Abstract
Aims
Endothelial SKCa and IKCa channels play an important role in the regulation of vascular function and systemic blood pressure. Based on our previous findings that small molecule activators of SKCa and IKCa channels (i.e. NS309 and SKA-31) can inhibit myogenic tone in isolated resistance arteries, we hypothesized that this class of compounds may induce effective vasodilation in an intact vascular bed, such as the coronary circulation.
Methods and results
In a Langendorff-perfused, beating rat heart preparation, acute bolus administrations of SKA-31 (0.01–5 µg) dose-dependently increased total coronary flow (25–30%) in both male and female hearts; these responses were associated with modest, secondary increases in left ventricular (LV) systolic pressure and heart rate. SKA-31 evoked responses in coronary flow, LV pressure, and heart rate were qualitatively comparable to acute responses evoked by bradykinin (1 µg) and adenosine (10 µg). In the presence of apamin and TRAM-34, selective blockers of SKCa and IKCa channels, respectively, SKA-31 and bradykinin-induced responses were largely inhibited, whereas the adenosine-induced changes were blocked by ∼40%; TRAM-34 alone produced less inhibition. Sodium nitroprusside (SNP, 0.2 μg bolus dose) evoked changes in coronary flow, LV pressure, and heart rate were similar to those induced by SKA-31, but were unaffected by apamin + TRAM-34. The NOS inhibitor L-NNA reduced bradykinin- and adenosine-evoked changes, but did not affect responses to either SKA-31 or SNP.
Conclusion
Our study demonstrates that SKA-31 can rapidly and reversibly induce dilation of the coronary circulation in intact functioning hearts under basal flow and contractility conditions.
Keywords: Coronary circulation, Endothelium, Ca2+-activated K+ channel, Vasodilation
1. Introduction
The contributions of endothelium-derived nitric oxide (NO) and prostacyclin (PGI2) to agonist-mediated vasodilation in the vasculature are well established; however, it is now apparent that additional cellular processes may be activated to evoke vasorelaxation via mechanisms not strictly dependent on NO and/or PGI2 synthesis.1–3 Early studies by several investigators (for review1) demonstrated that vasodilatory hormones evoked negative membrane hyperpolarization in isolated vascular endothelial cells, and subsequent studies indicated that this response was largely dependent upon the activation of small- and intermediate-conductance, Ca2+-activated K+ channels (SKCa and IKCa channels, respectively) that are prominently expressed in vascular endothelium.4 Functionally, we and others have demonstrated that pharmacologic blockade of SKCa and IKCa channels interfere with both agonist-evoked NO synthesis, hyperpolarization of vascular endothelium and smooth muscle, and subsequent vasorelaxation.5–10 Moreover, genetic disruption of IKCa channels in mice impairs agonist-evoked vasodilation, which is amplified when genes for both SKCa and IKCa channels are inactivated in the same animal.11–13 In addition to their potential contribution to stimulated NO production,14,15 it is therefore generally accepted that SKCa and IKCa channel activation generates an ‘endothelium-dependent hyperpolarization’ (EDH) signal that is readily conducted both along the endothelium and into the smooth muscle cell layer via gap junction-mediated, cell–cell communication, leading to smooth muscle cell hyperpolarization, the closure of voltage-gated calcium channels, and inhibition of vascular tone.16 Such observations imply that alterations of endothelial SKCa and IKCa activities can have profound effects on the control of vascular tone both locally and at a distance via multiple cellular pathways. Collectively, these data highlight the physiologic importance of EDH and SKCa and IKCa channels to the regulation of vascular tone16 and make it evident how impairment of these channels may contribute to endothelial dysfunction (for recent reviews17,18).
Positive gating modulators of SKCa and IKCa channels, such as NS309 and SKA-31, have been shown to augment agonist-induced inhibition of myogenic tone in isolated resistance arteries14,15,19 and to lower blood pressure following acute administrations in conscious mice and dogs.19,20 However, there has been limited information regarding the effects of such agents in distinct vascular beds, and it is not at all clear whether all vascular beds are affected in a similar manner by these activators. Moreover, it is unclear whether gender and/or hormonal influences, which are known to affect vascular reactivity and health,21,22 may also impact the effects of KCa channel activators. To address such issues, we have exploited the Langendorff-perfused, beating rat heart preparation to examine the direct effects of endothelial SKCa and IKCa channel activation on total flow in the coronary circulation using the direct channel activator SKA-31.19 The novel results of our study demonstrate that a SKCa and IKCa channel activator can evoke direct and robust vasodilation in the coronary circulation in both male and female rat hearts in the apparent absence of additional hormonal and neural inputs.
2. Methods
2.1. Experimental protocol
The experimental protocols used in this study were approved by the University of Calgary Animal Care Committee, and conform with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (8th edition, 2011). Sprague–Dawley rats (11–12 weeks of age) were injected intraperioneally with sodium pentobarbitol (50 mg/kg) to induce surgical anaesthesia (i.e. stage 3, loss of blink reflex), and then sacrificed by cervical dislocation. The heart was carefully excised and placed in ice-cold Krebs buffer (see Supplementary material online), followed by removal of superficial fat and connective tissues. The heart was then mounted on a blunted 16-gauge needle and perfused retrograde through the ascending aorta with Krebs buffer at constant pressure (i.e. 90 cm H2O). This forces the aortic valve to close and diverts perfusate into the coronary arteries via the coronary ostia. The flow rate was monitored via an inline Doppler flow probe and a Transonic T206 flow meter. The perfusate was maintained at 37°C by a circulating water bath and gassed continuously with 5% CO2 and 95% air. Iso-volumetric left ventricular (LV) developed pressure was measured with a fluid-filled latex balloon inserted into the left ventricle via the left atrium and connected to a pressure transducer (model 60–3002, Harvard Apparatus). The balloon volume was adjusted to obtain a diastolic pressure of ∼20 mmHg. LV pressure, heart rate, and coronary flow signals were digitally recorded using WinDaq data acquisition software. Langendorff-perfused hearts were allowed to equilibrate until heart rate and contractility reached steady state (i.e. 20 min or more), and drugs were acutely administered as 0.1 mL bolus injections into the perfusate via an injection port positioned upstream of the heart. In some experiments, hearts were treated with N-nitro-l-arginine (L-NNA, 0.1 mM), apamin (0.1 µM), and/or TRAM-34 (1 µM) for 25–30 min, and then drug exposures were repeated in the continued presence of the treatment.
