Abstract
Mucinous breast cancer (MBC) is mainly a disease of postmenopausal women. Pure MBC is rare and augurs a good prognosis. In contrast, MBC mixed with other histological subtypes of invasive disease loses the more favorable prognosis. Because of the relative rarity of pure MBC, little is known about its cell and tumor biology and relationship to invasive disease of other subtypes. We have now developed a human breast cancer cell line called BCK4, in which we can control the behavior of MBC. BCK4 cells were derived from a patient whose poorly differentiated primary tumor was treated with chemotherapy, radiation and tamoxifen. Malignant cells from a recurrent pleural effusion were xenografted in mammary glands of a nude mouse. Cells from the solid tumor xenograft were propagated in culture to generate the BCK4 cell line. Multiple marker and chromosome analyses demonstrate that BCK4 cells are human, near diploid and luminal, expressing functional estrogen, androgen, and progesterone receptors. When xenografted back into immunocompromised cycling mice, BCK4 cells grow into small pure MBC. However, if mice are supplemented with continuous estradiol, tumors switch to invasive lobular carcinoma (ILC) with mucinous features (mixed MBC), and growth is markedly accelerated. Tamoxifen prevents the expansion of this more invasive component. The unexpected ability of estrogens to convert pure MBC into mixed MBC with ILC may explain the rarity of the pure disease in premenopausal women. These studies show that MBC can be derived from lobular precursors and that BCK4 cells are new, unique models to study the phenotypic plasticity, hormonal regulation, optimal therapeutic interventions, and metastatic patterns of MBC.
Keywords: Mucinous breast cancer, Hormone receptors, Invasive lobular carcinoma, Xenografts, Estrogen
Introduction
Most invasive breast cancers are categorized as ductal (IDC), lobular (ILC), or a mixture of the two, but distinguishing among these can be challenging. Fifty to 80 % of breast cancers are classified as IDC “not otherwise specified” (IDC NOS) [1]. ILC comprise only 3–14 % of invasive breast cancers [2–5]. The incidence of ILC has increased over the past 20 years in women over 50 [6] potentially due to use of hormone replacement therapy [7, 8]. Compared to IDC, ILC occurs in older women and tends to be (1) larger at diagnosis; (2) estrogen receptor (ER) positive; (3) more differentiated; and (4) less vascularized [9]. Molecular discriminators between ILC and IDC include the lack of E-cadherin and positive cytoplasmic expression of delta catenin (p120 catenin) in ILC. Expression profiling has been performed to define markers unique to ILC and IDC [10–13] but the only common discriminator between the two tumor types appears to be loss of E-cadherin. Nevertheless, loss of E-cadherin alone is not sufficient to define ILC, as ~15 % of IDC also lack E-cadherin [14].
Among all breast cancers, 25 % contain phenotypic markers that allow sub-classification into rarer subtypes including apocrine, sebaceous, tubular, and mucinous breast cancers (MBC; also called colloidal or gelatinous). The definition of MBC is complex [1]. The World Health Organization does not recognize mucinous components if the non-mucinous invasive disease exceeds 50 %. If the non-mucinous component falls to 10–49 % then tumors are classified as “mixed,” and if the non-mucinous component is <10 % tumors are defined as “pure” MBC. The Royal College of Pathologists definition is similar; to be classified as pure MBC, ≥90 % of the tumor must be composed of epithelial cells floating in “a sea of” mucin [15].
The origin of “pure” and “mixed” MBC is unclear. Ductal versus mixed MBC is now largely referred to as a subtype of IDC, most often called “IDC with mucinous features” [16]. This is based on the assumption that extracellular mucin production is most often associated with IDC [1], despite the fact that ILC can also produce extracellular mucin [17–19]. Intracellular mucin production and/or signet ring cells are typically associated with an invasive component characteristic of ILC [5, 20–23]. However, in rare cases signet ring cells are associated with a variant of ductal carcinoma in situ [24] resembling diffuse gastric carcinomas [1]. Thus in mucinous disease, the distinctions between ILC and IDC remain unclear. The studies we describe here address these issues. Under current definitions, approximately 7 % of breast cancers in women over 75 years of age [25], and <1 % in women under 35, are classified as MBC; the overall rate is estimated to be 2–4 %. Because no clear definition of pure versus mixed MBC is used uniformly, their percent of all breast cancers is likely to be underestimated.
MBC are generally luminal-type tumors that are ER, progesterone (PR), and androgen (AR) receptor-positive, without HER2 overexpression and with a low S-phase fraction [26, 27]. Excessive extracellular mucin secretion is observed, but external lamina or basement membranes are not detected [28]. While mucin 1 (Muc1) is aberrantly overexpressed in ~90 % of all breast cancers [29], Muc2—a gel-forming secretory mucin expressed in the cytoplasm of pure and mixed MBC cases [30]—is not found in ILC and rarely in IDC [31], making it a good marker for mucinous components of all breast cancers. A diagnosis of MBC augurs a good prognosis with low probability of immediate metastasis [26]. However, both pure and mixed MBC can and do metastasize [32–37] with a propensity for late recurrences [35]. Invasive MBC has been diagnosed in association with ductal carcinoma in situ [38, 39] where it reportedly induces hyper-vascularization [39].
Cell lines are important models for studies of human breast cancer because they faithfully recapitulate gene expression patterns of tumors taken from patients [40]. Virtually all breast cancer lines currently in use are models of IDC, and ER+ cell lines that constitutively express PR protein in the absence of estradiol are rare. Models of ILC are also rare. To our knowledge only four—SUM44, MDA-MB-134 VI, IPH-926, and HCC2185—have been described, perhaps reflecting the rarity of this histologic subtype.
To date, no MBC cell lines exist of either the pure or mixed subtype. Here we report development of such a cell line we call “BCK4.” It was derived from a breast cancer patient who presented with a lymph node-positive, poorly differentiated adenocarcinoma. Her primary tumor was ER+, with PR and HER2 unknown. Following tumor resection, she received chemotherapy, radiation, and 5 years of tamoxifen. Three years later she was diagnosed with a recurrent pleural effusion and underwent therapeutic thoracentesis. BCK4 cells were developed from the pleural effusion. The cells are steroid receptor-positive, contain luminal cytokeratins, are of lobular origin, and grow into pure MBC when xenografted in the mammary glands of immunocompromised cycling mice. However, tumor growth is markedly accelerated and the phenotype switches to mixed MBC if mice are supplemented with continuous estradiol (E2). We propose that this effect of estrogens explains the rarity of pure MBC in premenopausal women and provides a mechanism through which estrogen-containing hormone replacement therapy stimulates the growth and progression of ILC. This new model will be extremely useful in studies of the molecular and tumor biology of MBC.
Materials and methods
Clinical background
The patient, a 65-year-old female, presented with a 3 cm, poorly differentiated adenocarcinoma with involved lymph nodes. The tumor was ER+; PR and HER2 status were unknown. After resection she received six cycles of cyclophosphamide, doxorubicin, and 5-fluorouracil, followed by radiation therapy and 5 years of tamoxifen. Three years after completing tamoxifen she presented with a malignant pleural effusion and underwent therapeutic thoracentesis. Immunocytochemical staining of the pleural cells showed them to be negative for ER (0 %) and HER2 (0 %) with focal PR expression (7 %). An Institutional Review Board approved the tissue acquisition protocol and patient-informed consent was obtained to acquire blood and tissue for research purposes.
Mouse explants of primary tumor cells and BCK4 development
All animal studies were performed according to a protocol approved by the University of Colorado Institutional Animal Care and Use Committee. Cells from the pleural effusion were transferred to flasks for 4 days to remove blood cells and other debris, then injected into mammary glands of ovariectomized nude mice supplemented with 17-beta-estradiol (E2) as described [41]. Tumors were grown for 12 weeks. Tumor xenografts were resected and cells were mechanically dispersed using a wire gauze and glass pestle. Cells were seeded into 6 well dishes, allowed to adhere and grown at 37 °C under 5 % CO2 in minimum essential medium (MEM) with Earle’s salts containing l-glutamine (292 µg/l) buffered with sodium bicarbonate (2.2 µg/l), insulin (6 ng/ml), non-essential amino acids (10 mM), and 5 % fetal calf serum. Cells were fed every 3–4 days. After ~14 months a stable cell line emerged, which was characterized and named BCK4. Short tandem repeat analysis performed at the University of Colorado Cancer Center Sequencing Core confirmed the human and unique genetic properties of the BCK4 cell line.
ZsGreen labeling of cells
BCK4 cells plated in a T75 flask were incubated with 15 ml of a retroviral vector encoding ZsGreen produced by Amphopack-293 cells (Clontech). Cells were transduced twice more and selected with G418. Green fluorescent cells were expanded and sorted for the brightest 10 % using a MoFlow cytometer as described [41].
BCK4 xenografts
ZsGreen-tagged BCK4 cells (5 × 106) in Matrigel were injected bilaterally into the 4th mammary glands of nonovariectomized NOD/SCID mice that had been implanted subcutaneously with a cellulose or E2-releasing pellet [41]. Tumors were grown for 22 weeks. Mice were injected intraperitoneally 2 h before sacrifice with 10 mg/kg of Bromodeoxyuridine (BrdU).
Three-dimensional (3D) assays
BCK4 cells (2 × 105) were plated onto Matrigel and treated with hormones. Colonies were paraffin embedded and sectioned as previously described [42] and stained with hematoxylin and eosin (H&E). Slides were imaged using a Nikon Eclipse E600 microscope (Nikon Corporation) coupled to an RGB-MS-C MicroColor camera (CRI, Inc.). Quantification of at least five fields and two wells per condition were performed to assess signet ring cell formation using Image Pro Plus 4.5 software (Media Cybernetics).
Immunocytochemistry (ICC) and antibodies
BCK4 cells were plated onto coverslips, fixed and ICC was performed as described [41]. Antibodies were: ER (Neomarkers, SP1) 1:100, PR (DAKO, 1294) 1:500, AR (Upstate, PG-21) 1:35, BrdU (BD Biosciences) 1:50, Her2 (Neomarkers, SP3) 1:50, CK5 (Novacastra, XM26) 1:100, CD10 (Epitomics, EP2998) 1:100, CK14 (Neomarkers) 1:200, CK18 (Calbiochem) 1:400, CK8/18 (Novocastra, 5D3) 1:100, CD117 (DAKO) 1:400, CD44 (Neomarkers 156-3C11) 1:200, EGFR (Epitomics E114) 1:12, E-Cadherin (DAKO) 1:25, p63 (Epitomics Y289) 1:250, Mammaglobin A (Abcam, 304-1A5) prediluted, Chromograinin A (Abcam) 1:60, Synaptophysin (Abcam) 1:100, Vimentin (Epitomics, SP20) 1:200, Vimentin (Abcam, V9) 1:50, α-SMA (Epitomics, E184) 1:400, p120 catenin (Epitomics, #2806) 1:250.
Immunohistochemistry (IHC)
IHC of paraffin sections was performed as described [41] using the antibodies above, plus an antibody to Muc2 (ab37433, Abcam) 1:200. Tumors were dual stained using DAB (ER, PR) and AP-conjugated permanent red (CK8/18) as previously described [43]. ZsGreen was visualized in paraffin sections as described [41]. BrdU labeling of paraffin sections and quantification of mitotic cells were performed as described [42]. Paraffin sections were stained for mucin using EZ Mucicarmine dye (Anatech Ltd.) and EZ Green (Anatech Ltd.) to counterstain nuclei according to the manufacturer’s instructions.
