Abstract
Purpose
Clinical staging of gastric cancer appears to be important more and more for tailored therapy. This study aimed to verify the accuracy of clinical T staging in a low-volume institute.
Materials and Methods
We retrospectively reviewed prospectively collected data of gastric cancer patients who underwent resection. A total of 268 patients of gastric cancer were enrolled from March 2004 to June 2012. These demographics, tumor characteristics, and clinical stages were analyzed for identification of diagnostic value of clinical T staging.
Results
The predictive values for pT1 of endoscopy and computed tomography were 90.0% and 89.4%, respectively. In detail, the predictive values of endoscopy for pT1a, pT1b, and pT2 or more were 87%, 58.5%, and 90.6%, respectively. The predictive values of computed tomography for pT1a, pT1b, and pT2 or more were 68.8%, 73.9%, and 84.4%, respectively. The factors leading to underestimation of pT2 or more lesions by gastroscopy were the middle third location, the size greater than 2 cm, and younger age. Those for overestimation of pT1 lesion by computed tomography were male, age more than 70 years, elevated type, and size greater than 3 cm.
Conclusions
Diagnostic accuracy of early gastric cancer was 90%, which is comparable to those of high volume center. In patients with early gastric cancer, limited gastrectomy or minimal invasive surgery can be safely introduced at a low volume center also. However, the surgeon of low-volume institute should consider the accuracy of clinical staging before extending the indication of limited treatment.
Keywords: Stomach neoplasms; Neoplasm staging; Gastroscopy; Technology, radiologic
Introduction
Gastric cancer is one of the most common malignant diseases in worldwide, and 5-year survival rate has been reported to be approximately 27~52%.(1-3) Recently, early detection and proper treatment of gastric cancer make the treatment outcome better. The depth of intramural tumor invasion and spreading beyond the gastric wall, the involvement of lymph nodes, and distant metastases are the most important prognostic factors in gastric cancer.(2,4) Among these, local extent of gastric cancer (so called T stage) is the most important factor in prognosis and for selection of therapeutic modality.(5-7) Because of early detection and improvement of survival, various tailored and limited therapies for early gastric cancer (EGC) have been introduced in many studies.(2-5) Therefore, the accurate clinical staging is required for tailored therapies of gastric cancer.
In Korea, gastric cancer occurs evenly in all geographical areas, but most patients with gastric cancer undergo surgical treatment at large-volume centers of the metropolitan area.(8) However, not a few patients are treated at low volume hospitals due to economic efficiency or better accessibility. Nevertheless, few studies have reported on the accuracy of clinical staging in low-volume centers that less than 80 gastrectomies per year for gastric malignancy were performed in. Therefore, this study aimed to assess the diagnostic accuracy of clinical staging of single low volume institute assessed by gastroscopy and stomach protocol computed tomography (S-CT) in comparison with that reported in high volume hospitals.
Materials and Methods
1. Patient selection
From March 2004 to June 2012, total 268 patients who diagnosed as gastric adenocarcinoma underwent laparoscopic or open gastrectomy at the Jeju National University Hospital. Endoscopic resection was indicated in our hospital if the following criteria were met: tumor confined to the mucosa; the tumor size smaller than 2 cm by endoscopic measurement; well or moderately differentiated adenocarcinoma; and no evidence of lymph node or distant metastases on abdominal computed tomography (CT) or endoscopic ultrasonography (EUS). The gastric cancers were total 282 lesions that include 160 EGCs and 122 advanced gastric cancers (AGC). The specimens obtained by surgical resection were histopathologically evaluated, and this histopathological data were used as reference standards for the T staging. Histologic T staging was based on the 7th edition of American Joint Committee on Cancer staging system of gastric cancer.(9-11)
Authors' criteria of clinical T staging were adopted in gastrointestinal inter-department conference after review of published articles.(12,13) Most of these cases were discussed in preoperative inter-department weekly conference, and the clinical stage by gastroscopy and S-CT was determined in this conference. Endoscopic diagnosis of T stage was reviewed by three endoscopists for whether the diagnosis supports the criteria, and final diagnosis was made by consensus of three endoscopists. All S-CT images were reviewed by two experienced radiologists and clinical stage was made by their consensus. The endoscopists and radiologists were blinded to each other's finding and pathologic data.