2.2. Data analysis
Drug-induced changes in coronary flow were quantified by first integrating the area beneath the flow tracing immediately prior to the time point of drug administration (basal value) and then the area beneath the curve following development of the full drug-induced response (drug); standard epochs of 100 s were used for each determination. Thus, an individual drug-induced response was always compared with a matched control measurement obtained immediately prior to drug perfusion, thereby minimizing any influence of tissue ‘run-down’ over the duration of a typical 2–3 h experiment. This same protocol was also used for the analysis of changes in LV systolic pressure and heart rate (see below). Drug-induced changes in flow were determined using the equation below and are expressed as a percentage increase above the basal flow:
The drug-associated change in average LV peak systolic pressure was calculated from 20 consecutive contractions observed in the presence of an administered drug and compared with the basal systolic pressure recorded immediately prior to the point of administration. The drug-associated change in heart rate was calculated from the LV pressure tracing by measuring the peak-to-peak intervals, and was compared with the average basal value measured immediately prior.
2.3. Statistical analysis
Data are presented as mean ± SEM. Statistically significant differences between different experimental results were evaluated using a two-tailed Student's t-test; significance was taken at P < 0.05. In some cases (e.g. Figures 3 and 4), data sets were analysed by one-way ANOVA and a Tukey's post hoc test.
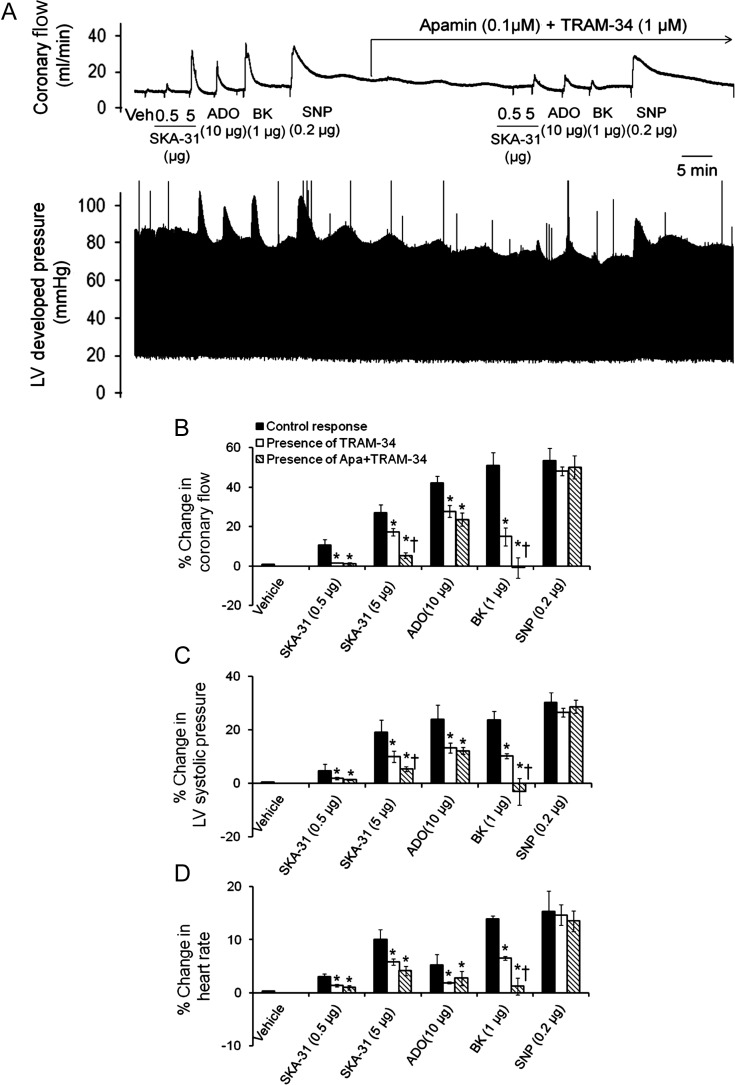
Figure 3.
Treatment with apamin and/or TRAM-34 inhibits the evoked responses to SKA-31, bradykinin, and adenosine, but not SNP, in male hearts. (A) Representative tracings of coronary flow (upper) and LV developed pressure (lower) in response to acute administrations of SKA-31 (0.5 and 5 µg), SNP (0.2 µg), bradykinin (BK, 1 µg), and adenosine (ADO, 10 µg) prior to and in the continued presence of apamin (0.1 µM) and TRAM-34 (1 µM). (B–D) Histograms quantifying SKA-31, BK, ADO, and SNP evoked increases in coronary flow (B), LV systolic pressure (C), and heart rate (D) under control conditions, and then in either the presence of apamin + TRAM-34 or TRAM-34 alone. Data are presented as mean ± SEM, *P < 0.05 vs. evoked response in the absence of apamin + TRAM-34.
Figure 4.
Exposure to apamin and/or TRAM-34 inhibits evoked responses to SKA-31, bradykinin, and adenosine, but not SNP, in female hearts. (A) Representative tracings of coronary flow (upper) and LV developed pressure (lower) in response to acute administration of SKA-31 (0.5 and 5 µg), SNP (0.2 µg), bradykinin (BK, 1 µg), and adenosine (ADO, 10 µg) in the absence and in the continued presence of apamin (0.1 µM) and TRAM-34 (1 µM). (B–D) Histograms quantifying SKA-31, BK, ADO, and SNP evoked increases in coronary flow (B), LV systolic pressure (C), and heart rate (D) under control conditions, and then in either the presence of apamin + TRAM-34, or TRAM-34 alone. Data are presented as mean ± SEM, *P < 0.05 vs. evoked response in the absence of apamin + TRAM-34.
