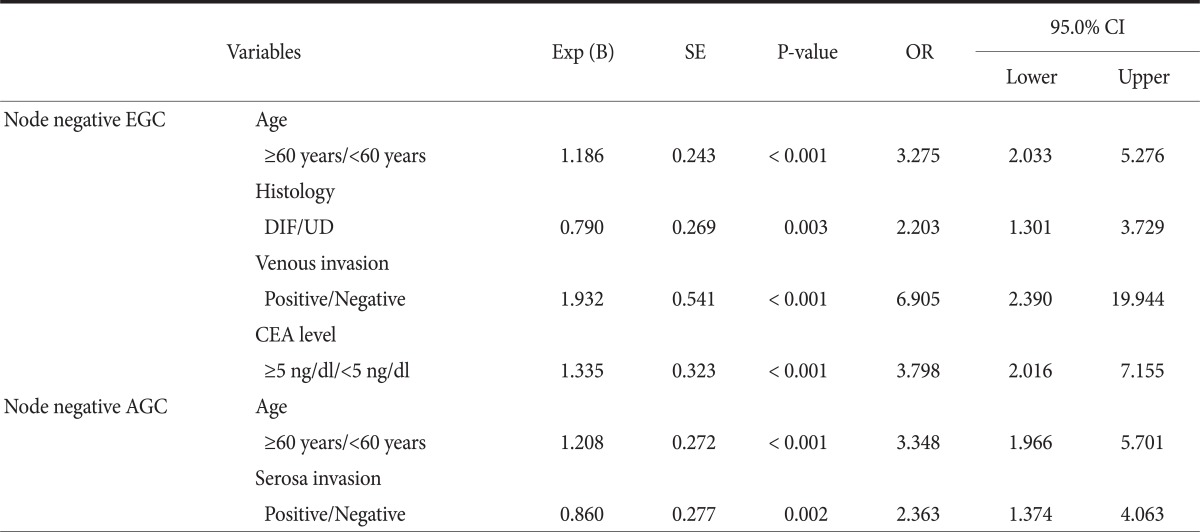
In the multi-variate analyses of prognosis factors regarding long-term survival, early gastric cancer without the lymph nodes metastasis differed significantly by age, histology, venous invasion, and CEA level. The advanced gastric cancer patients without lymph node metastasis differed significantly by age and serosa invasion (Table 4).
Table 4.
Multivariate survival analyses according to the nodal status in early and advanced gastric cancer patients
Exp (B) = exponentiation of the B; SE = standard error; OR = odds ratio; CI = confidence interval; EGC = early gastric cancer; DIF = differentiated adenocarcinoma; UD = undifferentiated adenocarcinoma; CEA = carcinoembryonic antigen; AGC = advanced gastric cancer.
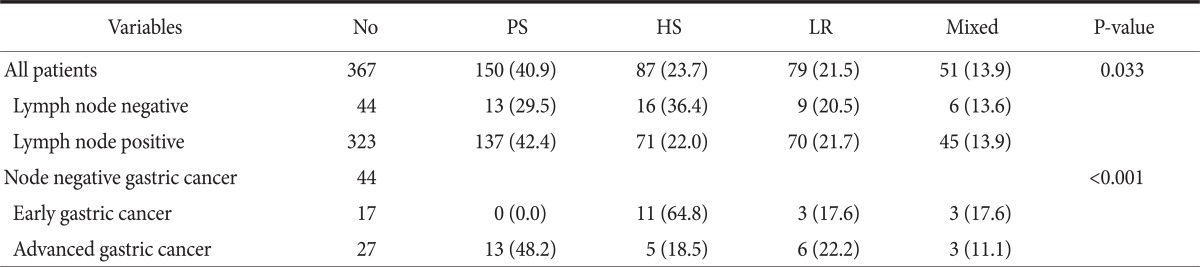
5. Recurrence patterns in gastric cancer patients who underwent curative resection
In the analysis of recurrence pattern depending upon whether or not lymph nodes were metastasized, hematogenous metastasis (36%) occurred the most in the negative lymph nodes metastasis group while peritoneal metastasis (42%) occurred the most in the positive lymph nodes metastasis group, with statistical significance. A recurrence pattern depending upon early gastric cancer and advanced gastric cancer was analyzed in the negative lymph nodes metastasis group and resulted in statistically significant difference between them (Table 5).
Table 5.
Recurrence patterns in gastric cancer patients who underwent curative resection
Values are presented as number or number (%). No = number; PS = peritoneal seeding; HS = hematogenous spread; LR = local recurrence.