Microscopy
Whole tumor micrographs were scanned with a Nikon Eclipse Ti inverted microscope using Nikon Instruments Software Elements and a Nikon ss-Fi1 camera for bright-field images or a Nikon Ds-Qi1MC camera for fluorescent images.
Karyotyping/SKY
Karyotyping/SKY was performed by the University of Colorado Cancer Center Cytogenetics Core as described [44]. Image acquisition used a SD300 Spectracube system (ASI) mounted in an Olympus BX60 microscope with a custom-designed optical filter (SKY-1, Chroma Technology). Conversion of the emission spectra to the display colors was achieved by assigning blue, green, and red to specific sections of the emission spectrum.
RT-PCR
Total RNA was prepared using the Qiagen mini kit as directed. RNA was reverse transcribed into cDNA using MULV reverse transcriptase as described [45]. Primers were: FKBP5 [45]; SGK (SGK1) [46]; DUSP1 fwd 5′-TT TGAGGGTCACTACCAG-3′, DUSP1 rev 5′-GAGATGA TGCTTCGCC-3′; HSD11B2 primers [45]; GAPDH primers [45]; pS2 fwd 5′-TTTGGAGCAGAGAGGAGGCAAT GG-3′, pS2 rev 5′-TGGTATTAGGATAGAAGCACCAG GG-3′; CathD fwd 5′-GTGCTTCACAGTCGTCTTC-3′, CathD rev 5′-GAGCCATAGTGGATGTCAAAC-3′. Semi-quantitative PCR was performed as described [45].
MTS assay
10,000 cells/well were plated into a 96 well plate and grown overnight. Cells were changed into phenol-red free MEM + steroid depleted serum with vehicle or hormone treatments (10 nM). MTS assays were performed using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. Absorbance was measured at 490 nm.
Transcription
Transfections used (PR) PRE2- and (ER) ERE2-luciferase/ promoters containing hormone response elements coupled to reporter constructs as previously described [46]. They were electroporated into 2.4 × 106 BCK4 cells in 4 cm cuvettes at 275 V. Transfected cells were treated with vehicle or hormones for 24 h, harvested, and luciferase assays were performed as described [46].
Results
Generation of the BCK4 cell line
Cells from the pleural effusion of a breast cancer patient were explanted bilaterally into the 4th mammary glands of an immunocompromised, ovariectomized mouse [41]. First generation tumors were formed and were resected. One was fixed and stained by IHC; the other was mechanically dispersed and cultured as described in “Materials and methods” section. Two novel stable and permanent lines were established from the dispersed cells: a mouse mammary stromal cell line, BJ3Z described previously [41], and a human breast cancer cell line named BCK4 described herein.
IHC characterization of the patient explant
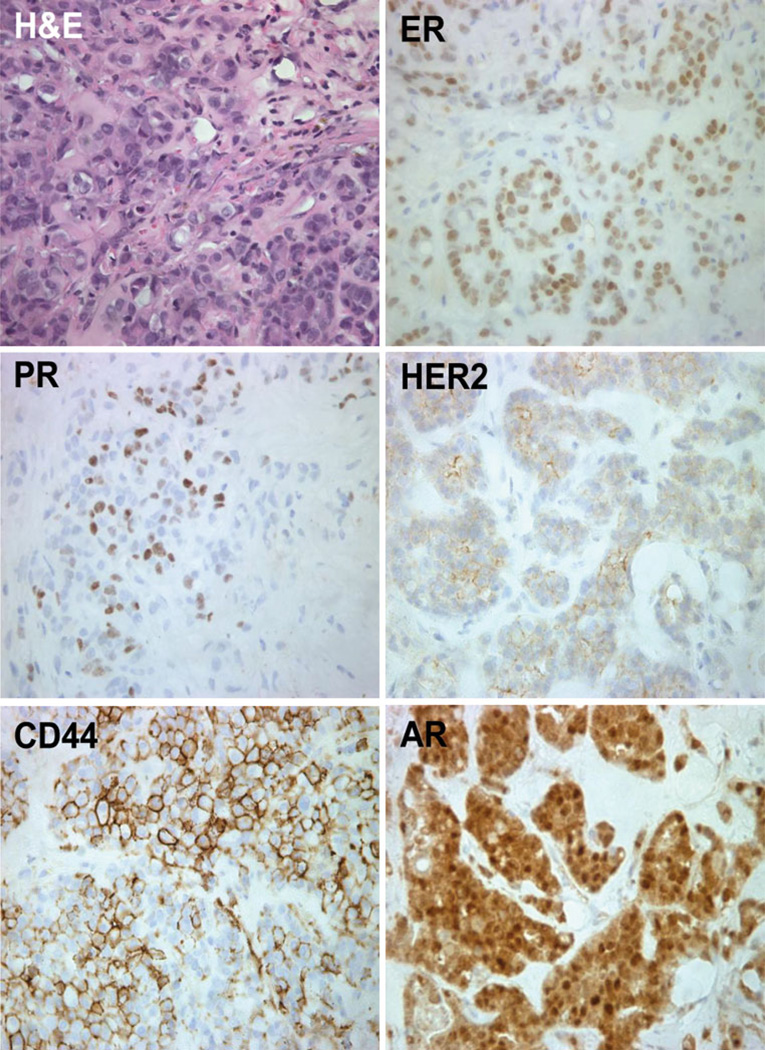
One xenografted tumor derived directly from the patient’s cells was fixed, paraffin embedded, sectioned, and stained with H&E or used for IHC (Fig. 1; Table 1). The xenograft was characteristic of an invasive adenocarcinoma with mucinous features. It was luminal as indicated by expression of ER, PR, and AR (Fig. 1); by cytokeratin (CK) 8/18 positivity (not shown); and by the absence of the basal markers, epidermal growth factor receptor (EGFR) and CK5 (not shown). The xenograft stained weakly for HER2 (Fig. 1) but quantification of this oncogene by FISH (not shown) indicated that it was not overexpressed. Xenografted cells also expressed CD44 (Fig. 1), a marker often associated with tumor initiating cells. The change in ER status of the xenograft tumor (ER+) compared to the patient’s pleural effusion (ER−) likely reflects technical artifacts in the clinical sample such as the small sample size used to measure ER and contamination of the effusion with normal lung epithelial cells.
Fig. 1.
Primary breast cancer xenograft tumor contains ER, PR, HER2, CD44, and AR. Breast cancer cells from a pleural effusion were grown in immunocompromised mice supplemented with estradiol for 12 weeks. H&E and immunostaining for ER, PR, HER2, CD44, and AR was performed as described
Table 1.
Immunohistochemical markers expressed in the patient explant, the BCK4 cell line, and the BCK4 tumors; pure mucinous (placebo supplemented mice; mucinous) and mixed mucinous (E2 supplemented mice; mucinous and invasive breast carcinoma (IBC)/invasive lobular carcinoma (ILC) regions)
| Antigen | Patient explant | BCK4 cells | BCK4 tumor |
||
|---|---|---|---|---|---|
| Pure mucinous | Mixed mucinous |
||||
| Mucinous | Mucinous | IBC (ILC) | |||
| ER | ++ | + | +⊥ | ++ | +++ |
| PR | + | + | − | + | +++ |
| AR | ++ | ++ | + | + | ++ |
| GR | nd | ++ | nd | nd | nd |
| Her2 | 1+ | 1+ | 1+ | 1+ | 1+ |
| EGFR | − | nd | ⊥ | ⊥ | − |
| E-cadherin | < 1 % | − | − | − | − |
| p120 catenin | cyto/nuc | cyto | cyto/nuc | cyto | cyto |
| CK8/18 | ++ | +++ | + | + | ++ |
| CD44 | ++ | + | ⊥ | ⊥ | + |
| CK5 | − | − | − | − | − |
| CK14 | nd | − | − | − | − |
| Ckit | nd | + | ++ | ++ | + |
| CD10 | nd | ++ | + | ⊥ | ⊥ |
| p63 | nd | ++ | + | ⊥ | ⊥ |
| αSMA | nd | ++ | ++ | ++ | ++ |
| Vimentin | nd | ⊥ | − | − | − |
| MAM-A | nd | nd | + | + | +⊥ |
| ChromograninA | nd | + | + | ⊥ | − |
| Synaptophysin | nd | ++ | + | ⊥ | − |
| Muc2 | nd | nd | + | +⊥ | ⊥ |
| N-cadherin | nd | nd | + | + | − |
Cellular staining designations are as follows: −, negative staining; ⊥, <5–10 % staining; +, >10–30 % staining; +⊥, >30–50 % staining; ++, >50–70 % staining; +++, >70–100 % staining; nd not determined, cyto/nuc cytoplasmic/nuclear expression, 1+ Her2 expression by FISH analysis
Characterization of the BCK4 cell line
We have extensively characterized the BCK4 cell line that grew out of the xenograft of the patient’s pleural effusion.
Karyotype
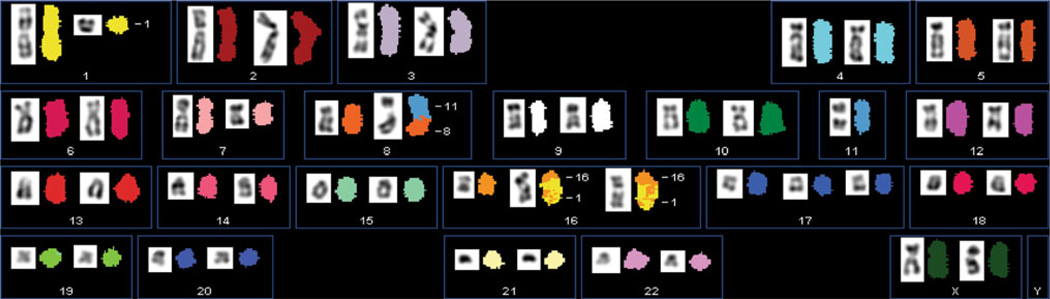
Metaphase chromosomes of BCK4 cells were analyzed by GTL banding (not shown) and Spectral Karyotyping (SKY) to assign their species of origin, define a modal chromosome number, and identify anomalies, if any (Fig. 2). The composite SKY karyotype shows 21 diploid and 1 triploid (chr17) chromosome, plus diploid X chromosomes demonstrating that the BCK4 cells are human, female, and relatively normal. The formal description—47,XX,del(1) (?p31;?q12),del(7)(q22),der(8)t(8;11)(p11;q13),11,der(16) t(1;16)(q12;q11.2)×2,+17—contains few anomalies. The BCK4 karyotype is much closer to normal than that of the commonly used T47D or MCF7 human breast cancer cells, which like 58 % of IDC with mucinous features [47] are aneuploid. The normality of BCK4 cells concurs with the observation that 96 % of pure MBC have diploid DNA by flow cytometry [47]. The karyotype of BCK4 cells is the first for any MBC. Importantly, the karyotype of the BCK4 cells is nearly identical to the karyotype of the original cells derived from the pleural effusion of the patient (Supplementary Fig. 1). Additionally, single tandem repeat analysis of the patient’s pleural effusion cells matched the BCK4 cell line (not shown).
Fig. 2.