2. Endoscopic staging
The endoscopes used in the study were mainly the GIF-H260 and GIF-Q260 (Olympus, Tokyo, Japan). A macroscopic classification of EGC lesions were classified as follows: type I (protruded), type IIa (superficial elevated), type IIb (flat), type IIc (superficial depressed), type III (excavated) and analyzed as two groups according to existence of ulceration.(12,13) Macroscopic classification of AGC lesions were followed as Borrmann's classification.
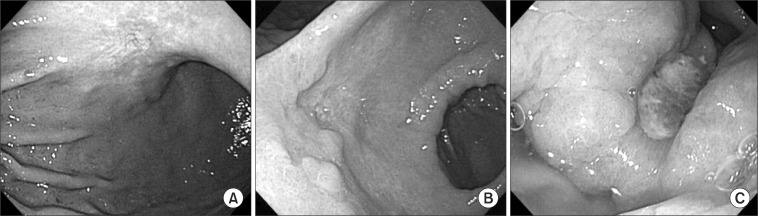
Endoscopic criteria for T staging were as follows (Fig. 1): criteria for mucosal (cT1a) cancer were a smooth surface protrusion, shallow and even depression with or without smooth tapering of converging folds, the erosion with slight marginal elevation, a flat or superficial spreading lesion, and a smooth surface protrusion with a size smaller than 3 cm (in the case of type I only); criteria for submucosal (cT1b) cancer were an irregular or nodular surface with or without abnormal converging folds such as clubbing, abrupt cutting, and fusion, subepithelial tumor-like protrusion without flexibility, deep ulceration with marked marginal elevation, and irregular protrusion (in the case of type I only); criteria for cT2 or more (AGC) were ulcerative lesions surrounded by a tumorous bank (dam formation) showing no distention after air inflation.(5,7)
Fig. 1.
Clinical T staging using gastroscopy. (A) A superficial spreading lesion with shallow and even depression corresponding to 'mucosa lesion'. (B) A deep ulceration with marked marginal elevation and abnormal converging folds corresponding to 'submucosa lesion'. (C) A ulcerative lesion surrounded by dam formation corresponding to 'advanced gastric cancer'.
3. S-CT staging
S-CT was performed with a 16 row multi-detector row computed tomography scanner (Sensation 16, Siemens Medical Systems, Erlangen, Germany) after administration of 10 mg of butyl scopolamine (Buscopan, Boehringer Ingelheim Korea, Seoul, Korea) and two packs of effervescent granules. The scanning protocol was as follows: 16×0.75 mm detector configuration; rotation time of 0.5 seconds; slice thickness of 1 mm; a pitch of 1.25; kVp and mAs of 120 and 160. Images were reconstructed at an interval of 0.7 mm for 3D imaging and 3 mm for clinical interpretation. CT images were obtained 70 seconds after injection of 120 ml of nonionic contrast material (iopromide, Ultravist 370, Schering, Berlin, Germany) at a rate of 3~4 ml/sec. During CT scanning, the patients were asked to be in 30° right anterior oblique position to obtain a better distension for the upper half of the stomach. After obtaining CT scan in a right anterior oblique position, the patients changed their postures to supine position to achieve an appropriate distension for the lower half of the stomach. CT images were then reconstructed using coronal and sagittal multiplanar reformation as well as axial images and 3D surface-shaded volume-rendering techniques. The radiologists used reconstructed images as well as transverse 2D images for the interpretation of T and N stages.