3. Results
3.1. SKA-31 increases coronary flow in isolated perfused rat hearts
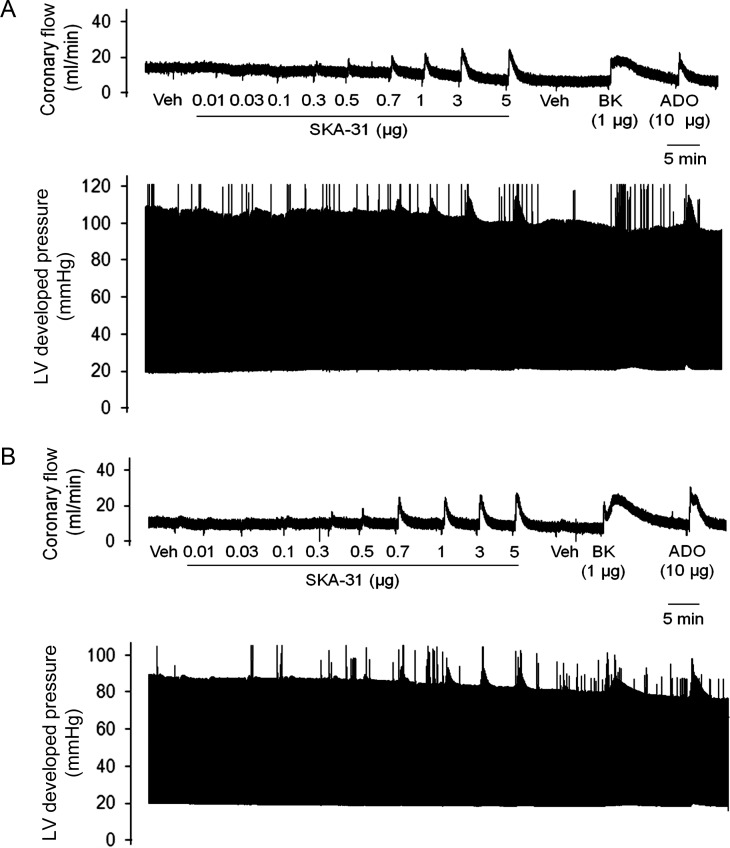
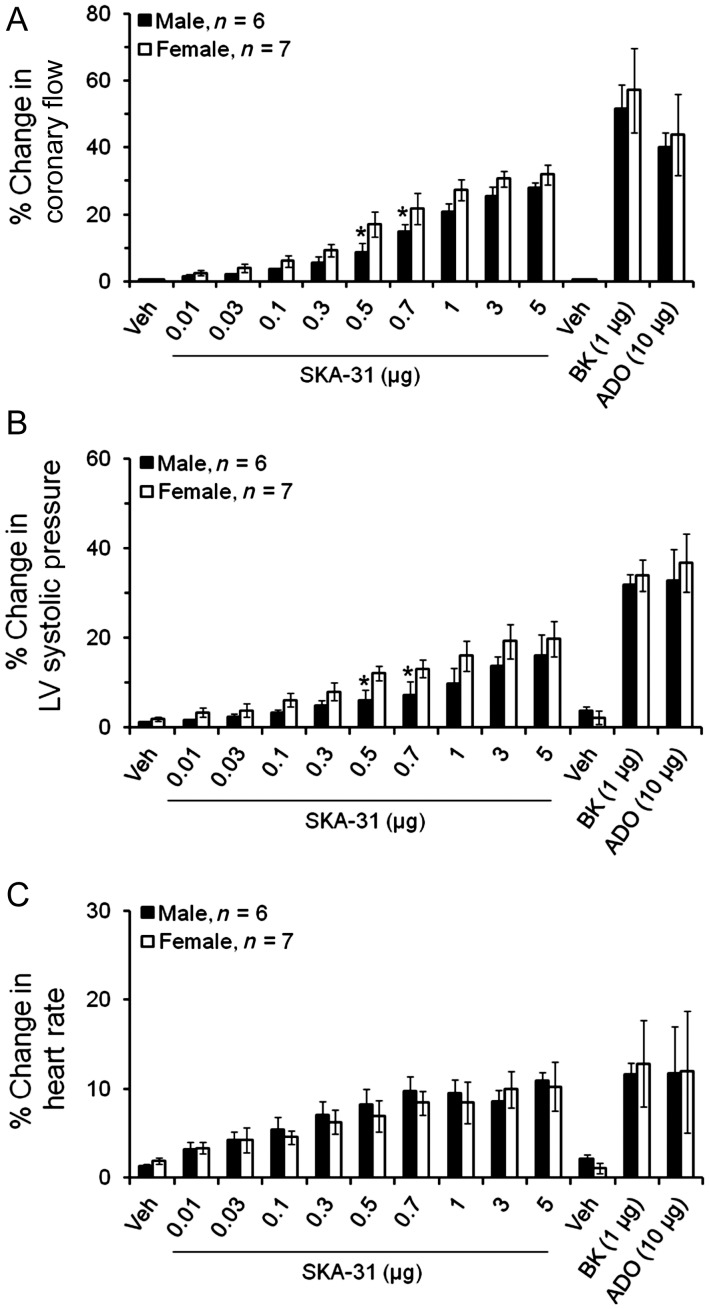
As published information on the actions of small molecule SKCa and IKCa channel activators within individual vascular beds is limited, we chose to examine the actions of SKA-31 on the coronary circulation of isolated, beating hearts from male and female rats. Moreover, gender and sex-related hormones are known to impact both vascular tone and coronary function,21,22 and the potential influence of these factors on the actions of SKCa/IKCa channel activators has not been examined. Experimentally, acute bolus administrations of SKA-31 (0.01–5 µg doses) delivered to either male or female hearts increased coronary flow above baseline levels in a dose-dependent manner (Figure 1A and B), and maximal increases in coronary flow were found to be similar in male (27.9 ± 1.6%) and female (31.8 ± 2.9%) preparations. Doses of SKA-31 > 5 µg did not produce further enhancement of coronary flow (data not shown). Interestingly, mid-range doses of SKA-31 (i.e. 0.5–1 µg) evoked increases in coronary flow that were significantly higher in female hearts compared with male hearts (Figure 2A). SKA-31 evoked responses were also comparable with the increases in coronary flow produced by bolus administrations of bradykinin (1 µg), and adenosine (10 µg) (Figure 1A and B). Bradykinin, an endothelium-dependent vasodilator,23 increased coronary flow (51.6 ± 7.1%) in male and (57.1 ± 12.5%) in female hearts (Figure 2A); similarly adenosine, another coronary artery dilator, produced comparable increases in coronary flow in male (39.9 ± 4.5%) and female (43.8 ± 12.1%) hearts (Figure 2A).