The BCK4 cell line exhibits a nearly normal karyotype. A xenograft tumor as grown in Fig. 1 was mechanically dispersed, cultured, and a permanent cell line, BCK4 was established. Spectral karyotyping of BCK4 cells was performed as described. Each chromosome has a unique color designation. The karyotype of the cells is 47,XX,del(1)(?p31;?q12),del(7)(q22),der(8)t(8;11)(p11;q13), 11,der(16)t(1;16) (q12;q11.2)×2,+17
Protein expression
To further characterize BCK4 cells, key biomarkers were analyzed by ICC, immunoblotting, and IHC (Supplementary Fig. 2; Table 1). Like the patient’s tumor explant, the BCK4 cell line is ER, PR, and AR-positive (Supplementary Fig. 2); ER and PR levels are similar to those in T47D cells, but AR levels are higher. Table 1 shows IHC semi-quantification for other markers. BCK4 cells weakly express Her2 protein but the HER2 gene is not overexpressed (not shown). The BCK4 cells are glucocorticoid receptor (GR)-positive and strongly express the epithelial and luminal specific marker CK8/18, but lack the basal markers vimentin, CK5 and CK14, putting these cells in the luminal category. Interestingly, the BCK4 cells lack E-cadherin and contain cytoplasmic/nuclear p120 catenin. This combination is indicative of lobular carcinomas [17] and is discussed further below. The patient explant contained less than 1.0 % E-cadherin positive cells, perhaps indicating that it was of a mixed lobular/ductal phenotype. If so, we speculate that the BCK4 cell line was generated from the lobular subpopulation.
Mucin producing breast cancers are often subclassified among neuroendocrine tumors [48], and indeed, BCK4 cells express chromogranin A and synaptophysin which are characteristic of such tumors (Table 1). Expression of mammaglobin-a (MAM-A) in BCK4 cells confirms their breast origin. They also express the tyrosine kinase receptor and proto-oncogene c-kit (CD117), which is a product of normal and benign breast tissues, but is often lost in breast cancers [49]. Unlike the patient xenograft, BCK4 cells express low levels of the putative tumor initiator cell marker CD44. Interestingly, BCK4 cells strongly express the myoepithelial markers CD10, p63, and α-smooth muscle actin (αSMA), which confer a poor prognosis to breast cancers regardless of ER or lymph node status [50], but may provide clues about the cells of origin in MBC.
Steroid receptor function
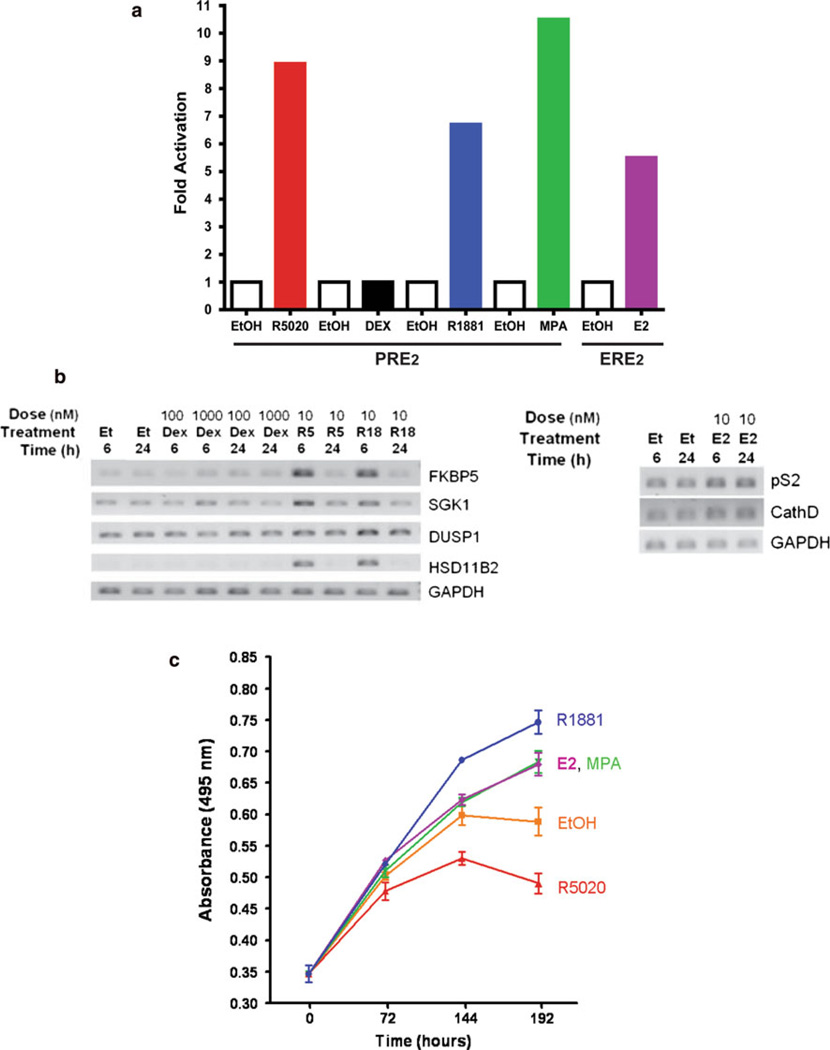
To determine whether the steroid receptors in BCK4 cells are functional, transcriptional and gene expression studies were performed. The ability of the endogenous receptors to regulate transcription was tested by transfecting BCK4 cells with a luciferase reporter driven by two copies of the cognate response element for PR, AR, or GR (here called progesterone response element; PRE2) or for ER (estrogen response element; ERE2). The transfected cells were then treated with ethanol control (EtOH) or the appropriate hormones for 24 h (Fig. 3a). The synthetic progestins R5020 (10 nM) or medroxyprogesterone acetate (MPA; 10 nM) stimulated PR-dependent PRE2 transcription 9.0- and 10.5-fold, respectively. AR-dependent transcription increased ~sevenfold in response to the synthetic AR, R1881 (10 nM). ER are also functional since estradiol (E2, 10 nM) stimulated transcription from the ERE2 reporter ~sixfold (Fig. 3a). Interestingly, despite the presence of GR proteins (not shown) and regardless of dose (1–1,000 nM), the glucocorticoid dexamethasone failed to stimulate transcription from the PRE2 promoter (Fig. 3a), or from an alternate canonical GR-dependent reporter driven by the mouse mammary tumor virus (MMTV) long terminal repeat (LTR; not shown). However, BCK4 cells transfected with an exogenous wild-type GR expression vector supported transcription from both PRE2 and MMTV response elements (not shown). This suggests that the cells harbor a flaw in endogenous GR protein(s) or that smaller inhibitory isoforms of GR predominate, but that the GR transcriptional machinery is functional.
Fig. 3.
AR, PR, and ER are functional, regulate endogenous genes and growth of BCK4 cells. a Transcription assays were performed with exogenous reporter constructs containing progesterone response elements (PRE2) or estrogen response elements (ERE2) as described. Cells were treated with vehicle (open bars) or hormone (solid bars) with the progestins R5020 or MPA, the glucocorticoid dexamethasone (DEX), the androgen R1881, or estradiol (E2). b Semi-quantitative PCR of BCK4 cells treated with vehicle (Et; EtOH) progestin (R5; R5020), androgen (R18; R1881), and glucocorticoid (Dex; Dexamethasone). Responsive genes were assayed at two different timepoints of hormone treatment, 6 or 24 h. Two different doses of Dex were used, 100 and 1,000 nM. GAPDH was used as an internal control. Semi-quantitative PCR analysis of BCK4 cells treated with vehicle (Et; EtOH) or estrogen (E2) for 6 or 24 h. Two estrogen responsive genes, pS2 and CathD were measured. GAPDH was used as a loading control. c MTS cell proliferation assay of BCK4 cells treated with vehicle (EtOH), estradiol (E2), androgen (R1881), progestin (R5020), or the progestin/androgen (MPA). Cells were assayed at 0, 72, 144, and 192 h of treatment
The ability of steroid receptors in BCK4 cells to regulate endogenous genes was tested by RT-PCR of several transcripts previously identified as steroid regulated in breast cancer cell lines [45, 51, 52]. Figure 3b shows that FK506 binding protein 5 (FKBP5), serum/glucocorticoid regulated kinase 1 (SGK1), dual specificity phosphatase 1 (DUSP1), and hydroxysteroid (11-beta) dehydrogenase 2 (HSD11B2) are all upregulated by 6 or 24 h of progestin or AR treatments, demonstrating again that PR and AR are functional. In contrast, as with the exogenous reporters, 6 or 24 h of dexamethasone failed to regulate GR responsive genes endogenously. Two classical ER regulated genes, pS2 and Cathepsin D (CathD), were upregulated by 6 and 24 h treatment with E2 (Fig. 3b, right panel).
Proliferation
Cell growth was measured by MTS uptake in viable cells. The basal doubling time of BCK4 cells is 4–5 days (~120 h; Fig. 3c, ethanol); much slower than that of other breast cancer cell lines, which tend to double in 24–48 h [53]. However, BCK4 cell growth is accelerated by the synthetic AR R1881, the mixed AR/progestin/glucocorti-coid MPA, and by E2. On the other hand, proliferation via PR appears to be inhibited by the pure synthetic progestin R5020 (Fig. 3c) suggesting that the MPA proliferative effect is mediated by AR, since the GR are non-functional.
BCK4 tumor xenografts
BCK4 cells were tumorigenic in immunocompromised mice. ZsGreen-tagged BCK4 cells were implanted bilaterally into the 4th mammary glands of ovariectomized (not shown) or intact immunocompromised mice, supplemented with placebo or E2-releasing pellets (Fig. 4) which raise circulating estrogen levels and render animals acyclic.
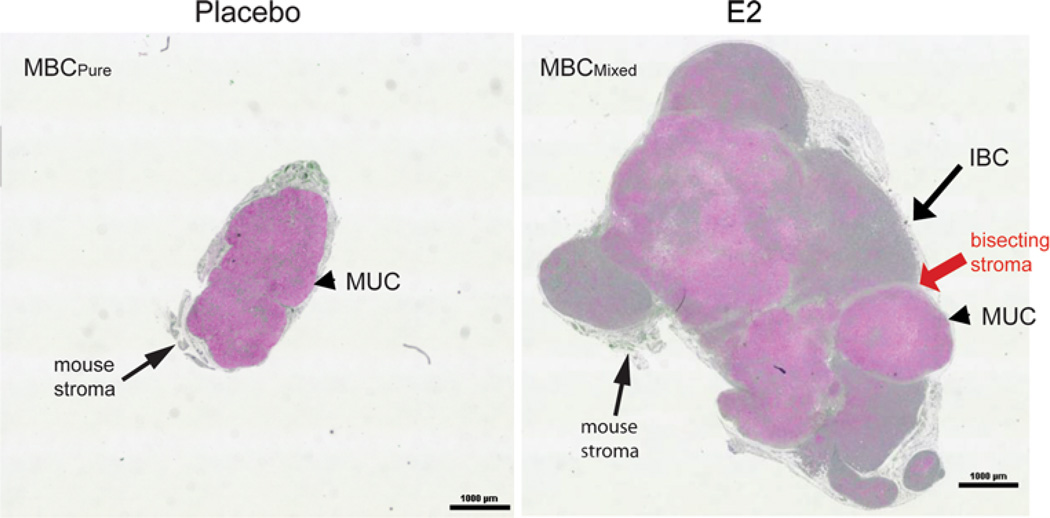
Fig. 4.