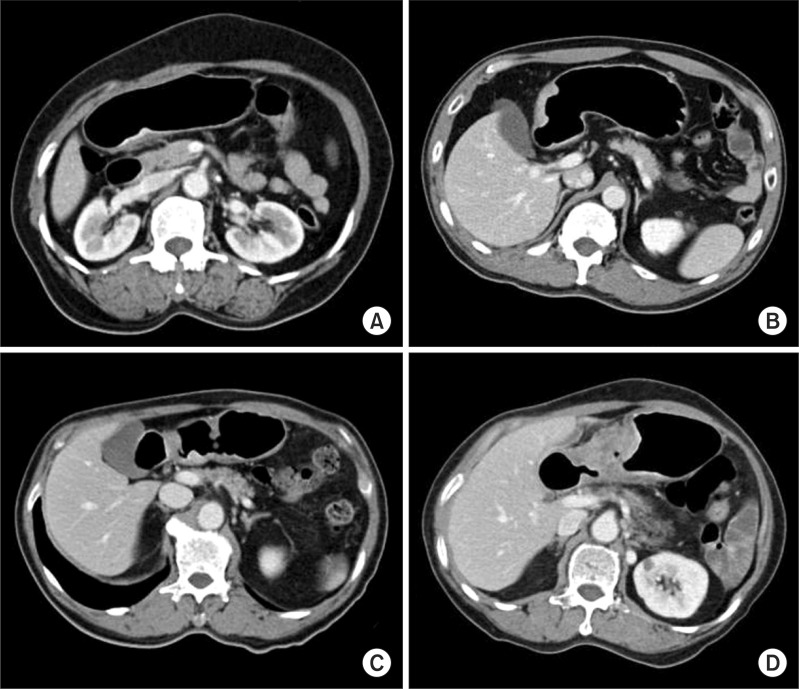
The clinical T stage of S-CT was determined by using the following criteria (Fig. 2): criteria for cT1a were a non-visualized lesion on CT gastrography, a visualized lesion without enhancing mucosal thickening, or the thickening of inner mucosal layer, as compared to the adjacent normal mucosal layer, with an intact low-density-stripe layer; criteria for cT1b were enhancing mucosal thickening into middle layer with intact outer layer or the disruption of low-density-stripe layer less than 50% of the thickness; criteria for cT2 were the disruption of low-density-stripe layer greater than 50% of the thickness without abutting on the outer layer, or a slightly high-attenuating or enhancing lesion that reaches into outer layer with smooth outline; criteria for subserosal tumor (cT3) were the impossibility of discrimination between enhancing gastric lesion and outer layer, and the presence of a smooth outer margin of the outer layer or a few small linear stranding in the perigastric fat plane; criteria for serosal tumor were gastric wall thickening with spiculation or nodular infiltration and preservation of fat plane between the gastric lesion and adjacent organ (definite cT4a) or with effacement of the fat plane without compression effect (probable cT4a); criteria for invasion to adjacent organ (cT4b tumor) were the obliteration of the fat plane between the gastric lesion and the adjacent organs (probable cT4b) or obvious tumor invasion (definite cT4b).(1,7,9,12)
Fig. 2.
Clinical T staging using stomach protocol computed tomography. (A) Enhancing mucosal thickening into middle layer with intact outer layers and a low-density-stripe layer corresponding to 'submucosal lesion'. (B) A enhancing lesion that reaches into outer layer with smooth outline corresponding to 'proper muscular lesion'. (C) Lesions without the discrimination between the enhancing gastric lesion and the outer layer with a few small linear stranding in the perigastric fat plane corresponding to 'subserosal lesion'. (D) Lesions with gastric wall thickening with spiculation and the preservation of fat plane between the gastric lesion and adjacent organ corresponding to 'serosal lesion'.
4. Statistical analysis
Quantitative results were expressed as the mean±standard deviation. Analysis was performed using chi-square test or Fisher's exact test for categorical variables. Multivariate analysis of the factors leading to under or over estimation was performed by Logistic regression test. The SPSS 11.0 software program (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. P-values of less than 0.05 were considered to indicate statistical significance.
Results
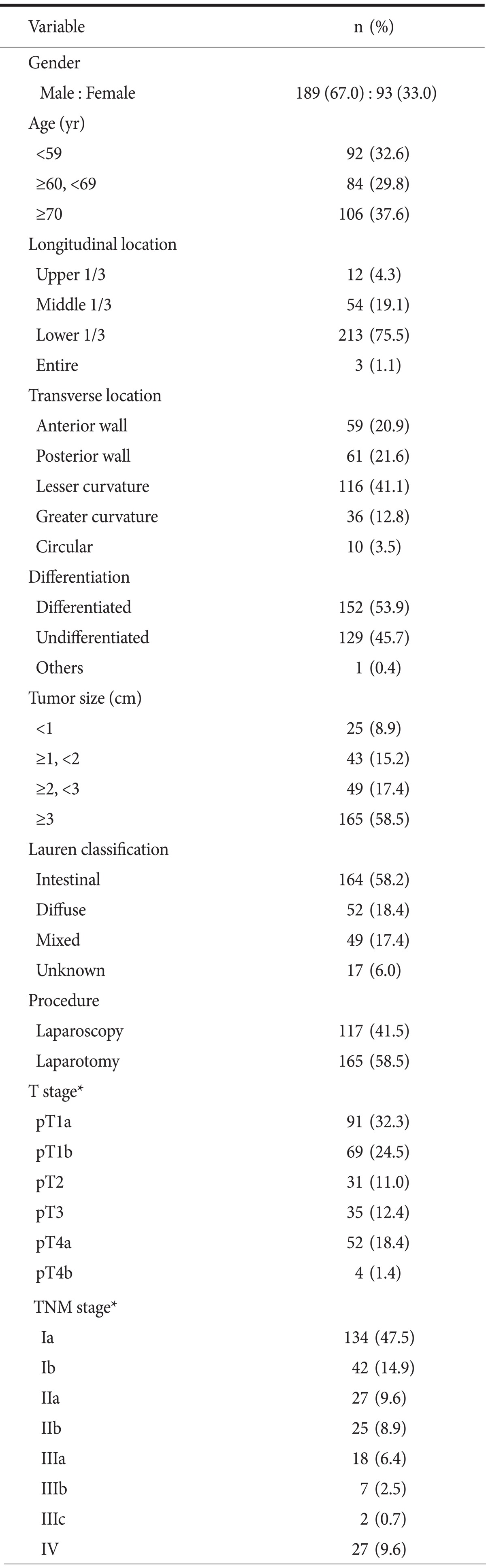
Clinical and pathologic characteristics of 282 lesions are summarized in Table 1. The study group consisted of 189 men and 93 women with a mean age of 64.4±11.4 years. Mean tumor size was 4.1±3.0 cm. 75.5% were located at lower third of stomach in longitudinal location and 41.1% were at lesser curvature in transverse location. Final diagnosis contains 160 EGC and 122 AGC lesions.