Figure 1.
SKA-31 transiently increases coronary flow in male and female hearts. (A and B) Simultaneous recordings of stimulus-evoked changes in coronary flow and LV developed pressure in isolated hearts from male and female rats, respectively. Changes in coronary flow were observed following acute single dose administrations of SKA-31, bradykinin (BK), and adenosine (ADO) in the amounts denoted beneath the flow tracing. Flow and pressure recordings in (A) and (B) are representative of six male and seven female heart preparations subjected to the same experimental protocol.
Figure 2.
Quantification of the drug-induced changes in coronary flow, LV systolic pressure, and heart rate following acute bolus administration of SKA-31 (0.01–5 µg), bradykinin (BK, 1 µg), adenosine (ADO, 10 µg), and solvent vehicle (Veh) in male and female hearts. Values are expressed as a percentage increase in a given parameter relative to the baseline value recorded immediately prior to the administration of a given compound. The histogram in (A) quantifies drug-induced changes in total coronary flow. (B and C) Evoked changes in LV systolic pressure and heart rate, respectively, in response to individual drug administrations. Data are presented as mean ± SEM, *P < 0.05 vs. female hearts.
3.2. SKA-31 evoked increases in coronary flow are associated with modest increases in LV systolic pressure and heart rate
In addition to the primary effects of SKA-31 on total coronary flow, we also observed transient increases in LV developed pressure following acute administrations of SKA-31, bradykinin, or adenosine (Figures 1A and B and 2B). A maximal dose of SKA-31 (5 µg) elevated LV systolic pressure 15.9 ± 4.8 and 19.8 ± 3.9% above baseline levels in male and female hearts, respectively. Both bradykinin and adenosine produced qualitatively similar increases in LV systolic pressure in male and female hearts and these responses were somewhat greater than those observed in the presence of SKA-31 (Figure 2B). The larger changes produced by bradykinin and adenosine in LV systolic pressure correlated closely with the larger increases in coronary flow produced by these agents compared with SKA-31. As shown in Supplementary material online, Figure S1, these drug-associated changes in developed LV pressure were also accompanied by increases in the rates of change of LV contraction and relaxation (i.e. +dP/dT and −dP/dT). Kinetic analysis of the flow and pressure recordings revealed that the drug-associated changes in coronary flow typically preceded changes in LV systolic pressure and heart rate (see below) by 2–3 s. These observations indicate that coronary vasodilation occurred as the primary response to drug administration, followed by secondary responses in LV developed pressure and heart rate.
Similar to LV systolic pressure, we also observed very modest increases (<13%) in the intrinsic beating rates of both male and female hearts following acute administration of SKA-31 (5 μg), bradykinin (1 μg), and adenosine (10 μg) (Figure 2C); no statistical difference was noted among the drug-induced heart rate responses.
3.3. Coronary flow is more sensitive to SKA-31 compared with LV pressure and heart rate
To evaluate the sensitivities of the SKA-31 evoked increases in coronary flow, LV systolic pressure, and heart rate, EC50 values for SKA-31 were determined by calculating dose–response curves (Supplementary material online, Figure S2). For male and female hearts, respectively, these values were 0.76 ± 0.09 vs. 0.54 ± 0.12 µg for coronary flow, 1.04 ± 0.12 vs. 0.69 ± 0.17 µg for LV systolic pressure and 0.79 ± 0.08 vs. 0.63 ± 0.05 µg for heart rate. No statistically significant differences were noted between the SKA-31-induced changes in these parameters for male and female hearts.
