Abstract
Orienting in large-scale space depends on the interaction of environmental experience and pre-configured, possibly innate, constructs. Place, head-direction and grid cells in the hippocampal formation provide allocentric representations of space. Here we show how these cognitive representations emerge and develop as rat pups first begin to explore their environment. Directional, locational and rhythmic organization of firing are present during initial exploration, including adult-like directional firing. The stability and precision of place cell firing continues to develop throughout juvenility. Stable grid cell firing appears later but matures rapidly to adult levels. Our results demonstrate the presence of three neuronal representations of space prior to extensive experience, and show how they develop with age.
The hippocampal cognitive map has been proposed as a Kantian synthetic a priori system, partly or wholly formed genetically, to serve as a scaffold for representing experiential information about the external environment (1). This suggests that the basic constituents of the cognitive map develop independently of spatial experience, or might even precede it, and is supported by the early development of spatial cognition in weanling rats (2-4). We tested this idea by looking for place cells (5) in hippocampal region CA1, and for grid (6) and directional cells (7) in medial entorhinal cortex (MEC) as pre-weanling rats first began to leave the nest and actively explore their environment ((8),typically on postnatal day 16 ‘P16’ in our experiment; see Fig. S1 for typical recording locations). We recorded 567 hippocampal pyramidal cells and 1514 medial entorhinal cells from 42 male rats between the ages of P16 and P30 as they foraged for food in an enclosure, using miniaturized microdrives and recording locations matched across ages (Fig. S2C-E). Cells were categorized as directional, place or grid cells if their spatial firing characteristics exceeded the 0.05 chance level of the relevant measures (spatial information (9) for place cells, gridness (6, 10) for grid cells, Rayleigh vector for directional cells) in spatially-shuffled data for the corresponding age and region (11).
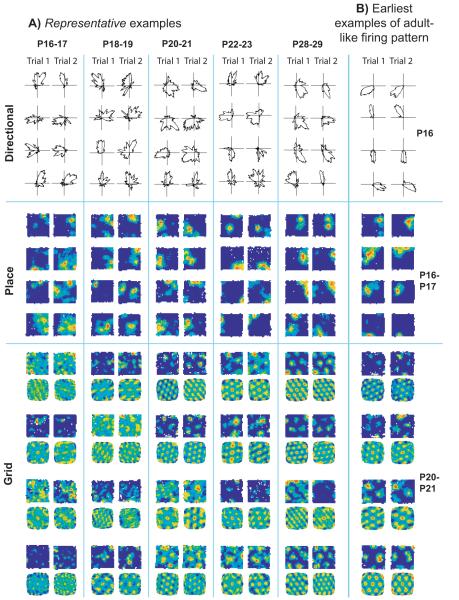
When do the three types of spatial firing first appear, and how do they develop with age? Fig. 1 shows (A) representative (see Fig 1 legend) examples of cells recorded across ages P16 to P29 and (B) the earliest examples of stable adult-like firing found in directional (P16), place (P16-17) and grid cells (P20-21), see Figs. S3-6 for the complete data set and Fig. S7 for the distributions of the relevant measures across all cells recorded in MEC and CA1. Adult-like proportions of directional cells were present from the earliest age point and these cells exhibited adult-like stability and quality (Figs. 2A and 3A. See S8A, B for statistical analysis). Somewhat higher proportions of directional cells were found near the border with parasubiculum (Fig. S9) than in the heart of MEC, but did not increase with age in either region. Interestingly, directional tuning was also tighter in this border region (Fig. S9C). This strong directional signal may originate in the presubicular head-direction cells, as preliminary recordings from this region revealed adult-like directional cells at very early ages (P14) there too (Fig. S9D-E).
Fig 1.
Development of directional, place, and grid cell firing with age. a) Four representative examples of each type of firing are shown across a range of ages for two consecutive trials (left and right columns): polar plots for directional firing, firing rate maps for place cells, firing rate maps (above) and spatial autocorrelograms (below) for grid cells. Example cells were chosen to have mean values of both the spatial firing criterion variable and inter-trial stability within one standard deviation of the appropriate group mean. b) The earliest examples of directional, place and grid firing corresponding to the classical examples found in adults.
Fig 2.