Table 1.
Demographic features of 282 gastric cancer lesions
*According to the 7th edition of the American Joint Committee on Cancer staging manual.
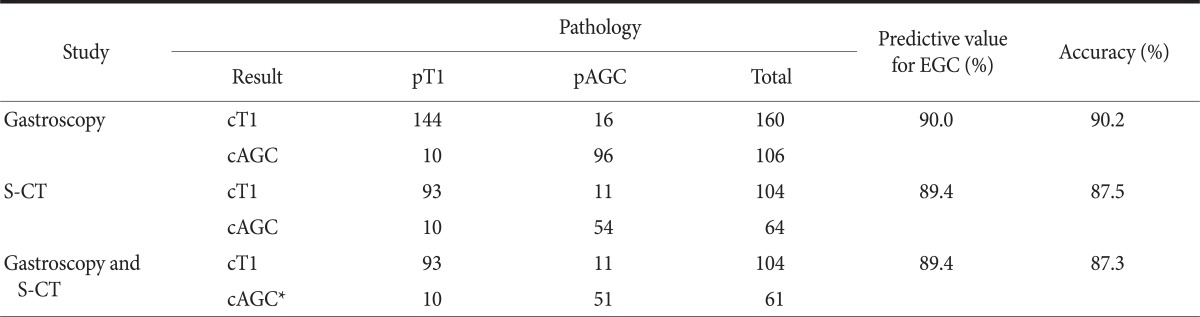
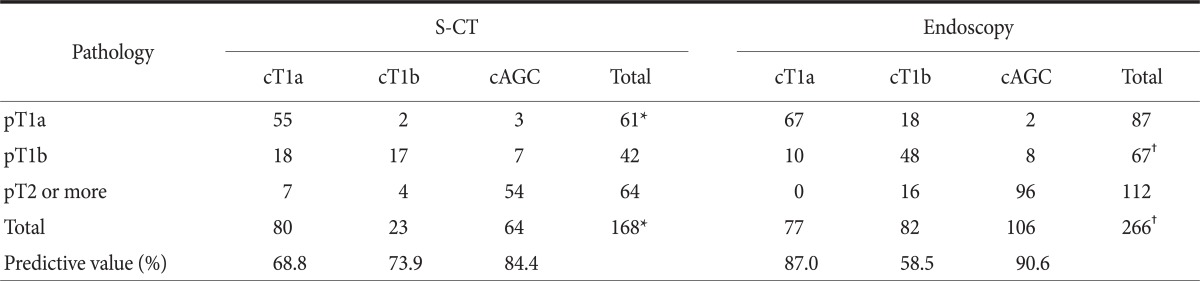
Of 282 lesions, 16 lesions were not able to be reviewed for the endoscopic T staging because of bad picture quality or no remained endoscopic picture, and these were excluded from endoscopic staging (Table 2). Of 266 lesions, 160 lesions were preoperatively diagnosed as EGC by gastroscopy. Predictive value and accuracy for EGC with gastroscopy was 90.0% and 90.2%, respectively. S-CT was taken in 168 lesions, and the cases examined by CT without stomach protocol were excluded from CT staging. Of these, 104 lesions were predicted as EGC. Predictive value and accuracy for EGC with S-CT were 89.4% and 87.5%, respectively. EGC lesions that were preoperatively diagnosed by both gastroscopy and S-CT were 104. Predictive value and accuracy with this combination method were 89.4% and 87.3%, respectively.