3.4. Apamin and/or TRAM-34 block the SKA-31 evoked increase in coronary flow
To verify the channel-selective actions of SKA-31, we assessed the SKA-31-induced increases in coronary flow (0.5 and 5 µg doses) in the absence and presence of apamin and TRAM-34, established blockers of SKCa and IKCa channels,24 respectively (Figures 3A and 4A). The effects of these blockers were also evaluated on the responses induced by bradykinin (1 µg), adenosine (10 µg), and the direct smooth muscle relaxant sodium nitroprusside (SNP) (0.2 µg). Following control responses to each compound, treatment of hearts with apamin (0.1 µM) + TRAM-34 (1 µM) for ∼25 min blocked the increase in coronary flow evoked by a 0.5 μg dose of SKA-31 and reduced the response to a 5 μg dose by ∼80% (Figures 3A and B and 4A and B). Interestingly, this treatment virtually abolished the bradykinin-induced increase in coronary flow, and revealed a modest vasoconstrictor action of bradykinin in the male coronary circulation. The adenosine-evoked response was decreased by ∼40% in both preparations. As anticipated, the SNP-induced increase in coronary flow was unaffected by apamin + TRAM-34, as these agents do not interfere directly with vascular smooth muscle function.8,24 As shown in Table 1, apamin + TRAM-34 treatment also produced a significant decrease (∼10%) in the level of basal coronary flow in these preparations, with a much weaker effect on heart rate. Treatment with the IKCa channel blocker TRAM-34 alone also inhibited drug-evoked increases in coronary flow, LV developed pressure, and heart rate (Figures 3B–D and 4B–D); however, the extent of inhibition was typically less compared with the combination of apamin + TRAM-34. Exposure to TRAM-34 also produced a very modest (∼5%) decrease in basal coronary flow, and had no effect on heart rate (Table 1). Importantly, control experiments revealed that the magnitudes of drug-induced increases in coronary flow evoked by repeated challenges with SKA-31, bradykinin, adenosine, and SNP were not significantly different when compared with initial responses (Supplementary material online, Figure S3). This finding demonstrates that the observed loss of drug responsiveness in the presence of apamin + TRAM-34 is not due to either tissue run-down or desensitization to the administered compounds.
Table 1.
Effect of treatment with L-NNA, TRAM-34, apamin + TRAM-34, and L-NNA + apamin + TRAM-34 on basal coronary flow and heart rate
| Treatment protocol | Basal flow (F2/F1) | Basal HR (R2/R1) |
|---|---|---|
| Control | 0.91 ± 0.015 | 0.98 ± 0.016 |
| TRAM-34 | 0.85 ± 0.017 | 0.95 ± 0.023 |
| Apamin + TRAM-34 | 0.82 ± 0.011* | 0.93 ± 0.014 |
| L-NNA | 0.68 ± 0.013* | 0.91 ± 0.017* |
| L-NNA + Apamin+ TRAM-34 | 0.64 ± 0.019* | 0.88 ± 0.012* |
Data represent the ratios of coronary flow and heart rate measurements obtained near the start of the experiment (i.e. after acute administration of vehicle only) and following a 25–30 min treatment with L-NNA (0.1 mM), apamin (0.1 μM), and/or TRAM-34 (1 μM). Control data were obtained from time control experiments (see Supplementary material online, Figure S3), in which no drug was added during the intervening treatment period. Data represent the means ± SEM calculated from four to nine male and female hearts in total.
*P < 0.05 vs. control as determined by one-way ANOVA and a Tukey's post hoc test.
Drug-associated changes in LV systolic pressure and heart rate were also inhibited by treatment with apamin and/or TRAM-34 in a manner similar to that observed for the drug-evoked increase in coronary flow. Qualitatively, apamin + TRAM-34 treatment blocked stimulated changes in LV systolic pressure, heart rate, and +dP/dT and −dP/dT associated with SKA-31, bradykinin, and adenosine administration, but did not affect the increase in these parameters associated with the SNP administration (Figures 3C and D, 4C and D, Supplementary material online, Figure S1).
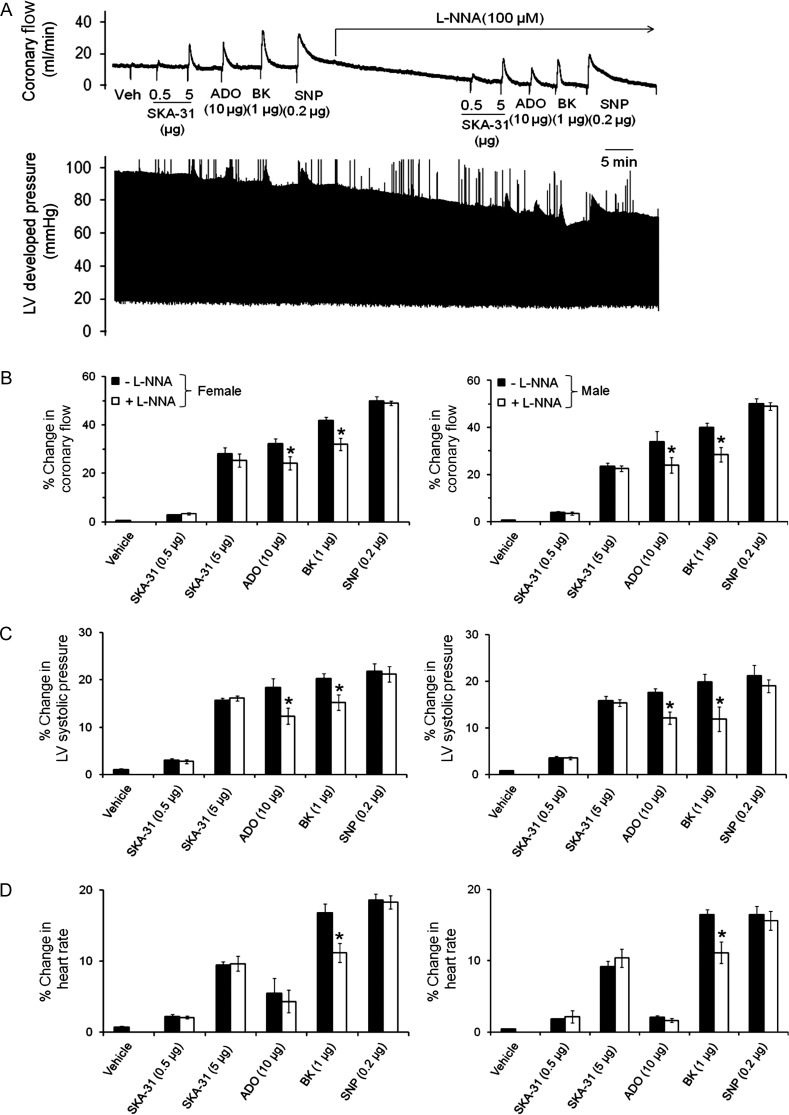
To examine the contribution of de novo NO production to the observed changes in cardiac performance, hearts were exposed to the NO synthase inhibitor L-NNA (0.1 mM, 25–30 min). As shown in Figure 5, L-NNA pre-treatment reduced the bradykinin- and adenosine-evoked increases in coronary flow by 25–30% in both male and female hearts, but did not affect increases induced by either SKA-31 or SNP. L-NNA treatment also caused a significant reduction in the basal coronary flow (∼20% below control), with a much weaker effect on heart rate (Table 1).