BCK4 cells are tumorigenic; estrogen causes a histologic switch. Images of whole tumors, grown in placebo (left MBCPure) or estrogen (E2, right MBCMixed) supplemented mice, stained with mucicarmine. Arrow denotes invasive breast carcinoma (IBC) component and arrowheads mucinous regions (MUC). Red arrow denotes stroma bisecting the IBC and MUC regions of the MBCMixed tumor
Tumorgenicity and effects of E2
BCK4 tumor growth was absolutely estrogen-dependent in ovariectomized mice (not shown). In normal cycling mice (placebo) only small tumors developed as shown by the cross section of a whole tumor (Fig. 4, left). In contrast, much larger (~sixfold) tumors formed if mice were continuously supplemented with E2 (Fig. 4, right). To identify mucinous components, paraffin sections were stained with mucicarmine (pink) and nuclei were counterstained green (Fig. 4). While the small tumors of placebo mice were pure MBC (MBCPure) composed entirely of cells floating in mucin (arrowhead), the much larger tumors derived from E2 supplemented mice were characteristic of an invasive breast carcinoma (long arrow) with mucinous features (arrowhead) (MBCMixed). These data demonstrate a heretofore unknown role for estrogens in breast cancers, namely their ability to switch MBCPure into MBCMixed that is more aggressive. The clinical implications are important because our model uses E2 levels that are physiologic [54] and observed in women taking HRT [55].
Ductal or lobular carcinoma?
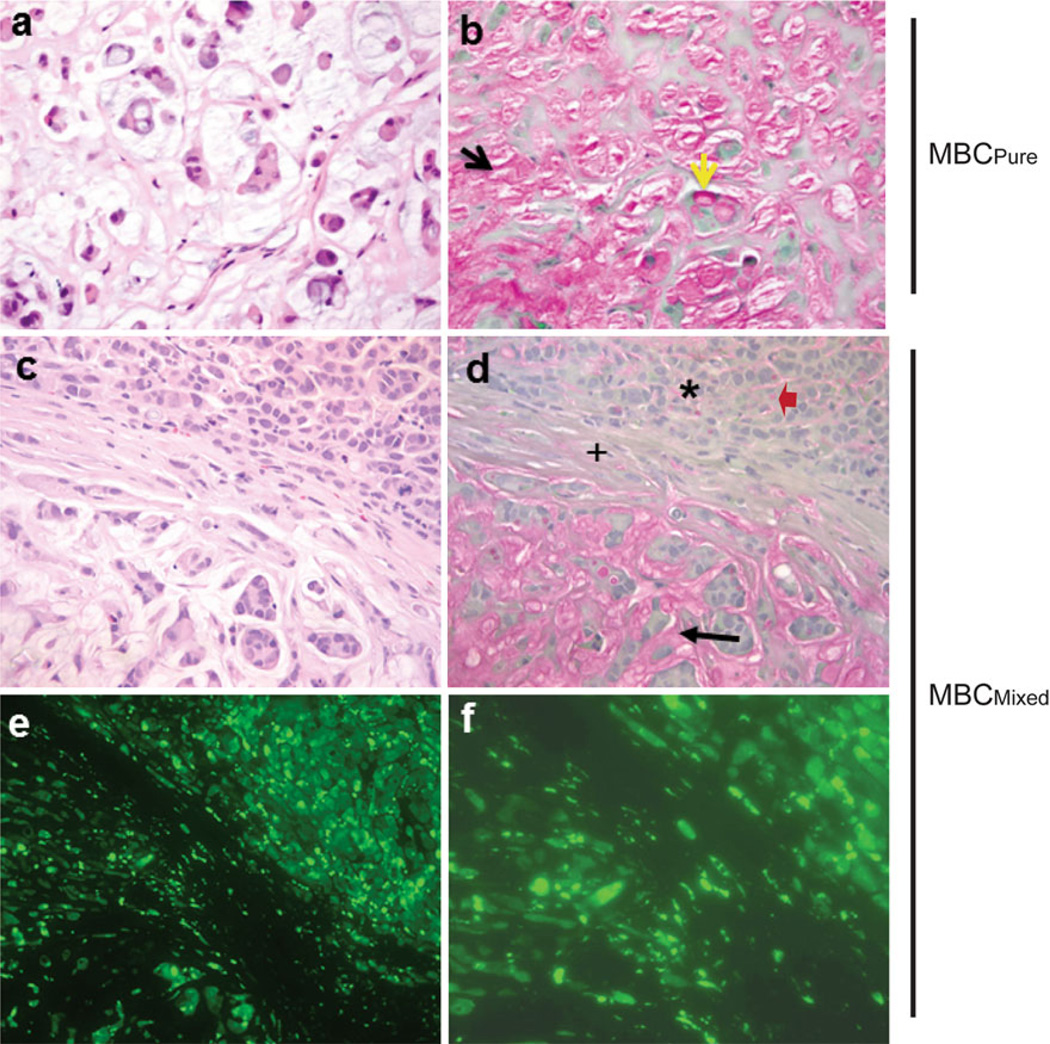
We next attempted to define whether the BCK4-derived tumors are of ductal (IDC) or lobular (ILC) origin, based on H&E and mucin staining. The slides were reviewed by a surgical pathologist. Figure 5 shows H&E (panel a) and mucicarmine (panel b) stains of a tumor from a placebo mouse. This tumor contains extracellular pools of mucin (panel b, black arrowhead), as well as intracellular mucin characterized by the presence of signet ring cells (yellow arrowhead). Collectively, the pathology is consistent with a diagnosis of MBCPure.
Fig. 5.
Estrogen induces regions of mucinous and invasive breast carcinoma; bisecting region is composed of human tumor cells. ×20 images of a H&E stain and b mucicarmine stain of MBCPure tumor from placebo supplemented mouse (yellow and black arrows designate intracellular and extracellular mucin, respectively); c H&E and d mucicarmine stains of MBCMixed tumor from E2 supplemented mouse (black arrow mucinous region; asterisk invasive breast carcinoma (IBC) region; plus region bisecting mucinous and IBC; red arrow cell containing intracytoplasmic mucin); e ×10 ZsGreen stain of bisecting tumor region of E2 supplemented mouse and f ×40 ZsGreen image of bisecting tumor region
In contrast, tumors from an E2 supplemented mouse are heterogeneous, containing areas of high (panel d, black arrow) and low (panel d, asterisk) mucin production. Within tumors, distinct subregions can be seen (panel c) consisting of corded areas that lack signet ring cells (top) or neoplastic cells floating in mucin pools (bottom) within the same tumor. Tumors also contain regions composed of solid nodules lacking signet ring cells (Supplementary Fig. 3, “+”); a feature of IDC. However, also within corded areas, some cells were arranged in single file and contain intracytoplasmic mucin; a feature of ILC (Fig. 5, panel d, red arrow). Since this low mucin producing region has characteristics of IDC and ILC, we refer to the solid region as invasive breast carcinoma (IBC). Finally, the mucinous and IBC regions are separated by bisecting “stroma” (Fig. 5, panel d (+)). However, this stroma is of human origin because the cells fluoresce green (Fig. 5, panels e and f) and only BCK4 cells express the ZsGreen tag. Taken together, the pathological data are indicative of an invasive mucinous carcinoma [56].
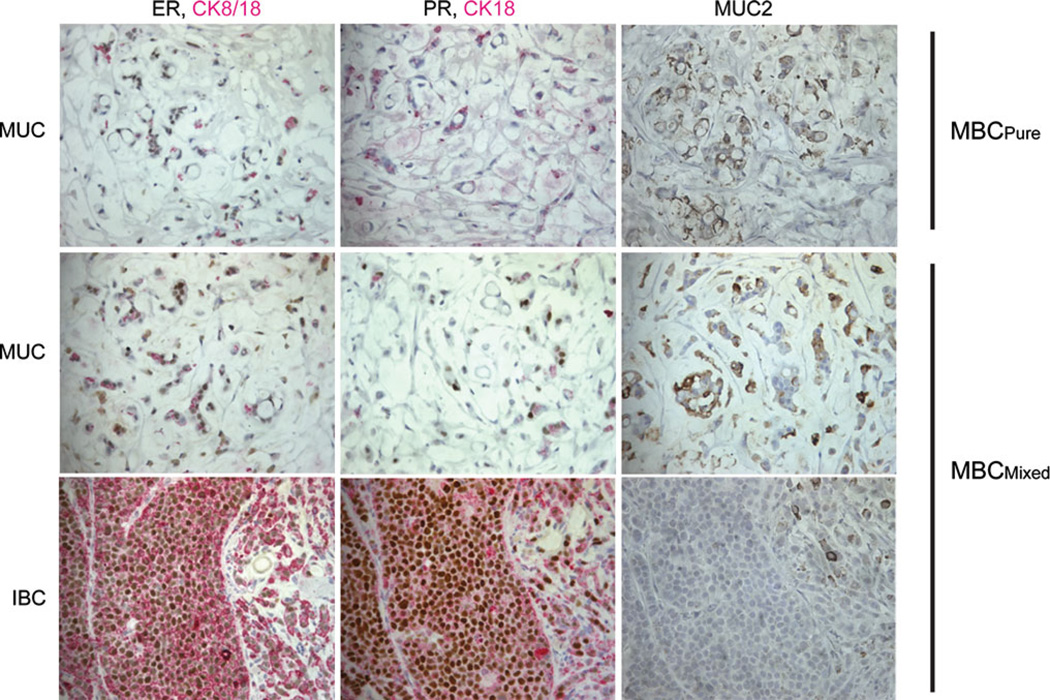
The MBCPure of control (placebo) cycling mice and the IBC plus mucinous (MUC) regions of MBCMixed tumors in E2-treated mice were characterized with the same panel of markers used for the clinical pulmonary explants and the cell line (Table 1; Fig. 6). ER (brown nuclei) expression was similar in the mucinous components (MUC) of MBCPure and MBCMixed, and ER increases in the IBC regions of the MBCMixed (Fig. 6, left column). Estrogen responsive PR proteins were completely absent in MBCPure, weakly up-regulated in the MUC region of MBCMixed, and strongly up-regulated in the IBC region of MBCMixed suggesting that the IBC component is preferentially stimulated by estrogenic signals (Fig. 6, middle column). Cytokeratins CK8/18 (epithelial markers, pink) were also up-regulated in the IBC region of MBCMixed compared to the MUC region and MBCPure tumor (Fig. 6). Other markers were not remarkably different among regions, except for the neuroendocrine markers, which were downregulated in the IBC regions of mixed tumors (Table 1). As expected, the tumors showed high levels of MUC2 staining (brown) in the mucinous regions but had low to no MUC2 in the IBC region (Fig. 6, right column). Interestingly, the EMT marker N-cadherin is expressed in the mucinous region of the mixed tumor but is absent in the IBC region (Table 1). These data suggest the possibility that as previously suggested [57], in MBCMixed tumors, the IBC subpopulation is derived from mucinous precursors in response to estrogen signaling.
Fig. 6.