Table 2.
Results of gastroscopic and S-CT in preoperative determination of early gastric cancer
S-CT = stomach protocol computed tomography; AGC = advanced gastric cancer; EGC = early gastric cancer. *Diagnosis of clinical stage was AGC by one of studies or both of them.
The depth of invasion was estimated as cT1a, cT1b and cT2 or more lesions, and these were matched with pathologic result (Table 3). By S-CT, 90.2% of 61 pT1a lesions were cT1a and 8.2% were overestimated. One unidentified lesion of EGC was pT1a. Of 42 pT1b lesions, 42.8% were understaged and 16.6% were overstaged. In pathologic AGC lesions, 17.1% of 64 lesions were understaged as cT1. Overall accuracy rate of diagnosis by S-CT was 75.0%. By endoscopy, 22.9% of pT1a lesions were overstaged. 14.9% and 11.9% of pT1b lesions were understaged and overstaged, respectively. An EGC lesion that could not be distinguished cT1a from cT1b was submucosal cancer. 14.2% of AGC lesions were understaged as T1b. Overall accuracy rate of endoscopic detailed T staging was 79.3%.
Table 3.
Comparison of pathologic T staging with clinical T staging assessed by S-CT and endoscopy
S-CT = stomach protocol computed tomography; AGC = advanced gastric cancer. *One unidentified lesion (cT1a or cT1b) by S-CT was pT1a cancer, not shown in Table. †One unidentified lesion (cT1a or cT1b) by gastroscopy was pT1b cancer, not shown in Table.
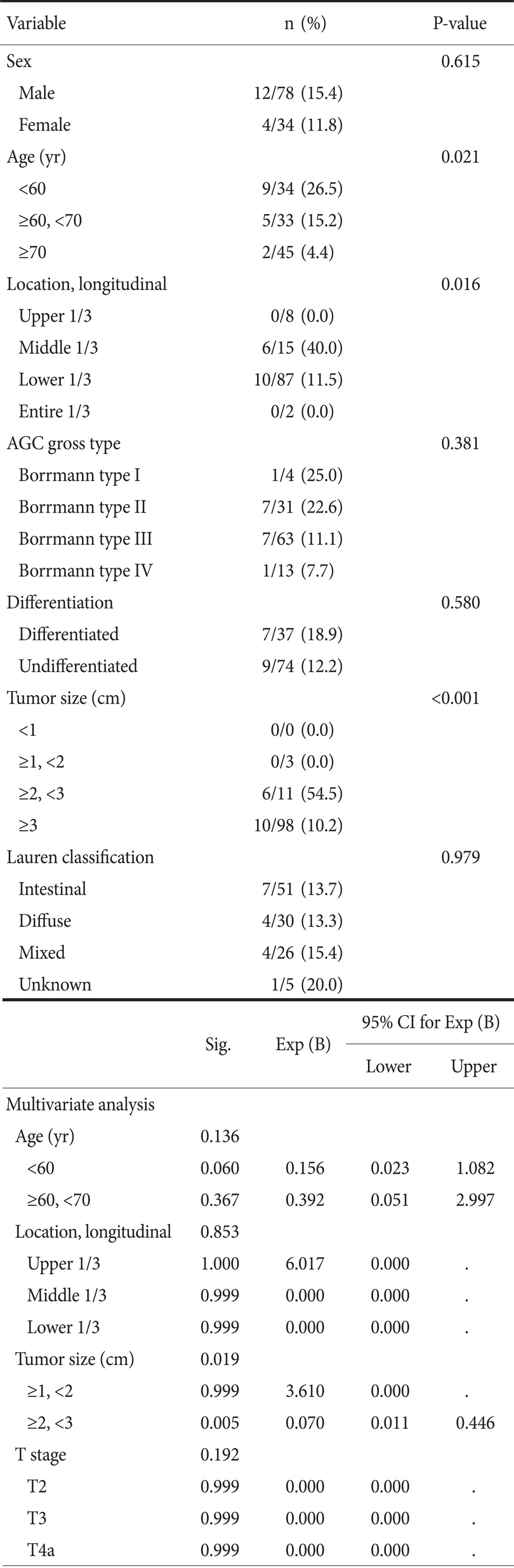
16 (14.3%) of 112 pathologic AGC lesions were understaged by gastroscopy (Table 4). Understaged patients were younger (P=0.021), and these lesions were located at middle thirds in longitudinal location (P=0.016) and these tumors' size tend to be larger than 2 cm in diameter (P=0.000). 9 (30%) of 30 proper muscle cancers were down-staged as cT1. In pT3 and pT4a cancers, 4 (12.9%) of 31 lesions and 3 (6.3%) of 47 lesions were down-staged as cT1, respectively. Lesions of proper muscle layer down-staged with statistical significance in comparison with other lesions (P=0.027). Multivariate analysis reveals that tumor size larger than 2 cm (P=0.024) is independent factor that affect underestimation by endoscopy. Age under 60 years tend to be underestimated but has no statistical significance.