Figure 5.
The NO synthase inhibitor L-NNA does not impact the SKA-31-evoked increase in coronary flow. (A) Representative tracings of coronary flow (upper) and LV developed pressure (lower) in a male rat heart in response to acute administration of SKA-31 (0.5 and 5 µg), SNP (0.2 µg), bradykinin (BK, 1 µg), and adenosine (ADO, 10 µg) in the absence and continued presence of 0.1 mM L-NNA. Histograms quantifying the SKA-31, BK, ADO, and SNP evoked changes in coronary flow, LV systolic pressure, and heart rate for male and female tissues are displayed in (B)–(D), respectively. Data are given as mean ± SEM, *P < 0.05 vs. evoked response in the absence of L-NNA.
To examine whether inhibition of eNOS in the presence of KCa channel blockade would have a synergistic effect on the adenosine-evoked increase in coronary flow, male and female hearts were treated with a combination of L-NNA + apamin + TRAM-34. In the presence of all three blockers, the adenosine-induced vasodilation was decreased slightly more compared with L-NNA alone (P < 0.05), but we did not observe a greater degree of inhibition compared with apamin + TRAM-34 (Supplementary material online, Figure S4). Vasodilatory responses to both SKA-31 and bradykinin were not affected to a greater extent by this triple combination of blockers compared with apamin + TRAM-34 treatment. Exposure to L-NNA + apamin + TRAM-34 also produced a similar decrease in basal coronary flow and heart rate compared with L-NNA alone (Table 1).
4. Discussion
In the present study, we have demonstrated that direct administration of SKA-31, a novel and selective activator of SKCa and IKCa channels, dose-dependently increases total flow in the coronary circulation of isolated, beating rat hearts perfused under constant pressure. The magnitude of SKA-31-induced coronary vasodilation was similar in male and female hearts at the ages examined (i.e. 12 weeks), with a modest trend towards greater sensitivity to SKA-31 in female hearts (Figure 2A). This latter observation is in line with reported differences in the regulation of vascular tone and coronary function in relation to gender and sex-related hormones.21,22 Our data further suggest that the coronary vasodilatation evoked by SKA-31 is directly responsible for the observed flow enhancement, and unlikely to be dependent upon the actions and/or presence of an endogenous vasodilatory agonist in this preparation. The observed SKA-31-evoked increase in coronary flow was comparable with vasodilatory responses evoked by bradykinin, adenosine, and SNP in male and female hearts, even though the mechanisms of action for these four agents differ at the cellular level. SKA-31 acts primarily as a positive gating modulator of SKCa and IKCa channels,19 which are expressed in vascular endothelium25,26 and largely absent in contractile vascular smooth muscle.27,28 Bradykinin is an endogenous and potent, endothelium-dependent vasorelaxant that acts via a GPCR to stimulate release of NO and PGI2, along with endothelial membrane hyperpolarization.23,29 Likewise, adenosine increases coronary flow by activating primarily the A2A GPCR subtype that is present on both endothelial and smooth muscle cells,30 leading to adenylyl cyclase stimulation and cAMP elevation. SNP, a therapeutic nitrovasodilator that releases NO following metabolic conversion31 and directly relaxes smooth muscle, was used to evaluate coronary smooth muscle function independent of the endothelium.
Selective blockade of endothelial SKCa and IKCa channels by apamin and TRAM-34, respectively, largely inhibited (∼80%) the increase in coronary flow evoked by a 5 μg dose of SKA-31 in male and female hearts, and these same blockers abolished the bradykinin-induced vasodilation (Figures 3A and B and 4A and B). While we anticipated that SKA-31-induced response would be impaired by apamin + TRAM-34, the observed abolition of the bradykinin-evoked vasodilation demonstrates the critical functional role of endothelial SKCa and/or IKCa channels play in this receptor-mediated effect. Our data are thus consistent with recent studies demonstrating the presence of both SKCa and IKCa channels in coronary arterial vessels25,26 and their involvement in agonist-evoked increases in coronary flow.32,33
Treatment of hearts with TRAM-34 alone allowed us to assess the selective contribution of IKCa channels to the observed drug-induced effects. As shown in Figures 3B–D and 4B–D, responses evoked by either adenosine or 0.5 μg SKA-31 were inhibited to the same extent by TRAM-34 alone compared with apamin + TRAM-34, suggesting that SKCa channel activity contributes very little to responses evoked by this low dose of SKA-31 or adenosine. Compared with TRAM-34 + apamin, treatment with TRAM-34 alone was less effective at blocking responses to bradykinin and 5 μg SKA-31, suggesting that these responses involve both SKCa and IKCa channel activation under our experimental conditions. The prominent calcium-mobilizing action of bradykinin in endothelium34,35 would thus be consistent with activation of both KCa channel types. SKA-31 is known to activate both SKCa and IKCa channels; however, this agent activates IKCa channels at ∼10-fold lower concentrations than SKCa channels,19 which is in line with our observations reported here.
In addition to stimulating cAMP production, adenosine also been shown to hyperpolarize the endothelium,36 and Ribeiro et al.37 have recently reported that adenosine-induced relaxation of pre-constricted porcine resistance arteries could be partially inhibited by either apamin or TRAM-34. In our study, the adenosine evoked increase in coronary flow was significantly reduced (∼40%) by apamin + TRAM-34 treatment in both male and female hearts (Figures 3A and B and 4A and B), which is consistent with the putative activation of endothelial SKCa and IKCa channels, as described above. The remaining adenosine-evoked vasodilation observed following apamin + TRAM-34 treatment likely occurs via direct effects of adenosine on coronary smooth muscle,30 and would not expected to be sensitive to SKCa and IKCa channel blockade. Exactly how endothelial adenosine receptors lead to enhancement of SKCa and/IKCa channel activity is unclear at present.