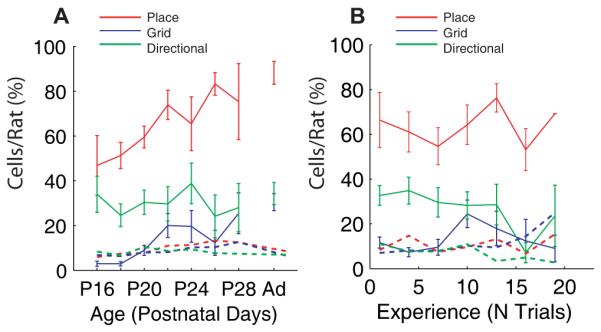
The proportions of cells per rat fulfilling the criteria for directional (green), place red), and grid (blue) cells as a function of age (a) or experience of the testing environment (b). Solid lines represent the mean percentage of cells per rat that fulfilled the spatial firing criteria, error bars represent ± S.E.M. over all rats. Dotted lines represent the p = 0.05 level for the mean percentage of cells for each cell type based on spike-shuffled data (see Supporting Methods).
Fig 3.
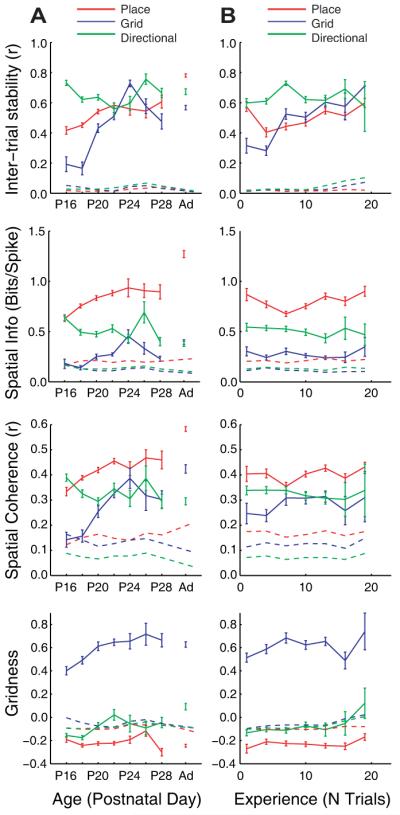
Spatial firing properties of the directional (green), place (red) and grid cells (blue) as a function of age (a) and experience of the testing environment (b). Shown (from top to bottom) are the stability (spatial correlation trial-to-trial), quality (locational or directional spatial information per spike), spatial coherence, and gridness of firing of the three cell types. For summary statistics, see Figure S8A. Dotted lines represent the p = 0.05 level of expected mean value of spatiality for each cell type based on spike-shuffled data, solid lines represent the mean over cells (± S.E.M.).
Significant proportions of place cells are also present initially (Fig. 2), but continue to increase towards adult levels throughout development. Although cells with multi-peaked firing were also found early in MEC, significant proportions of classical adult-like grid cells first appeared at around P20, and increased rapidly to near-adult levels by P22 (Fig. S8B).
A critical attribute of spatial representations is their stability over time which provides a reliable environmental representation suitable for long-term memory (12). The correlation of firing rate maps from one trial to the next is typically 0.6 or higher for all three cell types in the adult (see Fig. 3a). Stability measures during development show the same pattern as proportions of cells: directional firing has adult-like stability from the earliest age; stable place cell firing is also present initially but continues to improve throughout development; and stable grid cell firing is initially almost non-existent but develops rapidly at around P20 (Figs. 3A and S8A, B).
The quality of spatial encoding can be fairly compared across cell types by using the spatial information per spike (regarding location for place and grid cells and direction for directional cells (9)). As with the proportions of cells and stability of firing, the quality of spatial encoding is adult-like from the earliest age in directional cells, significantly high in early place cell firing but increasing throughout development, and reaches adult levels in grid cells at P24 (Figs. 3A and S8A, B).
We are able to dissociate the effects of age from effects of experience in the testing environment because we began recording from different animals at different ages. Effects of experience on place cell stability at P18-19, and grid cell firing stability at P22-30 were seen but these were weaker than the corresponding increases with age. All other effects reflect age alone (Figs 2B, 3B and S8A; Fig. S10 shows the independent effects of age and experience separately).