Table 4.
The clinicopathological features of 16 patients who were diagnosed as cT1 by gastroscopy among 112 patients with pT2 or more according to clinicopathological factors
CI = confidence interval; AGC = advanced gastric cancer.
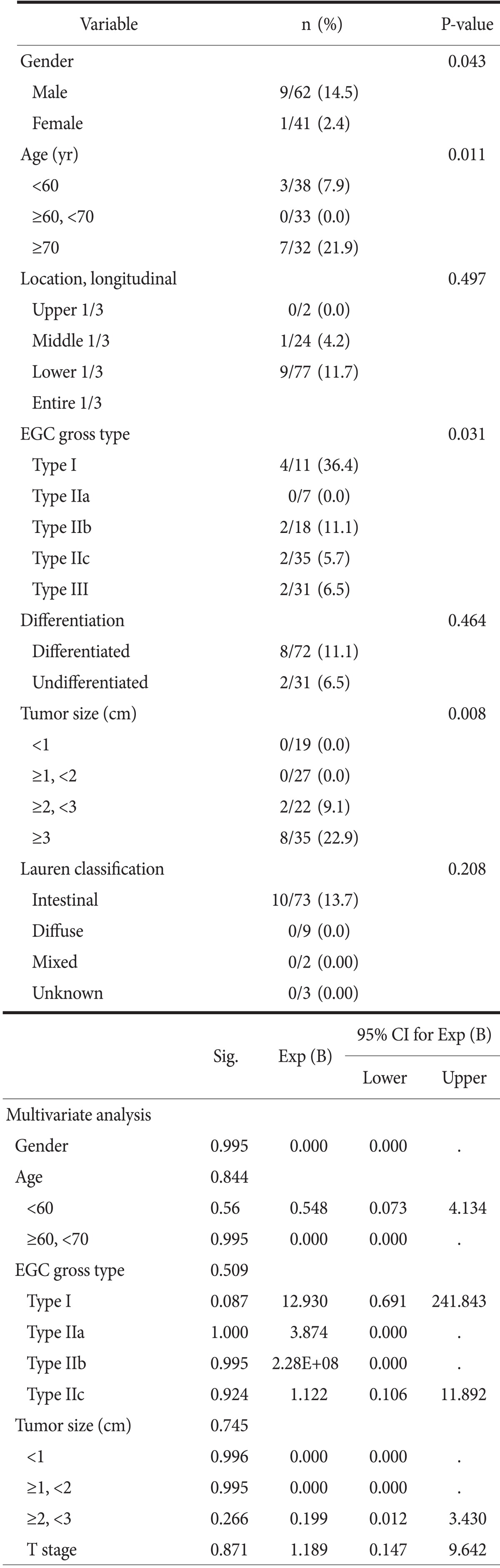
In pT1 cancers, overstaged lesions as AGC by S-CT were 10 cases (9.7%) and those characteristics are shown in Table 5. The lesions with male gender (P=0.043), age older than 70 years (P=0.011), macroscopic type I (P=0.031), or tumor size larger than 3 cm (P=0.008) are tend to be overestimated. Overestimated pT1a cancers were 3 (4.9%) of 61 lesions, and these of pT1b cancers were 7 (16.7%) of 42 lesions (P=0.048). However, there are no factors with statistical significance on multivariate analysis.
Table 5.
The clinicopathological features of 10 patients who were diagnosed as cT2 or more by S-CT among 103 patients with pT1 according to clinicopathological factors
S-CT = stomach protocol computed tomography; CI = confidence interval; EGC = early gastric cancer.