Estrogen induces regions of invasive breast carcinoma with increased ER and PR and decreased Muc2 expression. Immunohistochemistry was performed using antibodies directed IHC against ER, PR, or Muc2 (DAB, brown) CK8/18, CK18 (AP, Permanent red). Stains of MBCPure tumor and MBCMixed tumor with mucinous (MUC) and invasive breast carcinoma (IBC) regions are shown
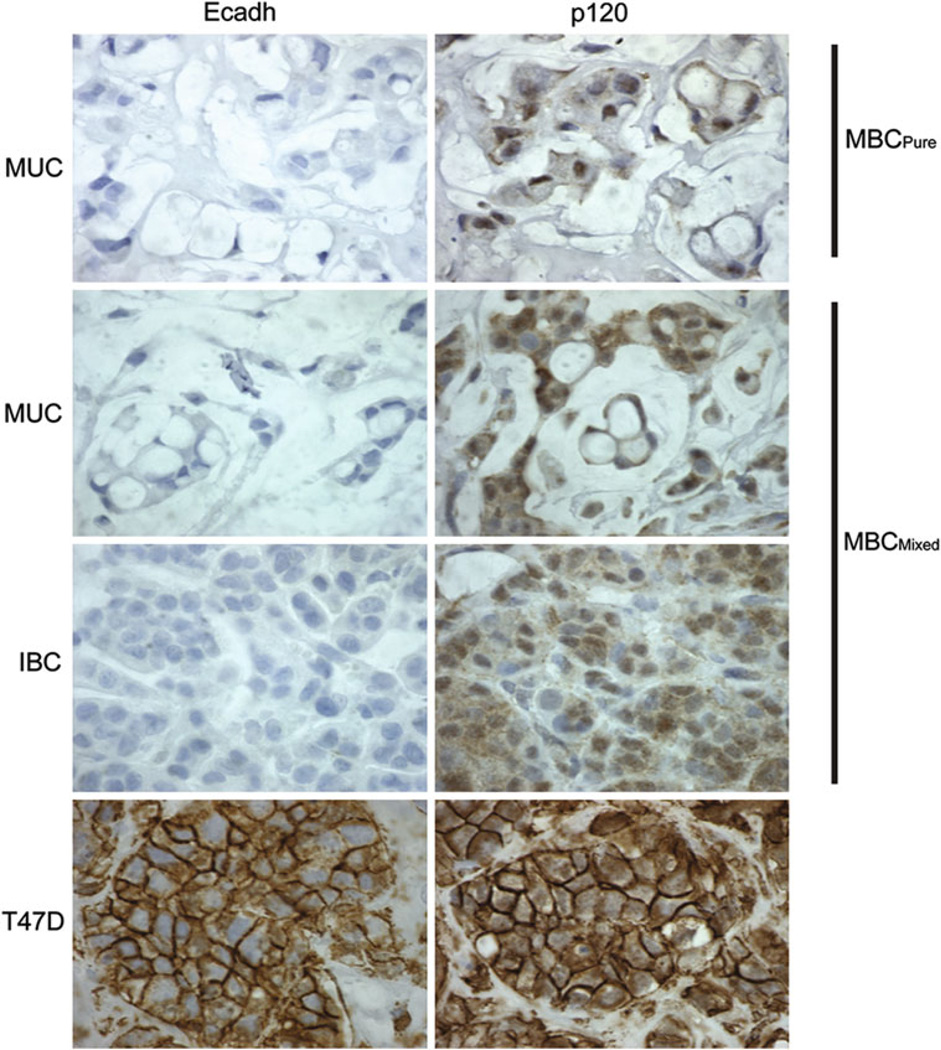
Whether the IBC is ductal (IDC) or lobular (ILC) could not be histologically determined since features of both extracellular and intracellular mucin were detectable. We therefore used molecular markers to discriminate between the two [58]. Loss of E-cadherin and cytoplasmic localization of p120 catenin characterize ILC [58]. E-cadherin was completely absent in MBCPure and MBCMixed in both the MUC and IBC regions (Fig. 7). As a positive control, a tumor derived from the IDC cell line, T47D, showed clear membranous E-cadherin staining. The absence of E-cadherin in BCK4 tumors suggested that they were lobular in origin. P120 catenin was expressed in both MBCPure and MBCMixed; however, its distribution was diffuse and cytoplasmic (Fig. 7), in contrast to the strong membranous staining of tumors derived from T47D cells. The combination of E-cadherin loss coupled with the cytoplasmic p120 catenin indicates that both the MBCPure and MBCMixed tumors originating from BCK4 cells were of lobular origin. While the MBCMixed tumor was called “invasive breast carcinoma (which can be ILC, IDC or a mixture of both) with mucinous features” by the reviewing pathologist, molecular analysis shows that the tumor contains features consistent with ILC. This staining is conserved in the BCK4 cell line. We therefore conclude that BCK4 cells are models of mucinous disease of lobular origin that will provide rare insight into the molecular dynamics of such tumors.
Fig. 7.
BCK4 tumors display lobular features. BCK4 tumors were stained with E-cadherin (Ecadh) or p120 catenin (p120). Staining of pure mucinous tumor (MBCPure) and mixed mucinous tumor (MBCMixed) mucinous region (MUC) and invasive regions (IBC) are shown (×100). A T47D tumor is shown as a control for membranous staining of both proteins
Phenotype switching by estrogens
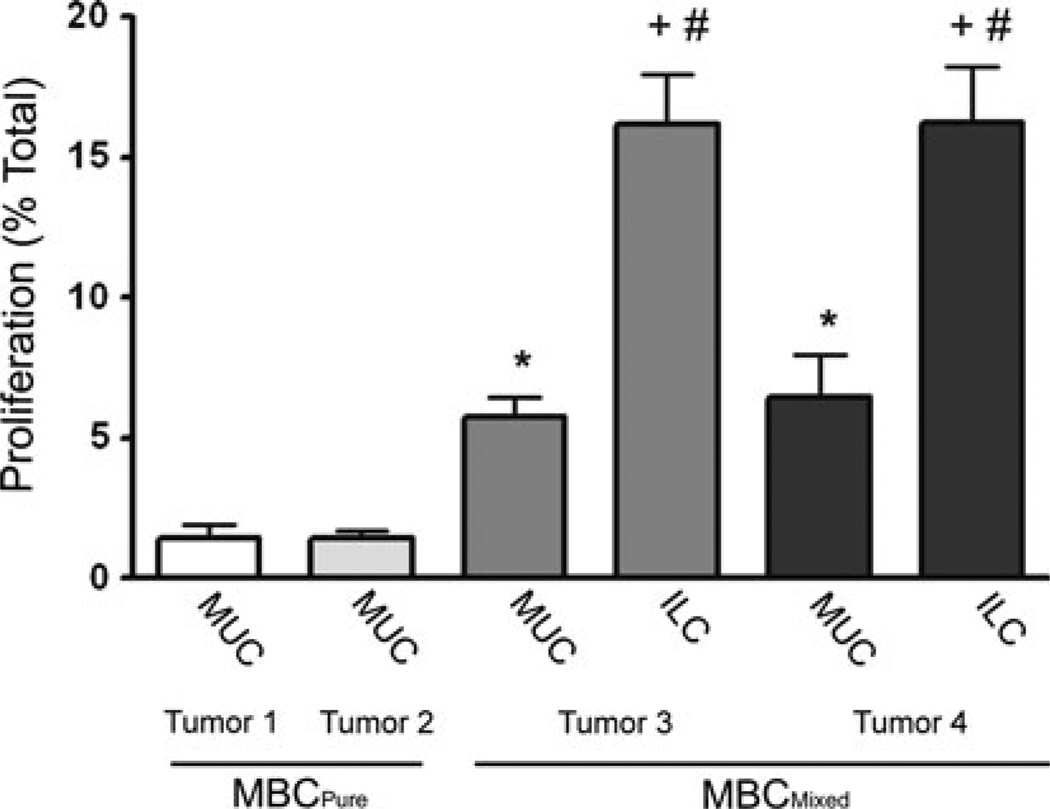
Our data also suggest the possibility that in the MBCMixed tumors, the ILC component is derived from MBCPure precursors in response to changed estrogen signaling patterns. To assess this, the proliferation rates of cells in different morphological regions of BCK4-derived tumors from two control (placebo) and two estrogen treated (E2) mice were quantified by BrdU incorporation during S-phase (Fig. 8). As expected from their slow growth, only 2 % of cells in the control MBCPure tumors were undergoing mitosis during the 2 h BrdU pulse. The MUC region of the two MBCMixed tumors from E2 supplemented mice exhibited an increased (to 5 %) proliferation rate that was statistically significant (p<0.05). Importantly, in the same mice, same tumors, and same hormonal environment, proliferation of the ILC regions exceeded 15 % (p<0.001). This strengthens our conclusion that in MBC, estrogens induce a phenotypic switch to ILC, and this is accompanied by increased proliferation in tumor subregions. The heterogeneous regional effects of estrogen within the same tumor are also of interest and have not been previously described.
Fig. 8.
Estrogen preferentially stimulates growth of ILC in mixed tumors. BrdU incorporation of tumors from placebo and E2 supplemented mice. Pure mucinous tumor (MBCPure) and mixed mucinous tumor (MBCMixed) showing mucinous region (MUC) and invasive lobular carcinoma (ILC). Five fields were quantified for each tumor and statistical significance was determined by an unpaired two tailed t test. *p < 0.014 for tumor 1 or tumor 2 MBCPure versus MBCMixed MUC region of tumor 3 or tumor 4; +p < 0.001 for tumor 3 or tumor 4 ILC versus tumor 1 or tumor 2 MBCPure; #p < 0.001 for tumor 3 or tumor 4 MUC region versus tumor 3 or tumor 4 ILC region
3D modeling in vitro
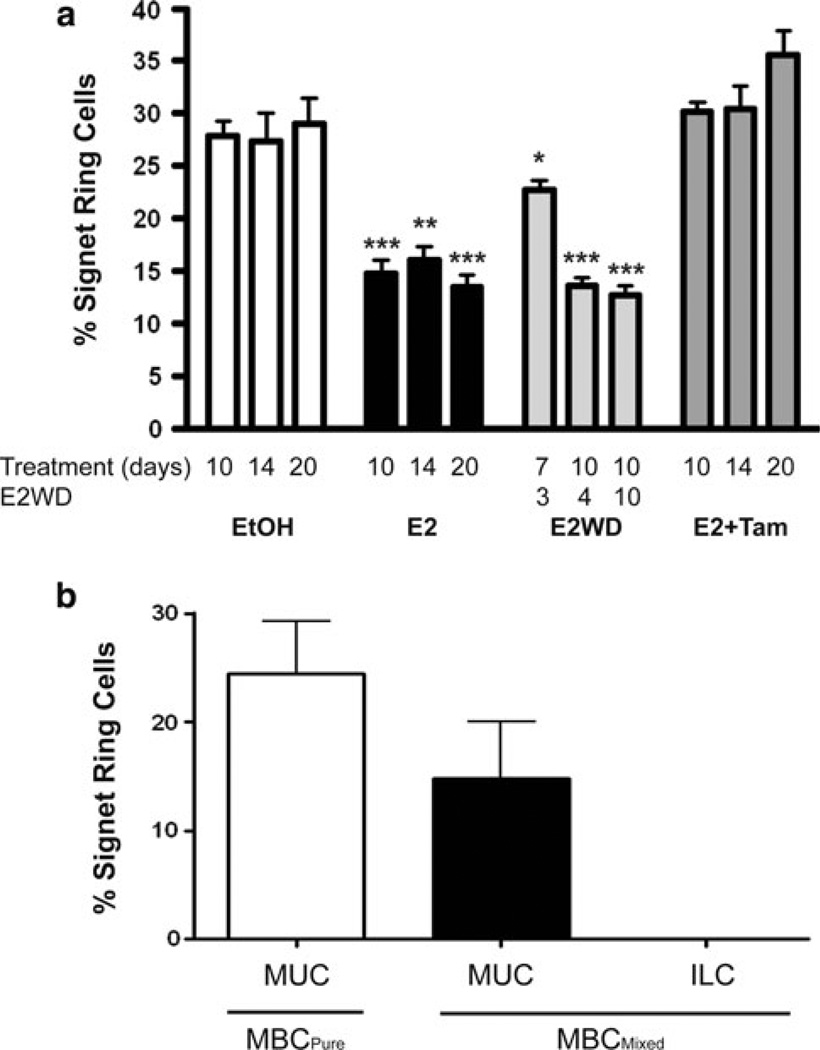
We established a 3D model to recapitulate BCK4 tumor growth in vitro and to analyze the promotion of the mixed phenotype by estrogen treatment. This model was also used to ask whether effects of E2 can be blocked by the anti-estrogen tamoxifen (Tam) or by E2 withdrawal (E2WD). For this, BCK4 cells were grown in a 3D-matrigel matrix and treated with vehicle (EtOH), E2, or E2 + Tam for 10, 14, or 20 days. Alternatively, cells were also treated for 7 or 10 days with E2 which was then withdrawn for 3, 4, or 10 days. Signet ring cell quantification was used to assess the mucinous phenotype. In control conditions, ~30 % of cells were signet ring. Ten to 20 days of E2 halved the number of mucin producing cells to ~15 % (Fig. 9a). This effect of E2 was completely prevented by co-treatment with tamoxifen. On the other hand, E2 treatment for 7 days followed by 3 days of E2 withdrawal partially restored signet ring cell number suggesting an initial degree of reversibility. Longer effects of E2 (10 days) were not reversible. We conclude that E2 effects are mediated directly via ER explaining the efficacy of tamoxifen. However, once the mixed phenotype is established, it is difficult to reverse. These results were confirmed in solid BCK4 xenografts (Fig. 9b) grown in the absence or in the presence of exogenous E2 supplementation. Under control conditions (placebo), the MBCPure tumors contained ~25 % signet ring cells; similar to the number in the 3D model. In E2 treated mice, mixed mucinous tumors formed. The MUC component of MBCMixed contained ~15 % signet ring cells (Fig. 9b) while the ILC component completely lacked signet ring cells.