Discussion
This study was carried out on 967 patients who had negative lymph nodes metastasis. The patients with early gastric cancer and advanced gastric cancer were 728 subjects and 239 subjects out of 967 patients, respectively. Whether or not having lymph nodes metastasis after curative gastrectomy of gastric cancer has been well know as a important prognostic factor related to the survival rate. (7,8) The results of the current study showed significant differences on the clinico-pathological analysis and long-term survival depending upon lymph nodes metastasis. That is because frequencies of lymph nodes metastasis are closely related to the depth of tumor invasion and biological behavior of the negative lymph node gastric cancer is relatively less aggressive than that of positive lymph node gastric cancer. Moreover, since recurrence pattern differs by lymph nodes metastasis (Table 5), more specialized strategies would expect to be prepared for follow-up examinations after the surgery if prognostic factors and recurrence characteristics are known.
Some researchers have shown that elderly patients with gastric cancer have an unfavorable prognosis. Especially in gastric cancer without lymph nodes metastasis, age of patients is an important prognostic factor as well as the depth of tumor invasion.(9-11) The current study also confirmed that being elderly was unfavorable for prognosis in all patient groups. However, since competing causes of death such as heart diseases and lung diseases were not excluded in this study, additional study would be required even though age was a statistically significant unfavorable prognosis factor.
This study was performed on patients with early gastric cancer without the lymph nodes metastasis and the venous invasion was an important prognosis factor. Saito et al.(12) and Hyung et al.(13) reported that the depth of tumor invasion and lymphovascular invasion are poor prognostic factors for overall survival in advanced gastric cancer patients. Gabbert et al.(14) suggested that invasions of gastric cancer cells in blood vessels, and lymphatics are high risk factors for recurrence and the degree of tumor invasion was higher in positive lymph node gastric cancer. That was also related to the degree of tumor invasion so that venous and lymphatic invasion rates were 4.0% and 11.2% in T1 (4th UICC) and 16.4% and 46.6% in T2 (4th UICC).
Saito et al.(12) reported that when the differentiation was not good, prognosis was not favorable in lymph node negative advanced gastric cancer patients. Even though lymph node negative advanced gastric cancer patients did not differ significantly for overall survival in the present study, we could confirm that undifferentiated gastric cancer is a significant prognostic factor for overall survival in lymph node negative early gastric cancer patients.
In gastric cancer patients, serum CEA and CA19-9 level etc are used for representative tumor marker examination, but they are not considered tumor markers regarding specific gastric cancer due to low sensitivity. Whereas Reiter et al.(15) reported that increase of serum CEA, and CA19-8 level before surgery was a prognostic factor in gastric cancer patients who underwent curative resection, Ucar et al.(16) mentioned that serum CEA and CA19-9 levels were not independent prognostic factors and only CA 72-4 level was an independent prognosis factor. Kwon et al.(17) emphasized that peritoneal irrigation solution tumor markers (CEA, CA19-9) reinforced significance as prognosis factors. Besides, Kim et al.(18) reported utilization of tumor markers as a purpose of recurrence diagnosis in gastric cancer patients who had curative gastrectomy. In the current study, we confirmed that serum CEA level was an independent prognosis factor in early gastric cancer patients with negative lymph nodes. However, it was not significant in early gastric cancer patients with metastatic lymph nodes and advanced gastric cancer patients.
In the present study, invasion of serosa by tumor cells was an important prognostic factor in advanced gastric cancer patients with negative lymph nodes. Bruno et al.(19) and Kooby et al.(20) reported that the depth of invasion was an important prognosis factor in gastric cancer patients with negative lymph nodes. Further Maehara et al.(21) and Kim et al.(22) also reported the size of tumor as well as invasion of serosa layer were important prognosis factors regarding the survival rate. Particularly, the recent 19th edition of Sabistone describes both positive lymph node metastasis and invasion of serosa layer as unfavorable prognosis factors.(23)
When the recurrence pattern was analyzed after the curative gastrectomy, there was a higher risk of hematogenous metastasis among lymph node negative patients than lymph node positive patients. The lymph node positive patients have the highest risk of peritoneal seeding. Particularly, hematogenous metastasis was significantly higher in early gastric cancer patients with negative lymph nodes (64.8%).
In patients with curative gastrectomy, the presence of lymph node metastasis has clinico-pathologically different characteristics as well as differences in survival rate. Particularly, early gastric cancer and advanced gastric cancer patients also showed different results in long-term survival rate according to the presence of lymph node metastasis. In early gastric cancer patients with negative lymph nodes, age, differentiation, venous invasion, and serum CEA level were important prognostic factors, while age and invasion of serosa layer were important in advanced gastric patients with negative lymph nodes. Further, given the apparent hematogenous metastasis in early gastric cancer patients with negative lymph nodes metastasis, more attention is required.