Discussion
EGC is on the rise due to improvements in diagnostic methods and changes in the concept of routine health examinations. Because of early detection and improvement of survival, various tailored and limited therapies for EGC have been introduced.(2-5,14) In EGC, as less invasive treatment is favored, endoscopic or laparoscopic treatment is widely accepted and concerned as a minimally invasive treatment modality.(14,15) For these minimally invasive treatment, preoperative clinical staging is very important for determining the treatment plans and predicting the prognosis.(1,5-7,15)
Patients who undergo gastric surgery at low volume institute are tend to be older, in lower socioeconomic state, and residents of rural area rather than metropolitan area.(16) Therefore, the patients have longer length of hospital stay and need more costs. There are a few reports about the surgical outcome of low volume hospital with acceptable result, but few studies report the diagnostic performance of low volume institute.(8) Therefore, authors evaluated diagnostic accuracy of clinical staging assessed by gastroscopy and S-CT of single low volume institute in comparison with high volume hospitals.
There have been not a few reports about diagnostic accuracy of gastroscopy and S-CT from high volume tertiary hospitals. Ahn et al.(7) demonstrated that predictive value and accuracy for EGC with gastroscopy were 87.4% and 83.4%, respectively. Predictive value and accuracy for EGC with S-CT were reported as 78~92.2% and 81.3~86.4%, respectively.(7,15) In this study, predictive value and accuracy for EGC with gastroscopy was 90.0% and 90.2%, and with S-CT were 89.4% and 87.5%, respectively. In our institute, diagnosing the gastric cancer lesion as EGC versus AGC, predictive value and accuracy were similar to those of other reports.
In detail, the reported predictive value and accuracy for T1a with gastroscopy were 82.0~83.2% and 73.7~82.7%, respectively, and for T1b with gastroscopy were 58.7~71.9% and 73.7~82.0%, respectively.(9) With S-CT, their predictive value and accuracy for T1a were 83.3~88.6% and 91.3~92.9%, respectively, and for T1b with S-CT were 87.1~92.0% and 90.6~92.1%, respectively.(9) However, when it was evaluated for detailed stages, diagnostic power for T1a and T1b lesions were decreased with S-CT comparing with those of high volume institutes. With gastroscopy, in this study, predictive value and accuracy for T1a were 87.0% and 77.0%, respectively, and for T1b lesions, predictive value and accuracy were 58.5% and 71.6%, respectively. With S-CT, our predictive value and accuracy for T1a were 68.8% and 90.2%, respectively, and for T1b lesions predictive value and accuracy were 73.9% and 40.5%, respectively.
Other studies have reported that the combined use of endoscopy and EUS was effective in predicting T stage, but EUS examination requires additional time and expense.(17,18) Overall accuracy for EGC of EUS in high volume hospitals was 76.2~95.6%. Their predictive value and accuracy of EUS for T1a lesions have reported as 77.8~80.3% and 69.0~79.9%, respectively. For T1b lesions, their predictive value and accuracy were reported as 38.5~51.6% and 68.5~79.6% respectively.(17,18) In our hospital, EUS was performed by three endoscopists from March 2009 and total 52 gastric cancers were evaluated. The overall accuracy for EGC of EUS was 88.5%. EGC lesions were correctly estimated in 37 (88%) of 42 lesions, AGC lesions were correctly estimated in 9 (90%) of 10 lesions. T1a lesion was correctly predicted in 12 (80%) of 15 lesions, and T1b and AGC lesions were correctly predicted in 11 (47.8%) of 23 lesions and 5 (35.7%) of 14 lesions, respectively. Overall accuracy rate for detailed T staging by EUS was 53.8%. Diagnostic accuracy of EUS for mucosal and submucosal cancer was similar to that of other reports of high volume centers. However, for submucosal cancer, the predictive value was lower than that of mucosal cancer regardless of the hospital volume. With the result, when we planning a limited resection on the basis of invasion depth, the risk for diagnostic accuracy must be considered.
Ahn et al.(7) demonstrated that the factors leading to underestimation of T stage with endoscopy were the upper third location, the size greater than 2 cm, and diffuse type of tumor. Those with S-CT were female sex, the upper third location and lesion size greater than 2 cm. Choi et al.(18) reported that clinicopathologic factors affecting the diagnostic accuracy of endoscopy were upper and middle third location, the size of greater than 2 cm and submucosal invasion.
