Abstract
Aims
α-Melanocyte-stimulating hormone (α-MSH), derived from the precursor molecule pro-opiomelanocortin, exerts potent anti-inflammatory actions in the vasculature, but its role in circulatory regulation remains unclear. Therefore, we sought to investigate whether α-MSH could regulate the local control of blood vessel tone.
Methods and results
Using in vivo and ex vivo methods to assess vascular reactivity, we found that α-MSH improved endothelium-dependent vasodilatation in the mouse aorta and coronary circulation without directly contracting or relaxing blood vessels. α-MSH promoted vasodilatation by enhancing endothelial nitric oxide (NO) formation and by improving sensitivity to endothelium-independent blood vessel relaxation. Using cultured human endothelial cells to elucidate the involved molecular mechanisms, we show that α-MSH increased the expression and phosphorylation of endothelial NO synthase in these cells. The observed effects were regulated by melanocortin 1 (MC1) receptors expressed in the endothelium. In keeping with the vascular protective role of α-MSH, in vivo treatment with stable analogues of α-MSH ameliorated endothelial dysfunction associated with aging and diet-induced obesity in mice.
Conclusion
The present study identifies α-MSH and endothelial MC1 receptors as a new signalling pathway contributing to the regulation of NO availability and vascular function. These findings suggest applicability of α-MSH analogues for therapeutic use in pathological conditions that are characterized by vascular dysfunction.
Keywords: Vasodilation, Nitric oxide, Endothelial function, Endothelial nitric oxide synthase, Melanocortin
1. Introduction
The melanocortin peptides, which include adrenocorticotropic hormone (ACTH) and α-, β-, and γ-melanocyte-stimulating hormones (MSH), are derived from a common precursor protein pro-opiomelanocortin (POMC). Melanocortins have well-established roles in the central regulation of energy homeostasis and cardiovascular functions, and mounting evidence demonstrates that they are also implicated in the control of inflammation.1,2 They exert their biological actions through a family of five related G-protein coupled melanocortin receptors (MC1–MC5).3 The MC1 receptor, predominantly expressed in peripheral tissues, is the classic MC receptor capable of binding α-MSH with high affinity and mediating melanin dispersion in melanocytes.4,5 MC1 receptors are also abundant in cells of the immune system where they mediate anti-inflammatory effects of α-MSH.6–8 Several recent studies have reported that endothelial cells of humans and rodents express MC1 receptors, as well as POMC, and seem to be capable of processing POMC into biologically active α-MSH.9–11 Although endothelial MC1 receptors have been investigated as anti-inflammatory targets, it is unexplored whether they could regulate the endothelium-dependent control of blood vessel tone.
The vascular endothelium plays a crucial role in the regulation of vascular tone by releasing several vasoactive substances, thereby adjusting regional blood flow in response to tissue demands.12,13 Nitric oxide (NO) produced by endothelial NO synthase (eNOS) upon shear stress and receptor activation is particularly important for the maintenance of vascular homeostasis. It relaxes vascular smooth muscle cells by activating guanylyl cyclase and increasing cGMP production.14 However, the importance of NO in vascular physiology is not solely determined by its direct vasodilating properties. It contributes to vascular homeostasis also by inhibiting vasoconstrictor influences, platelet aggregation, and proliferation of vascular smooth muscle cells.15,16 Owing to the multifaceted role of endothelium-derived NO, impairment of its availability is a predisposing and underlying factor for major cardiovascular disease states.
Along with the evident expression of MC1-R in the endothelium, we were inspired by an interesting clinical observation: a stable analogue of α-MSH, NDP-α-MSH (also known as afamelanotide or MT-I),17 currently investigated and developed for the treatment of skin diseases, was reported to cause headache and flushing as predominant side effects,18 typical reactions that are associated with vasodilatation. Given this, we hypothesized that α-MSH by interacting with the endothelial MC1-R might have a role as a regulator of vascular function. Here we identify that α-MSH promotes endothelium-dependent control of blood vessel tone by enhancing signalling through the NO-cGMP pathway. This is attributed to activation of eNOS mediated by endothelial MC1 receptors. These findings suggest that peripherally acting α-MSH analogues might represent a new therapeutic approach for the control of endothelial dysfunction in cardiovascular disease.
2. Methods
Full details of the methods used in this study are given in the online Supplementary material.
2.1. Mice and treatments
All animal studies were approved by the national Animal Experiment Board in Finland and conducted in accordance with the Directive 2010/63/EU of the European Parliament. To study acute and chronic effects of melanocortins, we administered NDP-α-MSH (0.3 mg/kg, i.p., 18 h before sacrifice) to 10-month-old and MT-II (0.3 mg/kg/day, i.p., for 3 weeks) to diet-induced obese C57Bl/6N mice.19 As a genetic model of chronic melanocortin activation, we studied transgenic mice overexpressing α-MSH under the universal CMV promoter (C57BL/6J- AwJ background after eight backcrosses).20 Mice were euthanized via CO2 asphyxiation and the selected blood vessels were harvested and used for ex vivo experimentation.