We have previously reported that the eNOS inhibitor L-NAME does not impair the inhibition of myogenic tone by an endothelial KCa channel activator in cremaster resistance arteries.15 Consistent with this earlier result, we observed that treatment with L-NNA (0.1 mM) did not impair the SKA-31 induced increase in coronary flow, but did significantly reduce flow responses to both adenosine and bradykinin (Figure 5A and B). As endothelial KCa channel activators primarily induce membrane hyperpolarization in small arteries without evoking calcium mobilization,14 these agents would not be expected to directly increase eNOS activity on their own and their vasorelaxant action should be largely insensitive to L-NNA treatment. In contrast, both bradykinin and adenosine would be expected to increase eNOS activity via calcium and/or protein phosphorylation-dependent pathways35,38 and thus, L-NNA treatment would be predicted to reduce the vasodilatory capacity of these hormones, as reported in Figure 5. The slightly greater inhibition of the adenosine-evoked vasodilation observed in the presence of L-NNA + apamin + TRAM-34 (Supplementary material online, Figure S4) further suggests that the adenosine-stimulated eNOS and endothelial KCa channel activities act synergistically to induce coronary vasodilation. The proportion of evoked vasodilation remaining in the presence of L-NNA + apamin + TRAM-34 likely reflects the relaxant effects of adenosine acting directly on vascular smooth muscle.
In addition to drug-induced increases in coronary flow, we also observed changes in LV developed pressure and heart rate associated with the stimulated vasodilatory responses. While IKCa channels do not appear to be expressed in the heart,39 SKCa channel expression and/or function has been described in different regions of the mouse heart40–42 and in the human atrium.40 Although apamin is reported to have little or no effect on action potential properties in ventricular myocytes40,43 and we did not observe significant apamin-induced changes in either myocardial contractility and/or automaticity, SKCa channel blockers may have important functional effects on cardiac performance under certain circumstances and SKCa blockade with NS8593 and UCL1684 has recently been reported to suppress acetylcholine- or pacing-induced atrial fibrillation in vitro and in vivo.44,45 However, since our preparations were not stressed by extreme pacing, we believe that a more plausible explanation for the changes in LV systolic pressure we observed in the presence of SKA-31, and other vasodilators (i.e. bradykinin, adenosine, and SNP), may be related to a phenomenon termed the ‘Gregg Effect’, in which stimulated increases in coronary microvascular filling or perfusion pressure are associated with an enhancement of ventricular contractility.46,47 This mechanical ‘cross-talk’ between the coronary vasculature and the myocardium is thought to involve the stimulation of stretch-activated cation channels in ventricular muscle, leading to enhanced calcium entry and calcium sensitivity of the contractile filaments.47,48 In addition, enhanced coronary perfusion is also reported to increase systolic ventricular stiffness and myocardial contractile force in ejecting hearts.49 It thus appears that drug-induced increases in coronary flow are primarily responsible for the observed enhancement of myocardial contractility, and that these changes are not due to direct effects of SKA-31 on the ventricular muscle itself. This interpretation is consistent with our observation that an increase in coronary flow temporally preceded contractility changes (e.g. see tracings in Figures 3–5), and that inhibition of the drug-induced increase in coronary flow by treatment with apamin + TRAM-34 also prevented changes in contractility. The latter result would not be expected if vasodilatory compounds were acting independently on both the vascular wall and myocardium. Only modest increases in heart rate were observed in the presence of SKA-31 (Figure 2C), indicating little effect of this agent on sino-atrial node automaticity. Such data are further consistent with the observed lack of effect of SKA-31 administration on heart rate in instrumented mice.19
In summary, the results of our study demonstrate that the novel SKCa and IKCa channel activator SKA-31 is an effective vasodilator in the coronary circulation of contractile myocardium of male and female rats, and can increase coronary flow to levels comparable to those produced by established coronary vasodilators, such as bradykinin and adenosine. As SKCa/IKCa channel activators not only promote vasodilation, but may also enhance agonist-evoked NO synthesis,14,15 this latter effect may prove therapeutically beneficial, as improved NO production should enhance vessel health by opposing pro-atherosclerotic processes and aberrant vascular wall remodelling.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by a grant-in-aid to A.B. from the Canadian Institutes of Health Research and an R21 award from the National Institutes of Health to H.W. (NS072585).