Fig. 9.
Long-term E2 causes signet ring cell loss. a 3D Matrigel cultures of BCK4 cells were treated 7–20 days with vehicle, E2 alone, or E2 plus tamoxifen (Tam). In one set of 7 or 10 day E2 treated colonies, E2 was withdrawn (E2WD) for 3 to 10 days. Five fields were quantified for each sample. Signet ring cells were quantified as the percent of total cells. * p < 0.05; **p < 0.01; ***p < 0.001. b BCK4 tumor xenografts, MBCPure and the MUC and ILC regions of MBCMixed, were assayed for signet ring cells. Five fields were quantified per tumor
We conclude that BCK4 cells model human MBC of lobular origin that produce intracellular and extracellular mucin and contain signet ring cells. However, whether the phenotype is MBCPure or MBCMixed can be regulated by estrogens. These data highlight the complexity of MBC; a complexity that we can now model and probe experimentally.
Discussion
Eleven different histologic subtypes of breast cancer have recently been classified based on gene expression profiling [27]. Included within these are MBC, which are more complex and heterogeneous than originally anticipated. The molecular data further sub-classified MBC according to the criteria of Capella et al. [59] into Type A which contain 60–90 % extracellular mucin and Type B which contain 33–75 % extracellular mucin [59]. Type B tumors also contain signet ring cells [59]. Cluster analysis of gene expression data placed Type A and Type B tumors in the luminal centroid [27]; not surprising since MBC are ER+[26]. What was surprising was that the majority of Type A tumors which are enriched for extracellular mucin clustered with ILC [27]. In contrast, Type B tumors containing signet ring cells clustered with neuroendocrine tumors. Both types of MBC differ from IDC NOS, however [48]. This suggests that all MBC, whether pure or mixed, may be erroneously categorized as a subtype of IDC if only histological markers such as mucin localization are considered. Further evidence that MBC differ from IDC NOS is based on the fact that MBC rarely contain mutations in phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) or v-akt murine thymoma viral oncogene homolog 1 (AKT1); both of which are commonly observed in IDC NOS [60].
While reports of ILC that produce extracellular mucin may be rare, three cases have recently been reported. In all three cases tumors expressed ER and PR, contained intra-cellular and extracellular mucin, were signet ring cell positive and exhibited loss of E-cadherin [17–19]. Thus more detailed molecular analyses of MBC may reveal that many are of lobular origin [27] but are currently underestimated because the presence of extracellular mucin is considered to be a marker of IDC. Expression profiling studies with larger numbers of MBCPure or MBCMixed have not been done, thus the prevalence of ILC within MBC remains unknown. One study using tissue arrays examined 40 cases of MBCPure or MBCMixed; all tumors contained at least some E-cadherin with membranous p120 catenin, which led to their characterization as IDC [17]. However, some ILC are E-cadherin positive as are some of the MBC that cluster with them by expression profiling analysis [27]. Thus the presence of E-cadherin does not rule out that MBC are mixed with ILC. It is clear that more definitive molecular markers need to be identified for MBCMixed to determine whether the more invasive regions are IDC or ILC.
The presence of signet ring cells in mucin producing tumors likely signifies that they are of lobular origin. Signet ring cell carcinomas (SRCC) are classified as a subtype of breast cancer. Tumors are classified as such when at least 20 % of the total tumor is composed of signet ring cells [22, 23, 61]. Mucin producing signet ring cells are present in ILC [5, 20, 22] and MBC [59] leading many to conclude that SRCC is a variant of ILC [23, 61, 62] but two studies suggest that SRCC are entities distinct from MBC and ILC [24, 63]. However, three lines of evidence suggest that signet ring cells are of lobular origin (1) the majority of SRCC are negative for E-cadherin [64], (2) signet ring cells contain intracellular mucin, and 3) SRCC metastasize to serosal surfaces [23] resembling the metastatic pattern for ILC. This is clinically important because the percentage of signet ring cells within tumors can determine the propensity for recurrence. Patients with stage I breast cancer whose tumors contain as few as 10 % signet ring cells have an increased risk of recurrence compared to patients whose tumors have lower numbers of signet ring cells [22].
The ductal or lobular origins of MBCPure and MBCMixed have been unclear to date. Since the lumen of ductal carcinomas in situ (DCIS) often contain mucin [39], some speculate that MBCPure originates from DCIS [30]. Others speculate based on loss of heterozygosity analysis that MBCPure originate from IDC [30]. However, since mucin production is also associated with lobular carcinoma in situ [65], it is entirely possible that MBCPure are derived from lobular precursors. While both IDC and ILC originate in the terminal ductal lobular unit of the breast, they likely arise via different pathways [66]. This is reinforced by the fact that ILC lack basal cytokeratins such as CK5 are commonly observed in IDC [67]. Expression profiling studies contrasting ILC from IDC [10–13] show that many functional pathways, including ones involved in immune responses, cell adhesion and motility, and lipid/fatty acid transport and metabolism differ between the two. However, only downregulation of the gene encoding E-cadherin (CDH1) in ILC was common among them. Of note is the loss of E-cadherin expression in the BCK4 cells which correlates with translocations in chromosome 16 of their karyotype (Fig. 2). Loss of heterozygosity at chromosome 16 which disrupts the CDH1 gene is common among ILC [68]. As supported by the data of Weigelt et al. [27], our model suggests that some MBCPure are inherently lobular and that in MBCMixed, the ILC subpopulation can be derived from MBCPure under conditions that may involve estrogen signaling. This requires further study, as does the role of the mesenchymal-like fibroblastic cells in MBCMixed, apparently also of BCK4 origin, located in stromal-like bands separating MUC regions from ILC regions in our estrogenized tumors (Fig. 5). Similar bisecting stroma is observed in MBC of patients [56] and in ILC, giving us confidence in the power of BCK4 cells as investigative tools.
MBC tends to be a disease of older women. The prevalence of MBCPure is ~7 % in women older than 75 years (median age 71 [69]) and ~1 % in women less than 35 years [25]. Similarly, patients with MBCPure tend to be older (median age 55) than patients with MBCMixed (median age 47) or IDC NOS [26, 34, 35, 70, 71]. Explanations for this remarkable difference are unclear but our data suggest that steroid hormones are involved. One study that analyzed the role of postmenopausal hormone replacement therapy on breast cancer risk by histologic type reported a non-significant trend for increased MBC in current estrogen users compared to never users. Interestingly, there was a trend for decreased MBC in current combined estrogen plus progestin users [72]. Furthermore in postmenopausal women, endogenous estrogen is largely unopposed by PR while both estrogen and PR are present in premenopausal women. The presence of PR may explain the low prevalence of MBC in premenopausal women. The hypothesis that progestins, unlike the case for more common breast cancer types, are protective against MBC development in premenopausal women can be studied using the BCK4 model.
With regard to ER, no significant differences in ER status have been reported between MBCPure and MBCMixed [34], or in the immunohistochemical pattern of ER expression in mucinous compared to invasive regions of MBCMixed [73]. However, in line with our data (Table 1) MBCMixed tend to have higher ER levels than MBCPure as measured biochemically [73]. Also of interest is the increased expression of PR in the more aggressive and proliferative ILC tumor regions. High PR expression typically correlates with a more “differentiated” phenotype and a better prognosis in breast tumors. The infiltrating regions of MBCMixed are more proliferative than the mucinous regions [38] and proliferation is generally higher in MBCMixed than in MBCPure [33]. Our studies confirm this (Fig. 8).
The standard of care for MBCPure, like most of the other uncommon histologic subtypes, is not differentiated from the overall management of breast cancer. Increased understanding of the role of tumor biology, which we have shown can be influenced by histologic subtype in the case of MBC, is changing the treatment landscape. Clinically validated genomic tools for determining the appropriate use of chemotherapy in early stage disease and multiple reports on the differential responsiveness to available therapies by tumor biologic characteristics are now available (reviewed in [74]). However, some of these tests may not be reliable for rare histological subtypes because of the presence of inflammatory cells and/or stroma within these tumors [75]. Thus, despite these gains in knowledge for breast cancer overall, the ability to “personalize” treatment decision making in rarer histologic subtypes remains a research goal. Our unique cell line and its demonstrated plasticity in the face of estrogens suggests that it may be important to elucidate differences between MBC mixed with IDC or ILC as a histologic means of predicting for different therapeutic responses and long-term outcomes. The prognostic and predictive differences between ILC and IDC have been recently reviewed and are not always consistent [76]. We hypothesize that tumor plasticity and mixed subcomponents may influence these clinical results and deserve further study.
The postmenopausal patient in our study was given 6 cycles of cyclophosphamide, doxorubicin, and 5FU followed by radiation and 5 years of tamoxifen. Three years later metastatic disease recurred as a pleural effusion. Using cells derived from the pleural effusion we developed a cell line that forms a MBCPure in mice exposed to cycling estrogens, but switches to MBCMixed with ILC subpopulations when exposed to continuous physiologic estrogens (Fig. 4). Our BCK4 tumors, like their clinical MBC counterparts [77] co-express AR along with ER and PR (Supplementary Fig. 2) and may be stimulated to grow by ARs (Fig. 3). Given the diversity of anti-endocrine therapies available, including selective ER modulators, a pure ER downregulator and aromatase inhibitors, it is of clinical interest to determine if histologic aspects of MBC, such as their mucinous components and/or biologic aspects like AR expression, influence responses to different hormonal therapies. Moreover, while optimal treatment regimens for patients with MBC remain unclear, BCK4 cells may provide excellent models to study existing and potential novel therapeutic interventions.