2.2. Vascular reactivity analysis
Segments (2 mm in length) of mouse thoracic aortae and small mesenteric arteries (80–100 μm in diameter) were studied by wire myography for contractile responses to phenylephrine (PE), and endothelium-dependent and -independent relaxations to acetylcholine (ACh) and sodium nitroprusside (SNP), respectively. After mounting, vessels were equilibrated, normalized, and contracted repeatedly with 62 mmol/L KCl until maximal and reproducible contractions were obtained.21,22 To measure vasodilatation, arterial rings were pre-contracted with prostaglandin F2α (aorta) or PE (mesenteric artery) and the contraction was adjusted to 50–80% of the reference contraction to 62 mmol/L KCl.23 Effects of NOS blockade on vascular responses were tested by applying L-NNA (100 μmol/L for 30 min).
2.3. NO release and NOS activity assay
NO (nitrate + nitrite, NOx) concentrations in cell-culture medium were determined by fluorometric detection (Cayman Chemicals). Aortic samples were homogenized in 25 mM Tris–HCl and incubated at room temperature for 60 min in the presence of calmodulin (0.1 µm), NADPH (1.2 mm), and CaCl2 (0.7 mm) according to the manufacturer's instructions (Cayman Chemicals). The NO synthesizing activity was determined by quantifying the rate of the conversion of [3H]l-arginine to [3H]l-citrulline. NOS activity is expressed as dissociations per minute per mg protein.
2.4. cGMP content of aortic rings
Aortic segments were equilibrated (37°C, 30 min) in Krebs bicarbonate buffer and then treated with NDP-α-MSH (1 μmol/L) for 60 min. The tissue samples were homogenized in 5% trichloroacetic acid and assayed for cGMP content with an enzyme immunoassay (Cayman Chemicals). The cGMP levels of equilibrated, untreated rings were taken as the control.
2.5. Echocardiography
For Doppler echocardiography, mice were anaesthetized with isoflurane (induction 3%, maintenance 1.5%) and assigned to scanning with an Acuson Sequoia C512 instrument equipped with a 15 MHz linear transducer 15L8 (Siemens Acuson). Diastolic peak and mean flow velocities in the middle left coronary artery were recorded under colour Doppler guidance during baseline (1.5% isoflurane) and hyperaemic flow conditions (2.5% isoflurane).24,25 Coronary flow reserve (CFR) was calculated as the ratio of hyperaemic to baseline flow velocity. All measurements were done off-line in a blinded manner.
2.6. Isolated heart perfusion and coronary flow
Mice were anaesthetized with pentobarbital sodium (50–100 mg/kg) and administered intravenous heparin (0.2 mL of 1000 IU/mL). Pedal and tail withdrawal reflexes were monitored to ensure adequate depth of anaesthesia. Thereafter, the heart was isolated, mounted according to the Langendorff method and retrogradely perfused through the aorta at a constant pressure (60 mmHg) with Krebs bicarbonate buffer. After equilibration, coronary vasodilator responses to ACh and bradykinin were assessed by timed collection of the outflow perfusate. After the experiments, the hearts were weighed and coronary vasodilator responses were expressed as increase in mL/min/g of wet tissue weight.
2.7. Cell culture
Primary human endothelial cells (HUVEC) were isolated from freshly collected umbilical cords.26 The protocol for preparing a primary human cell culture was approved by The Joint Ethics Committee of Turku University and Turku University Central Hospital, and conforms with the principles outlined in the Declaration of Helsinki. HUVECs and transformed HUVEC-derived endothelial cells (EA.hy926) were incubated in complete medium (M199 and DMEM, respectively) supplemented with 10% FBS. After confluence, cells were stimulated as indicated in the graph legends and used for mRNA and protein analyses.
2.8. Quantitative RT–PCR and western blotting
Total RNA from cell-culture samples was extracted (Qiagen) and reverse-transcribed (Applied Biosystems). Quantitative RT–PCR was performed using SYBR Green protocols (Kapa Biosystems) on an Applied Biosystems 7300 Real-Time PCR system. Samples were run in triplicate. Primer sequences are given in Supplementary material online, Tables S1 and S2. mRNA expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the comparative ΔCt method and are presented as relative transcript levels (2−ΔΔCt).
2.9. Western blot analysis
Cell-culture samples were lysed in RIPA buffer supplemented with a protease and phosphatase inhibitor cocktail (Thermo Scientific). Aliquots of total protein were size-fractioned by SDS–PAGE and transferred onto nitrocellulose membranes. Membranes were probed with specific antibodies for eNOS, phospho(Ser1177)-eNOS and Mn-SOD (BD Biosciences). The results for total protein expression were normalized to β-actin (Sigma) to correct for loading.
2.10. Chemicals
The selective MC1-R antagonist MSG606 was synthesized as previously described.27 All other drugs and reagents were purchased from Sigma-Aldrich except PGF2α and U46619 which were from Cayman Chemicals.