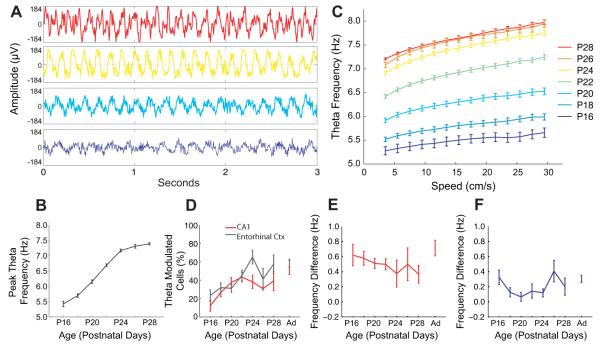
In adult rats, the firing of place and grid cells is carefully timed to the theta rhythm (‘phase precession’(13, 14)) and theta oscillations may play a role in the creation of place (13) and grid (15, 16) firing. How does the temporal organization of the hippocampal formation develop? As the rat pups moved around the environment, theta activity was present in the local field potential at the earliest recording time point of P16. Theta frequency increased with age and with running speed (as in adults (17, 18)). Theta amplitude showed a similar profile. See Fig. 4 and also (19). Both the slope and intercept of the frequency-speed relation increased with age (P < 0.001, Fig. 4C). Significant proportions of cells in CA1 and MEC showed theta modulated firing from the earliest ages. The proportions in each region were approximately equal, started from around 20% at P16 and were not significantly different from adult levels by P22 (Fig. 4D).
Fig 4.
Movement-related theta rhythmicity of the local field potential (LFP) and cell firing. a-c) LFP theta is present at the earliest age recorded, increases in frequency with age (a, b), and increases with running speed at every age (c). Samples of LFP (a) and mean theta frequency for periods of translational motion are shown versus age (b) and running speed (c). Error bars show S.E.M., a) and c) are color coded by age (key top right). d) Theta-modulated firing is present at the earliest ages in CA1 and MEC, and the proportions of theta-modulated cells increases with age in both areas (CA1: r = 0.34, p = 0.01; MEC: r = 0.36, p < 0.001), and are not significantly different from adult by P22 (p = 0.74, Tukey’s HSD multiple comparisons). The theta-band modulation of firing in CA1 place cells (e) and entorhinal grid cells (f) is higher in frequency than the ongoing LFP theta, and the frequency difference does not change significantly with age (Place Cells: r = 0.05, p = 0.28; Grids: r = 0.08, p = 0.32).
Finally, the frequency of modulation (indicated by the power spectrum of the spike-train autocorrelogram) of grid and place cell firing was slightly higher than the simultaneously recorded local field potential theta (Fig. 4E,F), consistent with the presence of phase precession. We did not systematically record on the linear track to test for this, but can report one observation of phase precession in 5 CA1 cells as early as P17 (Fig. S11).
Could any of our results reflect changes in behavior, such as speed of movement (17, 18)? Speed of movement initially slows between P16 and P20 and then rises steadily producing a u-shaped pattern with age (Fig. S12A), mirrored by an n-shaped pattern in the number and duration of periods of immobility (Fig. S12B-D). Coverage of the environment and distribution of heading directions did not vary across the recording period (Fig. S13). The observed monotonic changes in spatiality seem unlikely to reflect these factors, which we confirmed by filtering the data to equate median speeds across all ages (Fig. S12E-G). Changes in the quality of tetrode recordings with age can also be discounted (Fig. S2F-G), as can changes in more general properties such as firing rates or percentage of complex spikes (Fig. S14).
Because early directional firing is stable relative to the environment, it must use environmental cues as well as interoceptive inputs. In addition, directional modulation remains parallel across the whole environment, even during the rat’s first ever exploration of the recording environment (Fig. S15A), indicating that, as in the adult (7), it does not reflect a simple association to a single cue. Furthermore, when tested in 2 visually different environments, the preferred directions of simultaneously recorded cells rotated together coherently (Fig. S15B-C). These observations suggest the presence of functional sensory input to MEC at P16, and that directional firing could not be produced solely by experience-dependent plasticity driven by movements within the nest prior to exploration. The timing of development of the different spatial cell types suggests that ontogeny recapitulates phylogeny insofar as the directional signal originates in the brainstem, the place signal originates in archicortex (i.e. hippocampus proper) and the grid cells originate in neo/transcortex.
Stable directional firing, place cell firing and theta-band temporal organization occur prior to significant proportions of adult-like grid cells. Thus these three factors may combine in the creation of stable adult-like grid cell firing (15, 16) around P20-24, in concert with physiological development (20) and spatial experience during P16-22. Once formed, simultaneously-recorded grids have similar wavelengths and orientations, as in the adult (6) (Fig. S16), consistent with the presence of coherent ensembles (21). In addition, the appearance of place cell firing with trial-to-trial stability prior to stable grid cell firing implies that place cell firing can be driven by inputs other than grid cells, including environmental inputs, such as boundary vector cells (22-24) or local cues (24), acting together with the (highly stable) directional cells. Our results, together with evidence that place cell firing is not abolished by entorhinal cortex lesions (25), call into question the hypothesis that EC grid cells are the only spatial input to the place cells. Both place and grid cell firing continues to develop after P20, and more rapidly for grid than place cells. Although both systems appear to be interdependent in the adult (26), their differential developmental time courses suggests that interconnectivity develops after pups begin to explore. For example, the presence of theta-modulated firing in both regions at the earliest ages suggests that each area possesses oscillatory machinery, such as that required for phase precession (16, 27). Controlled rearing studies will be required to further disentangle the dependencies of these different spatial representations on each other and on experiential and innate processes.