Clinical T stage of gastric cancer, which is classified as cT1a, cT1b and over cT2, is the most important factor that affects the decision of therapeutic modality. According to this classification, endoscopic diagnosis of this study was most accurate in predicting the AGC lesions, and S-CT diagnosis was too. T1b lesions were more accurately diagnosed by S-CT than endoscopy. These results are different from clinical tendency that clinicians give more consideration to endoscopic result than S-CT result in EGC and S-CT result than endoscopic result in AGC. So we analyzed risk factors of misdiagnosis when clinicians follow the tendency. In this study, 16 lesions cT1 by gastroscopy in 112 pathologic AGC were analyzed. The risk factors for underestimation of AGC by endoscopy were younger age, T2 lesions and tumor size larger than 2 cm. Multivariate analysis shows no significance in those factors but tumor size 2 to 3 cm. 10 lesions clinical AGC by S-CT in 103 pT1 lesions were analyzed and male gender, macroscopic type I EGC, tumor size larger than 3 cm and T1b lesions are the factors that affect overstaging by S-CT. And by multivariate analysis there were no significant statistical values. These results may be due to small number of cases. When establishing the preoperative clinical staging, these factors must be considered.
This study has limitations. First, it is a retrospective study during long period of 8 years. Second, the estimation of diagnostic accuracy of clinical N staging was not done in this study. Further evaluation will be needed about clinical N stage. Third, the number of patient who underwent EUS was not sufficient because the examination was possible from March 2009. Further evaluation will be needed about diagnostic performance of EUS of more patients.
In conclusions, diagnostic accuracy of EGC was 90%, which is comparable to those of high volume center. In patients with EGC, limited gastrectomy or minimal invasive surgery can be safely introduced at a low volume center also. However, the surgeon of low-volume institute should consider the accuracy of clinical staging before extending the indication of limited treatment.
References
- 1.Kumano S, Murakami T, Kim T, Hori M, Iannaccone R, Nakata S, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology. 2005;237:961–966. doi: 10.1148/radiol.2373041380. [DOI] [PubMed] [Google Scholar]
- 2.Cidón EU, Cuenca IJ. Gastric adenocarcinoma: is computed tomography (CT) useful in preoperative staging? Clin Med Oncol. 2009;3:91–97. doi: 10.4137/cmo.s2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, et al. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg. 2010;14:958–964. doi: 10.1007/s11605-010-1195-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917–927. doi: 10.1016/j.gie.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 6.Park JM, Ahn CW, Yi X, Hur H, Lee KM, Cho YK, et al. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11:109–115. doi: 10.5230/jgc.2011.11.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim SH, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–27. doi: 10.1002/jso.21170. [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Park J, Kim SG, Choi S, Yoon S, Lee S. Feasibility of gastric cancer surgery at low volume hospitals. J Gastric Cancer. 2010;10:234–240. doi: 10.5230/jgc.2010.10.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, Park YK, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. 2012;22:654–662. doi: 10.1007/s00330-011-2283-3. [DOI] [PubMed] [Google Scholar]
- 10.Santiago JM, Sasako M, Osorio J. TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer. Cir Esp. 2011;89:275–281. doi: 10.1016/j.ciresp.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Dikken JL, van de Velde CJ, Gönen M, Verheij M, Brennan MF, Coit DG. The New American Joint Committee on Cancer/International Union Against Cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy. Ann Surg Oncol. 2012;19:2443–2451. doi: 10.1245/s10434-012-2403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59:619–626. doi: 10.1016/s0016-5107(04)00169-5. [DOI] [PubMed] [Google Scholar]
- 13.Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997;46:212–216. doi: 10.1016/s0016-5107(97)70088-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang SJ, Ahn EJ, Park SH, Kim JH, Park JM. The early experience of laparoscopy-assisted gastrectomy for gastric cancer at a low-volume center. J Gastric Cancer. 2010;10:241–246. doi: 10.5230/jgc.2010.10.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin RM, Lee JH, Lee MS, Park DJ, Kim HH, Yang HK, et al. Analysis of diagnostic performance of CT and EUS for clinical TN staging of gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:177–185. [Google Scholar]
- 16.Lee JA, Park JH, Lee EJ, Kim SY, Kim Y, Lee SI. High-quality, low-cost gastrectomy care at high-volume hospitals: results from a population-based study in South Korea. Arch Surg. 2011;146:930–936. doi: 10.1001/archsurg.2011.81. [DOI] [PubMed] [Google Scholar]
- 17.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Is endoscopic ultrasonography indispensable in patients with early gastric cancer prior to endoscopic resection? Surg Endosc. 2010;24:3177–3185. doi: 10.1007/s00464-010-1112-0. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–713. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]