Survival of patients with MBCPure is better than that of patients with MBCMixed [32, 47, 78, 79]; indeed the later has the same prognosis as IDC [26, 30, 69, 80]. Nevertheless, even a diagnosis of MBCPure may underestimate the risk of eventual (>12 years later) recurrence [81]. Thus despite its seemingly innocuous local behavior, MBCPure can and does metastasize [71]. As with other breast cancers, large tumor size (>4 cm) or the presence of lymph node metastases is associated with decreased survival for either MBCPure or MBCMixed [35]. The incidence of metastases in MBCMixed (29–72 %) is higher than that of MBCPure (0–26 %) [32–37] and both MBC types tend to be multifocal [69, 82]. Understanding what may influence the incidence of these unique histologic subtypes and under-standing which cases merit additional therapy and what the therapeutic targets might be are important issues. We propose that BCK4 cells are a novel and unique resource to investigate MBC specifically, and the plasticity of breast cancers in general.
In summary, MBC is a unique histology that we demonstrate as having plasticity between ductal and lobular components and highlights the opportunities and challenges of applying biologic understanding to traditional nomenclature. MBC of lobular origin may be much more common than originally realized. Furthermore whether MBC are ductal or lobular in origin cannot be easily discriminated solely on tumor appearance, or even by immunohistochemistry. The lobular identity of many MBC may require a panel of markers yet to be identified. We have developed a new human breast cancer cell line that models mucinous disease, and as xenografts gives rise to pure MBC or MBC mixed with ILC, depending on the estrogenization state of the mouse. The model recapitulates many aspects of clinical MBC including their histological appearance, luminal subtype, and protein marker expression. BCK4 cells are the first model of MBC, specifically those of lobular subtype. These cells will be useful for indepth studies of hormone regulated growth, MBC phenotypic plasticity, the biology of mucinous ILC, potential therapeutic interventions, and pattern of metastatic spread of this poorly understood breast cancer subtype.
Supplementary Material
Acknowledgments
These studies were supported by The Avon Foundation for Women (BMJ, KBH), NIH NCI CA026869, the National Foundation for Cancer Research, and the Breast Cancer Research Foundation (KBH). We are grateful to the University of Colorado Cancer Center Sequencing and Cytogenetics Core laboratories for technical support, to Dr. Hany Abdel-Hafiz for providing PCR primers for pS2 and CathD, and we thank Robert W. Burke for helpful discussions. All authors edited and approved the final manuscript.
Abbreviations
- MBC
Mucinous breast cancer
- PR
Progesterone receptor
- ER
Estrogen receptor
- AR
Androgen receptor
- GR
Glucocorticoid receptor
- ILC
Invasive lobular carcinoma
- IDC
Invasive ductal carcinoma
- IDC NOS
Invasive ductal carcinoma not otherwise specified
- MBCPure
Pure mucinous breast cancer
- MBCMixed
Mixed mucinous breast cancer
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-012-2377-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Purevsuren Jambal, Division of Endocrinology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8106, Aurora, CO 80045, USA.
Melanie M. Badtke, Division of Endocrinology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8106, Aurora, CO 80045, USA
J. Chuck Harrell, Department of Genetics, University of North Carolina, Chapel Hill, NC 27599, USA.
Virginia F. Borges, Division of Medical Oncology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8117, Aurora, CO 80045, USA
Miriam D. Post, Department of Pathology, University of Colorado, Anschutz Medical Campus, Aurora, CO 80045, USA
Grace E. Sollender, Division of Endocrinology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8106, Aurora, CO 80045, USA
Monique A. Spillman, Department of Obstetrics and Gynecology, University of Colorado, Anschutz Medical Campus, Aurora, CO 80045, USA
Kathryn B. Horwitz, Division of Endocrinology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8106, Aurora, CO 80045, USA Department of Pathology, University of Colorado, Anschutz Medical Campus, Aurora, CO 80045, USA.
Britta M. Jacobsen, Email: Britta.Jacobsen@ucdenver.edu, Division of Endocrinology, Department of Medicine, University of Colorado, Anschutz Medical Campus, Mail Stop 8106, Aurora, CO 80045, USA.
References
- 1.Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. World Health Organization Classification of Tumours. [Google Scholar]
- 2.Henson D, Tarone R. A study of lobular carcinoma of the breast based on the third national cancer survey in The United States of America. Tumori. 1979;65(2):133–142. doi: 10.1177/030089167906500201. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW. Infiltrating lobular carcinoma of the breast. Histopathology. 1982;6(2):149–161. doi: 10.1111/j.1365-2559.1982.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 4.Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979;3(6):467–488. doi: 10.1111/j.1365-2559.1979.tb03029.x. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrecher JS, Silverberg SG. Signet-ring cell carcinoma of the breast. The mucinous variant of infiltrating lobular carcinoma? Cancer. 1976;37(2):828–840. doi: 10.1002/1097-0142(197602)37:2<828::aid-cncr2820370231>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000;88(11):2561–2569. [PubMed] [Google Scholar]
- 7.Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000;88(11):2570–2577. doi: 10.1002/1097-0142(20000601)88:11<2570::aid-cncr20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor IF, Shembekar MV, Shousha S. Breast carcinoma developing in patients on hormone replacement therapy: a histological and immunohistological study. J Clin Pathol. 1998;51(12):935–938. doi: 10.1136/jcp.51.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, Holmberg SB, Lindtner J, Snyder R, Thurlimann B, Murray E, Viale G, Castiglione-Gertsch M, Coates AS, Goldhirsch A. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006–3014. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Langerod A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Karesen R, Botstein D, Borresen-Dale AL, Jeffrey SS. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15(6):2523–2536. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigelt B, Geyer FC, Natrajan R, Lopez-Garcia MA, Ahmad AS, Savage K, Kreike B, Reis-Filho JS. The molecular underpinning of lobular histological growth pattern: a genomewide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J Pathol. 2010;220(1):45–57. doi: 10.1002/path.2629. [DOI] [PubMed] [Google Scholar]
- 12.Bertucci F, Orsetti B, Negre V, Finetti P, Rouge C, Ahomadegbe JC, Bibeau F, Mathieu MC, Treilleux I, Jacquemier J, Ursule L, Martinec A, Wang Q, Benard J, Puisieux A, Birnbaum D, Theillet C. Lobular and ductal carcinomas of the breast have distinct genomic and expression profiles. Oncogene. 2008;27(40):5359–5372. doi: 10.1038/onc.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, Hajduch M, Murray P, Kolar Z. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser micro-dissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turashvili G, Bouchalova K, Bouchal J, Kolar Z. Expression of E-cadherin and c-erbB-2/HER-2/neu oncoprotein in high-grade breast cancer. Cesk Pathol. 2007;43(3):87–92. [PubMed] [Google Scholar]
- 15.Ellis IO, Pinder SE, Bobrow L, et al. Classifying invasive carcinomas Pathology reporting of breast disease. vol. no 58. Sheffield: NHSBSP publications: The Royal College of Pathologists; 2005. pp. 61–69. [Google Scholar]
- 16.Rosen PP. Rosen’s breast pathology. 3rd edn. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 17.Yu J, Bhargava R, Dabbs DJ. Invasive lobular carcinoma with extracellular mucin production and HER-2 overexpression: a case report and further case studies. Diagn Pathol. 2010;5:36. doi: 10.1186/1746-1596-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa M, Mohammadi A, Masood S. Lobular carcinoma of the breast with extracellular mucin: new variant of mucin-producing carcinomas? Pathol Int. 2009;59(6):405–409. doi: 10.1111/j.1440-1827.2009.02385.x. [DOI] [PubMed] [Google Scholar]
- 19.Haltas H, Bayrak R, Yenidunya S, Kosehan D, Sen M, Akin K. Invasive lobular carcinoma with extracellular mucin as a distinct variant of lobular carcinoma: a case report. Diagn Pathol. 2012;7(1):91. doi: 10.1186/1746-1596-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslow A, Brancaccio ME. Intracellular mucin production by lobular breast carcinoma cells. Arch Pathol Lab Med. 1976;100(11):620–621. [PubMed] [Google Scholar]
- 21.Gad A, Azzopardi JG. Lobular carcinoma of the breast: a special variant of mucin-secreting carcinoma. J Clin Pathol. 1975;28(9):711–716. doi: 10.1136/jcp.28.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost AR, Terahata S, Yeh IT, Siegel RS, Overmoyer B, Silverberg SG. The significance of signet ring cells in infiltrating lobular carcinoma of the breast. Arch Pathol Lab Med. 1995;119(1):64–68. [PubMed] [Google Scholar]
- 23.Merino MJ, Livolsi VA. Signet ring carcinoma of the female breast: a clinicopathologic analysis of 24 cases. Cancer. 1981;48(8):1830–1837. doi: 10.1002/1097-0142(19811015)48:8<1830::aid-cncr2820480821>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Hull MT, Seo IS, Battersby JS, Csicsko JF. Signet-ring cell carcinoma of the breast: a clinicopathologic study of 24 cases. Am J Clin Pathol. 1980;73(1):31–35. doi: 10.1093/ajcp/73.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Rosen PP, Lesser ML, Kinne DW. Breast carcinoma at the extremes of age: a comparison of patients younger than 35 years and older than 75 years. J Surg Oncol. 1985;28(2):90–96. doi: 10.1002/jso.2930280204. [DOI] [PubMed] [Google Scholar]
- 26.Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17(5):1442–1448. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 27.Weigelt B, Horlings HM, Kreike B, Hayes MM, Hauptmann M, Wessels LF, de Jong D, Van de Vijver MJ, Van’t Veer LJ, Peterse JL. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216(2):141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 28.Adsay NV, Merati K, Nassar H, Shia J, Sarkar F, Pierson CR, Cheng JD, Visscher DW, Hruban RH, Klimstra DS. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003;27(5):571–578. doi: 10.1097/00000478-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Zotter S, Hageman PC, Lossnitzer A, van den Tweel J, Hilkens J, Mooi WJ, Hilgers J. Monoclonal antibodies to epithelial sialomucins recognize epitopes at different cellular sites in adenolymphomas of the parotid gland. Int J Cancer Suppl. 1988;3:38–44. doi: 10.1002/ijc.2910410809. [DOI] [PubMed] [Google Scholar]
- 30.Kato N, Endo Y, Tamura G, Katayama Y, Motoyama T. Mucinous carcinoma of the breast: a multifaceted study with special reference to histogenesis and neuroendocrine differentiation. Pathol Int. 1999;49(11):947–955. doi: 10.1046/j.1440-1827.1999.00975.x. [DOI] [PubMed] [Google Scholar]