Supplementary Material
References
- 1.Busse R, Fleming I, Hecker M. Signal transduction in endothelium-dependent vasodilatation. Eur Heart J. 1993;14(Suppl.1):2–9. [PubMed] [Google Scholar]
- 2.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. TIPS. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 3.Grgic I, Kaistha A, Hoyer J, Köhler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses—relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. doi:10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflügers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. doi:10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 5.Sheng J-Z, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. doi:10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- 6.Stankevicius E, Lopez-Valverde V, Rivera L, Hughes AD, Mulvany MJ, Simonsen U. Combination of Ca2+-activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br J Pharmacol. 2006;149:560–572. doi: 10.1038/sj.bjp.0706886. doi:10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol (Lond) 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. doi:10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to endothelium. Am J Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- 9.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+ channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. doi:10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K+ channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol. 2003;138:1031–1035. doi: 10.1038/sj.bjp.0705171. doi:10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. doi:10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 12.Brahler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. doi:10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 13.Köhler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. doi:10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 14.Dalsgaard T, Kroigaard C, Misfeldt M, Simonsen U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br J Pharmacol. 2010;160:1496–1508. doi: 10.1111/j.1476-5381.2010.00803.x. doi:10.1111/j.1476-5381.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng J-Z, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar dilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. doi:10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socha MJ, Behringer EJ, Segal SS. Calcium and electrical signalling along endothelium of the resistance vasculature. Basic Clin Pharmacol Toxicol. 2011;110:80–86. doi: 10.1111/j.1742-7843.2011.00798.x. doi:10.1111/j.1742-7843.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. doi:10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalsgaard T, Kroigaard C, Simonsen U. Calcium-activated potassium channels - a therapeutic target for modulating nitric oxide in cardiovascular disease? Expert Opin Ther Targets. 2010;14:825–837. doi: 10.1517/14728222.2010.500616. doi:10.1517/14728222.2010.500616. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, et al. Naphthol[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. doi:10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, et al. Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol. 2012;165:223–234. doi: 10.1111/j.1476-5381.2011.01546.x. doi:10.1111/j.1476-5381.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orshal JM, Khalil RA. Gender, sex hormones and vascular tone. Am J Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. doi:10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 22.Ng MKC. New perspectives on Mars and Venus: Unravelling the role of androgens in gender differences in cardiovascular biology and disease. Heart Lung Circ. 2007;16:185–192. doi: 10.1016/j.hlc.2007.02.108. doi:10.1016/j.hlc.2007.02.108. [DOI] [PubMed] [Google Scholar]
- 23.Mombouli JV, Bissiriou I, Agboton V, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: a key mediator of the vasodilator action of bradykinin. Immunopharmacology. 1996;33:46–50. doi: 10.1016/0162-3109(96)00083-5. doi:10.1016/0162-3109(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 24.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. doi:10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 25.Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. doi:10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Félétou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. doi:10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle. Circ Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. doi:10.1161/01.RES.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. doi:10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 29.Antonio A, Rocha e Silva M. Coronary vasodilation produced by bradykinin on isolated mammalian heart. Circ Res. 1962;11:910–915. doi: 10.1161/01.res.11.6.910. doi:10.1161/01.RES.11.6.910. [DOI] [PubMed] [Google Scholar]
- 30.Berne RM. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. doi:10.1161/01.RES.47.6.807. [DOI] [PubMed] [Google Scholar]
- 31.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–1467. doi: 10.1161/01.cir.87.5.1461. doi:10.1161/01.CIR.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 32.Paolocci N, Pagliaro P, Isoda T, Saavedra FW, Kass DA. Role of calcium-sensitive K+ channels and nitric oxide in in vivo coronary vasodilation from enhanced perfusion pulsatility. Circulation. 2001;103:119–124. doi: 10.1161/01.cir.103.1.119. doi:10.1161/01.CIR.103.1.119. [DOI] [PubMed] [Google Scholar]
- 33.Kurian MM, Berwick ZC, Tune JD. Contribution of IKCa channels to the control of coronary blood flow. Exp Biol Med. 2011;236:621–627. doi: 10.1258/ebm.2011.010351. doi:10.1258/ebm.2011.010351. [DOI] [PubMed] [Google Scholar]
- 34.Schilling WP. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 1989;257:H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- 35.Yi FX, Zhang AY, Campbell WB, Zou AP, Van Breeman C, Li P-L. Simultaneous in situ monitoring of intracellular Ca2+ and NO in endothelium of coronary arteries. Am J Physiol. 2002;283:H2725–H2732. doi: 10.1152/ajpheart.00428.2002. [DOI] [PubMed] [Google Scholar]
- 36.Mehrke G, Pohl U, Daut J. Effects of vasoactive peptides on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol (Lond) 1991;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro AS, Fernandes VS, Orensanz LM, Martinez MP, Recia P, Martinez-Saenz A, et al. Mechanisms involved in the adenosine-induced vasorelaxation to the pig prostatic small arteries. Purinergic Signal. 2011;7:413–425. doi: 10.1007/s11302-011-9238-7. doi:10.1007/s11302-011-9238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyatt AW, Steinert JR, Wheeler-Jones CP, Morgan AJ, Jugden D, Pearson JD, et al. Early activation of the p42/p44MAPK pathway mediates adenosine-induced nitric oxide production in human endothelial cells: a novel calcium-insensitive mechanism. FASEB J. 2002;16:1584–1594. doi: 10.1096/fj.01-0125com. doi:10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 39.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. doi:10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. doi:10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 41.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2 and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. doi:10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol (Lond) 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. doi:10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagy N, Szuts V, Horvath Z, Seprenyi G, Farkas A, Acsai K, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. doi:10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, et al. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. doi:10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- 45.Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, et al. Effects on atrial fibrillation in aged hypertensive rats by Ca2+-activated K+ channel inhibition. Hypertension. 2011;57:1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613. doi:10.1161/HYPERTENSIONAHA.111.170613. [DOI] [PubMed] [Google Scholar]
- 46.Gregg DE. Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circ Res. 1963;13:497–500. doi: 10.1161/01.res.13.6.497. doi:10.1161/01.RES.13.6.497. [DOI] [PubMed] [Google Scholar]
- 47.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86:1263–1308. doi: 10.1152/physrev.00029.2005. doi:10.1152/physrev.00029.2005. [DOI] [PubMed] [Google Scholar]
- 48.Lamberts RR, van Rijen MH, Sipkema P, Fransen P, Sys SU, Westerhof N. Coronary perfusion and muscle lengthening increase cardiac contraction: different stretch-triggered mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H1515–H1522. doi: 10.1152/ajpheart.00113.2002. [DOI] [PubMed] [Google Scholar]
- 49.Iwamoto T, Bai XJ, Downey HF. Coronary perfusion related changes in myocardial contractile force and systolic ventricular stiffness. Cardiovasc Res. 1994;28:1331–1336. doi: 10.1093/cvr/28.9.1331. doi:10.1093/cvr/28.9.1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.