References
- 1.Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999;85:2119–2123. doi: 10.1002/(sici)1097-0142(19990515)85:10<2119::aid-cncr4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Kim DW, Kwon SJ, Won CK. Clinical review of early gastric cancer with lymph node metastasis. J Korean Surg Soc. 1995;49:344–351. [Google Scholar]
- 3.Kim W, Park CH, Park SM, Park WB, Lim KW, Kim SN. Prognostic significance of lymphatic and perineural invasions in patients with gastric cancer who have no lymph node and serosal involvement. J Korean Gastric Cancer Assoc. 2001;1:77–82. [Google Scholar]
- 4.Huh H, Hyung WJ, Chen J, Choi SH, Noh SH. Implication of lymphatic or blood vessel invasion in early gastric cancer. J Korean Surg Soc. 2003;64:134–139. [Google Scholar]
- 5.Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35:409–414. doi: 10.1016/j.ejso.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Ha TK, Kwon SJ. Clinicopathologic characteristics according to the type of recurrence in curative-resected gastric cancer patient. J Korean Gastric Cancer Assoc. 2007;7:23–30. [Google Scholar]
- 7.Liu C, Zhang R, Lu Y, Li H, Lu P, Yao F, et al. Prognostic role of lymphatic vessel invasion in early gastric cancer: a retrospective study of 188 cases. Surg Oncol. 2010;19:4–10. doi: 10.1016/j.suronc.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4:137–140. doi: 10.1007/BF02303796. [DOI] [PubMed] [Google Scholar]
- 10.Houry S, Amenabar J, Rezvani A, Huguier M. Should patients over 80 years old be operated on for colorectal or gastric cancer? Hepatogastroenterology. 1994;41:521–525. [PubMed] [Google Scholar]
- 11.Takeda J, Tanaka T, Koufuji K, Kodama I, Tsuji Y, Kakegawa T. Gastric cancer surgery in patients aged at least 80 years old. Hepatogastroenterology. 1994;41:516–520. [PubMed] [Google Scholar]
- 12.Saito H, Kuroda H, Matsunaga T, Fukuda K, Tatebe S, Tsujitani S, et al. Prognostic indicators in node-negative advanced gastric cancer patients. J Surg Oncol. 2010;101:622–625. doi: 10.1002/jso.21562. [DOI] [PubMed] [Google Scholar]
- 13.Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–567. doi: 10.1007/BF02573892. [DOI] [PubMed] [Google Scholar]
- 14.Gabbert HE, Meier S, Gerharz CD, Hommel G. Incidence and prognostic significance of vascular invasion in 529 gastric-cancer patients. Int J Cancer. 1991;49:203–207. doi: 10.1002/ijc.2910490210. [DOI] [PubMed] [Google Scholar]
- 15.Reiter W, Stieber P, Reuter C, Nagel D, Cramer C, Pahl H, et al. Prognostic value of preoperative serum levels of CEA, CA 19-9 and CA 72-4 in gastric carcinoma. Anticancer Res. 1997;17:2903–2906. [PubMed] [Google Scholar]
- 16.Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 17.Kwon SJ, Lee WS, Kim HJ. Prognostic significance of tumor markers in sera and peritoneal washings in gastric cancer patients. J Korean Surg Soc. 2000;58:58–66. [Google Scholar]
- 18.Kim SY, Ha TK, Kwon SJ. Clinical significance of tumor markers in gastric cancer patients after curative resection. J Korean Gastric Cancer Assoc. 2009;9:136–142. [Google Scholar]
- 19.Bruno L, Nesi G, Montinaro F, Carassale G, Boddi V, Bechi P, et al. Clinicopathologic characteristics and outcome indicators in node-negative gastric cancer. J Surg Oncol. 2000;74:30–32. doi: 10.1002/1096-9098(200005)74:1<30::aid-jso7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Kooby DA, Suriawinata A, Klimstra DS, Brennan MF, Karpeh MS. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–835. doi: 10.1097/01.SLA.0000072260.77776.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maehara Y, Tomoda M, Tomisaki S, Ohmori M, Baba H, Akazawa K, et al. Surgical treatment and outcome for node-negative gastric cancer. Surgery. 1997;121:633–639. doi: 10.1016/s0039-6060(97)90051-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim DY, Seo KW, Joo JK, Park YK, Ryu SY, Kim HR, et al. Prognostic factors in patients with node-negative gastric carcinoma: a comparison with node-positive gastric carcinoma. World J Gastroenterol. 2006;12:1182–1186. doi: 10.3748/wjg.v12.i8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahvi DM, Krantz SB. Stomach. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston Textbook of Surgery. 19th ed. Philadelphia: ELSEVIER; 2012. pp. 1182–1226. [Google Scholar]