Our results put the development of the place and directional systems considerably earlier than previously reported (28) and have general implications for the interaction of innate and experientally-acquired knowledge in spatial cognition. The fact that the expression of some types of spatial learning ability continues to improve for a long time after the components of the cognitive map are relatively mature suggests that the rate limiting step may be the use of spatial signals by the rest of the brain. On the other hand, some types of spatial behavior in rats such as spatial orientation based on enclosure geometry (29) is likely directly controlled by head-direction cells, so that our evidence for a very early, perhaps pre-configured, directional firing would be consistent with the very early appearance of this behavior in humans (30).
Supplementary Material
Acknowledgments
We thank Caswell Barry for use of adult grid cell data, Ali Jeewajee and Caswell Barry for assistance with data analysis, Stephen Burton and Rosa Varriale for technical assistance, Colin Lever and Julija Krupic for helpful discussions. This work was supported by the EU SpaceBrain grant, the Wellcome Trust, the Medical Research Council, UK, the Royal Society and a Research Councils UK academic fellowship to FC.
References and Notes
- 1.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; 1978. [Google Scholar]
- 2.Schenk F. Behav. Neural Biol. 1985;43:69. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- 3.Rudy JW, Stadler-Morris S, Albert P. Behav. Neurosci. 1987;101:62. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Nadel L, Wilson L, Kurtz EM. In: Developmental time and timing. Turkewitz G, Devenny DA, editors. Erlbaum; Hillsdale,NJ: 2009. pp. 233–252. [Google Scholar]
- 5.O’Keefe J, Dostrovsky J. Brain Res. 1971;34:171. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 6.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Nature. 2005;436:801. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 7.Taube JS, Muller RU, Ranck JB., Jr. J. Neurosci. 1990;10:420. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerrish CJ, Alberts JR. Dev. Psychobiol. 1996;29:483. doi: 10.1002/(SICI)1098-2302(199609)29:6<483::AID-DEV1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. Adv. Neural Inf. Process Syst. vol. 5. Morgan Kaufmann; San Mateo, CA: 1993. pp. 1030–1037. [Google Scholar]
- 10.Barry C, Hayman R, Burgess N, Jeffery KJ. Nat. Neurosci. 2007;10:682. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- 11.Materials and methods are available as supporting material on Science online.
- 12.Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Nature. 2002;416:90. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- 13.O’Keefe J, Recce ML. Hippocampus. 1993;3:317. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 14.Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Nature. 2008;453:1248. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- 15.Burgess N, Barry C, O’Keefe J. Hippocampus. 2007;17:801. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. Science. 2007;315:1719. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivas J, Gaztelu JM, Garcia-Austt E. Exp. Brain Res. 1996;108:113. doi: 10.1007/BF00242908. [DOI] [PubMed] [Google Scholar]
- 18.Jeewajee A, Barry C, O’Keefe J, Burgess N. Hippocampus. 2008;18:1175. doi: 10.1002/hipo.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblanc MO, Bland BH. Exp. Neurol. 1979;66:220. doi: 10.1016/0014-4886(79)90076-1. [DOI] [PubMed] [Google Scholar]
- 20.Burton BG, Economo MN, Lee GJ, White JA. J. Neurophysiol. 2008;100:3144. doi: 10.1152/jn.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Nat. Rev. Neurosci. 2006;7:663. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 22.Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. J. Neurosci. 2009;29:9771. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Science. 2008;322:1865. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 24.Savelli F, Yoganarasimha D, Knierim JJ. Hippocampus. 2008;18:1270. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Cauter T, Poucet B, Save E. Eur. J. Neurosci. 2008;27:1933. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- 26.Brun VH, et al. Neuron. 2008;57:290. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Harvey CD, Collman F, Dombeck DA, Tank DW. Nature. 2009;461:941. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin PD, Berthoz A. Hippocampus. 2002;12:465. doi: 10.1002/hipo.10021. [DOI] [PubMed] [Google Scholar]
- 29.Cheng K. Cognition. 1986;23:149. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 30.Hermer L, Spelke ES. Nature. 1994;370:57. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.