- 31.Chu JS, Chang KJ. Mucin expression in mucinous carcinoma and other invasive carcinomas of the breast. Cancer Lett. 1999;142(1):121–127. doi: 10.1016/s0304-3835(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 32.Fentiman IS, Millis RR, Smith P, Ellul JP, Lampejo O. Mucoid breast carcinomas: histology and prognosis. Br J Cancer. 1997;75(7):1061–1065. doi: 10.1038/bjc.1997.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre S, Cunha F, Bernardo M, Meneses e Sousa J, Cortez F, Soares J. Mucinous carcinoma of the breast: a pathologic study of 82 cases. J Surg Oncol. 1995;58(3):162–167. doi: 10.1002/jso.2930580305. [DOI] [PubMed] [Google Scholar]
- 34.Paramo JC, Wilson C, Velarde D, Giraldo J, Poppiti RJ, Mesko TW. Pure mucinous carcinoma of the breast: is axillary staging necessary? Ann Surg Oncol. 2002;9(2):161–164. doi: 10.1007/BF02557368. [DOI] [PubMed] [Google Scholar]
- 35.Norris HJ, Taylor HB. Prognosis of mucinous (gelatinous) carcinoma of the breast. Cancer. 1965;18:879–885. doi: 10.1002/1097-0142(196507)18:7<879::aid-cncr2820180716>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Toikkanen S, Kujari H. Pure and mixed mucinous carcinomas of the breast: a clinicopathologic analysis of 61 cases with long-term follow-up. Hum Pathol. 1989;20(8):758–764. doi: 10.1016/0046-8177(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 37.Silverberg SG, Kay S, Chitale AR, Levitt SH. Colloid carcinoma of the breast. Am J Clin Pathol. 1971;55(3):355–363. doi: 10.1093/ajcp/55.3.355. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen BB. Human mucinous breast carcinomas and their lymph node metastases. A histological review of 247 cases. Pathol Res Pract. 1985;180(4):377–382. doi: 10.1016/S0344-0338(85)80110-2. [DOI] [PubMed] [Google Scholar]
- 39.Gadre SA, Perkins GH, Sahin AA, Sneige N, Deavers MT, Middleton LP. Neovascularization in mucinous ductal carcinoma in situ suggests an alternative pathway for invasion. Histopathology. 2008;53(5):545–553. doi: 10.1111/j.1365-2559.2008.03152.x. [DOI] [PubMed] [Google Scholar]
- 40.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24(3):227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 41.Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66(16):8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- 42.Pinto MP, Badtke MM, Dudevoir ML, Harrell JC, Jacobsen BM, Horwitz KB. Vascular endothelial growth factor secreted by activated stroma enhances angiogenesis and hormone-independent growth of estrogen receptor-positive breast cancer. Cancer Res. 2010;70(7):2655–2664. doi: 10.1158/0008-5472.CAN-09-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, Horwitz KB, Sartorius CA. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128(1):45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Bokhoven A, Caires A, Maria MD, Schulte AP, Lucia MS, Nordeen SK, Miller GJ, Varella-Garcia M. Spectral karyotype (SKY) analysis of human prostate carcinoma cell lines. Prostate. 2003;57(3):226–244. doi: 10.1002/pros.10291. [DOI] [PubMed] [Google Scholar]
- 45.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen BM, Jambal P, Schittone SA, Horwitz KB. ALU repeats in promoters are position-dependent co-response elements (coRE) that enhance or repress transcription by dimeric and monomeric progesterone receptors. Mol Endocrinol. 2009;23(7):989–1000. doi: 10.1210/me.2009-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toikkanen S, Eerola E, Ekfors TO. Pure and mixed mucinous breast carcinomas: DNA stemline and prognosis. J Clin Pathol. 1988;41(3):300–303. doi: 10.1136/jcp.41.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigelt B, Geyer FC, Horlings HM, Kreike B, Halfwerk H, Reis-Filho JS. Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type. Mod Pathol. 2009;22(11):1401–1414. doi: 10.1038/modpathol.2009.112. [DOI] [PubMed] [Google Scholar]
- 49.Ko CD, Kim JS, Ko BG, Son BH, Kang HJ, Yoon HS, Cho EY, Gong G, Ahn SH. The meaning of the c-kit proto-oncogene product in malignant transformation in human mammary epithelium. Clin Exp Metastasis. 2003;20(7):593–597. doi: 10.1023/a:1027323210736. [DOI] [PubMed] [Google Scholar]
- 50.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Dexter T, Davies S, Bulmer K, Ford E, Parry S, Budroni M, Palmieri G, Neville AM, O’Hare MJ, Lakhani SR. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64(9):3037–3045. doi: 10.1158/0008-5472.can-03-2028. [DOI] [PubMed] [Google Scholar]
- 51.Wan Y, Nordeen SK. Overlapping but distinct gene regulation profiles by glucocorticoids and progestins in human breast cancer cells. Mol Endocrinol. 2002;16(6):1204–1214. doi: 10.1210/mend.16.6.0848. [DOI] [PubMed] [Google Scholar]
- 52.Ghatge RP, Jacobsen BM, Schittone SA, Horwitz KB. The progestational and androgenic properties of medroxyprogesterone acetate: gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast Cancer Res. 2005;7(6):R1036–R1050. doi: 10.1186/bcr1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karey KP, Sirbasku DA. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988;48(14):4083–4092. [PubMed] [Google Scholar]
- 54.Sartorius CA, Shen T, Horwitz KB. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat. 2003;79(3):287–299. doi: 10.1023/a:1024031731269. [DOI] [PubMed] [Google Scholar]
- 55.Waaseth M, Bakken K, Dumeaux V, Olsen KS, Rylander C, Figenschau Y, Lund E. Hormone replacement therapy use and plasma levels of sex hormones in the Norwegian Women and Cancer postgenome cohort—a cross-sectional analysis. BMC Womens Health. 2008;8:1. doi: 10.1186/1472-6874-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molavi D, Argani P. Distinguishing benign dissecting mucin (stromal mucin pools) from invasive mucinous carcinoma. Adv Anat Pathol. 2008;15(1):1–17. doi: 10.1097/PAP.0b013e31815e52aa. [DOI] [PubMed] [Google Scholar]
- 57.Wilson TE, Helvie MA, Oberman HA, Joynt LK. Pure and mixed mucinous carcinoma of the breast: pathologic basis for differences in mammographic appearance. AJR Am J Roentgenol. 1995;165(2):285–289. doi: 10.2214/ajr.165.2.7618541. [DOI] [PubMed] [Google Scholar]
- 58.Dabbs DJ, Bhargava R, Chivukula M. Lobular versus ductal breast neoplasms: the diagnostic utility of p120 catenin. Am J Surg Pathol. 2007;31(3):427–437. doi: 10.1097/01.pas.0000213386.63160.3f. [DOI] [PubMed] [Google Scholar]
- 59.Capella C, Eusebi V, Mann B, Azzopardi JG. Endocrine differentiation in mucoid carcinoma of the breast. Histopathology. 1980;4(6):613–630. doi: 10.1111/j.1365-2559.1980.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 60.Kehr EL, Jorns JM, Ang D, Warrick A, Neff T, Degnin M, Lewis R, Beadling C, Corless CL, Troxell ML. Mucinous breast carcinomas lack PIK3CA and AKT1 mutations. Hum Pathol. 2012 doi: 10.1016/j.humpath.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Eltorky M, Hall JC, Osborne PT, el Zeky F. Signet-ring cell variant of invasive lobular carcinoma of the breast. A clinicopathologic study of 11 cases. Arch Pathol Lab Med. 1994;118(3):245–248. [PubMed] [Google Scholar]
- 62.Raju U, Ma CK, Shaw A. Signet ring variant of lobular carcinoma of the breast: a clinicopathologic and immunohistochemical study. Mod Pathol. 1993;6(5):516–520. [PubMed] [Google Scholar]
- 63.Harris M, Vasudev KS, Anfield C, Wells S. Mucin-producing carcinomas of the breast: ultrastructural observations. Histopathology. 1978;2(3):177–188. doi: 10.1111/j.1365-2559.1978.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 64.Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121(6):884–892. doi: 10.1309/A09E-RYMF-R64N-ERDW. [DOI] [PubMed] [Google Scholar]
- 65.Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17(4):491.3–496.3. doi: 10.3322/canjclin.32.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8(1):47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 67.Khilko N, Wang J, Wei B, Hicks DG, Tang P. Invasive lobular carcinomas do not express basal cytokeratin markers CK5/6, CK14 and CK17. Breast Cancer (Auckl) 2010;4:49–55. doi: 10.4137/BCBCR.S5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huiping C, Sigurgeirsdottir JR, Jonasson JG, Eiriksdottir G, Johannsdottir JT, Egilsson V, Ingvarsson S. Chromosome alterations and E-cadherin gene mutations in human lobular breast cancer. Br J Cancer. 1999;81(7):1103–1110. doi: 10.1038/sj.bjc.6690815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111(3):541–547. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 70.Clayton F. Pure mucinous carcinomas of breast: morphologic features and prognostic correlates. Hum Pathol. 1986;17(1):34–38. doi: 10.1016/s0046-8177(86)80152-6. [DOI] [PubMed] [Google Scholar]
- 71.Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, Ditkoff BA, Schnabel FR. Pure mucinous carcinoma of the breast. Am J Surg. 2004;187(4):528–532. doi: 10.1016/j.amjsurg.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 72.Reeves GK, Beral V, Green J, Gathani T, Bull D Million Women Study C. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7(11):910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 73.Shousha S, Coady AT, Stamp T, James KR, Alaghband-Zadeh J. Oestrogen receptors in mucinous carcinoma of the breast: an immunohistological study using paraffin wax sections. J Clin Pathol. 1989;42(9):902–905. doi: 10.1136/jcp.42.9.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prat A, Ellis MJ, Perou CM. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol. 2012;9(1):48–57. doi: 10.1038/nrclinonc.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Acs G, Esposito NN, Kiluk J, Loftus L, Laronga C. A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX recurrence scores in low-grade invasive breast carcinomas. Mod Pathol. 2012;25(4):556–566. doi: 10.1038/modpathol.2011.194. [DOI] [PubMed] [Google Scholar]
- 76.Kounalakis N, Diamond J, Rusthoven K, Horn W, Jindal S, Wisell J, Klein CE, Elias A, Finlayson C, Borges VF. Diagnosis of invasive lobular carcinoma in a young woman presenting with pleomorphic lobular carcinoma in situ on core biopsy. Oncology (Williston Park) 2011;25(4):351–356. [PubMed] [Google Scholar]
- 77.Cho LC, Hsu YH. Expression of androgen, estrogen and progesterone receptors in mucinous carcinoma of the breast. Kaohsiung J Med Sci. 2008;24(5):227–232. doi: 10.1016/S1607-551X(08)70146-3. [DOI] [PubMed] [Google Scholar]
- 78.Melamed MR, Robbins GF, Foote FW., Jr Prognostic significance of gelatinous mammary carcinoma. Cancer. 1961;14:699–704. doi: 10.1002/1097-0142(199007/08)14:4<699::aid-cncr2820140404>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 79.Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer. 1988;61(5):989–996. doi: 10.1002/1097-0142(19880301)61:5<989::aid-cncr2820610522>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 80.Stanley MW, Tani EM, Skoog L. Mucinous breast carcinoma and mixed mucinous-infiltrating ductal carcinoma: a comparative cytologic study. Diagn Cytopathol. 1989;5(2):134–138. doi: 10.1002/dc.2840050205. [DOI] [PubMed] [Google Scholar]
- 81.Rosen PP, Wang T-Y. Colloid carcinoma of the breast. Analysis of 64 patients with long term followup. Am J Clin Pathol. 1980;73:304. [Google Scholar]
- 82.Pusztai L, Sotiriou C, Buchholz TA, Meric F, Symmans WF, Esteva FJ, Sahin A, Liu ET, Hortobagi GN. Molecular profiles of invasive mucinous and ductal carcinomas of the breast: a molecular case study. Cancer Genet Cytogenet. 2003;141(2):148–153. doi: 10.1016/s0165-4608(02)00737-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.