2.11. Statistical analyses
All data are expressed as means ± SEM. Differences between two groups were analysed by unpaired Student's t-test. Comparisons between three or more groups were made by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests. For two independent factors, two-way ANOVA was used. Concentration–response curves were generated using nonlinear regression and Emax and EC50 values were compared using the extra sum-of-squares F-test. A two-tailed P-value of <0.05 was considered statistically significant.
3. Results
3.1. α-MSH enhances endothelium-dependent vasodilatation without directly relaxing blood vessels
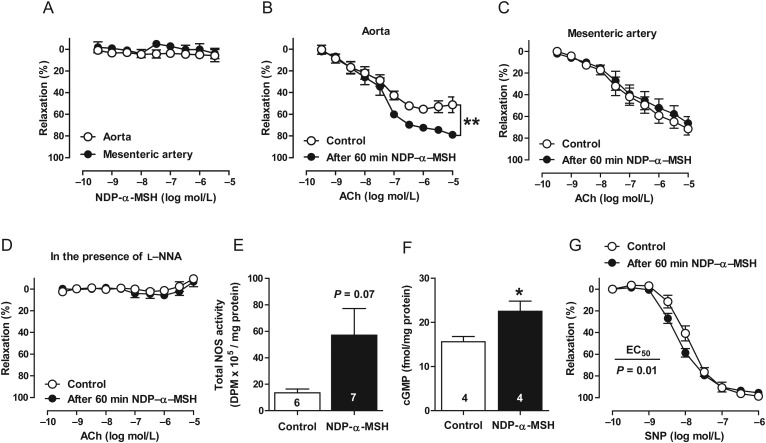
To test the hypothesis that melanocortins regulate endothelium-dependent control of blood vessel tone, isolated rings of the mouse aorta and small mesenteric arteries were studied by wire myography for vascular reactivity. First, we investigated whether NDP-α-MSH could directly modulate vascular tone of isolated arteries, and applied NDP-α-MSH to pre-constricted mouse aortae or mesenteric arteries. We observed, however, no vasoconstriction or vasorelaxation in mouse aortae or mesenteric arteries (Figure 1A). Next, to determine whether α-MSH could modulate endothelial function, we pre-treated isolated arteries with NDP-α-MSH (1 μmol/L) for 60 min and assessed the responses to endothelium-dependent vasodilatation. In vitro pre-treatment with NDP-α-MSH significantly enhanced aortic responses to acetylcholine (ACh) (Figure 1B). These responses were confined to large arteries as the same pre-treatment failed to improve ACh responses in mesenteric arteries (Figure 1C). NOS inhibition with L-NNA abrogated ACh-evoked relaxation and the effect of NDP-α-MSH on the ACh-responses (Figure 1D), implying that increased NO formation contributes to the improved endothelium-dependent relaxation. Consistently, NOS activity tended (P = 0.07) to be increased and cGMP level was markedly enhanced in NDP-α-MSH-treated aortae (Figure 1E and F). In addition, NDP-α-MSH treatment increased the sensitivity of the vascular smooth-muscle layer to endothelium-independent relaxation evoked by the NO donor SNP (Figure 1G). Collectively, α-MSH seems to be devoid of direct effects on blood vessel tone, but it substantially enhances NO-dependent endothelial function and endothelium-independent vasorelaxation.
Figure 1.
α-MSH enhances endothelium-dependent relaxation but has no immediate effect on blood vessel tone. (A) NDP-α-MSH caused no direct vasodilatation in the mouse aorta or mesenteric artery. n = 6 per vessel type. Endothelium-dependent relaxations of mouse aorta (B) and mesenteric artery (C) in control preparations and in preparations pre-treated with 1 μmol/L NDP-α-MSH for 60 min. n = 8 per group in both graphs. Arteries were isolated from 10-month-old mice. (D) ACh-evoked relaxation in the aorta after NOS inhibition with L-NNA. n = 6 per group. NOS activity (E) and cGMP level (F) in NDP-α-MSH-treated aortae. *P < 0.05 vs. control. (G) Endothelium-independent relaxation responses to SNP in the aorta. Emax and EC50 were estimated by nonlinear regression and compared using the extra sum-of-squares F-test, where **P < 0.01 vs. control. n = 6 per group.
3.2. In vivo treatment with α-MSH analogues ameliorates endothelial dysfunction in aged and obese mice
To further examine the vascular effects of melanocortins, we subjected 10-month-old C57Bl/6N mice to acute (0.3 mg/kg, 18 h before sacrifice) treatment with NDP-α-MSH and diet-induced obese (DIO) mice to chronic (0.3 mg/kg/day, for 3 weeks) treatment with a more stable analogue of α-MSH, MT-II, to obtain extended duration of action. With the selected dosage regimen, MT-II had no effect on body weight or adiposity of DIO mice (see Supplementary material online, Table S3). As a genetic model of chronic melanocortin activation, we studied 5-month-old transgenic MSH-OE mice overexpressing α-MSH. Aged and obese mice were selected for the pharmacological interventions based on our initial findings showing that melanocortins enhance endothelial function. Hence, we sought to investigate whether melanocortins could ameliorate the endothelial dysfunction present in these mice (see Supplementary material online, Figure S1).
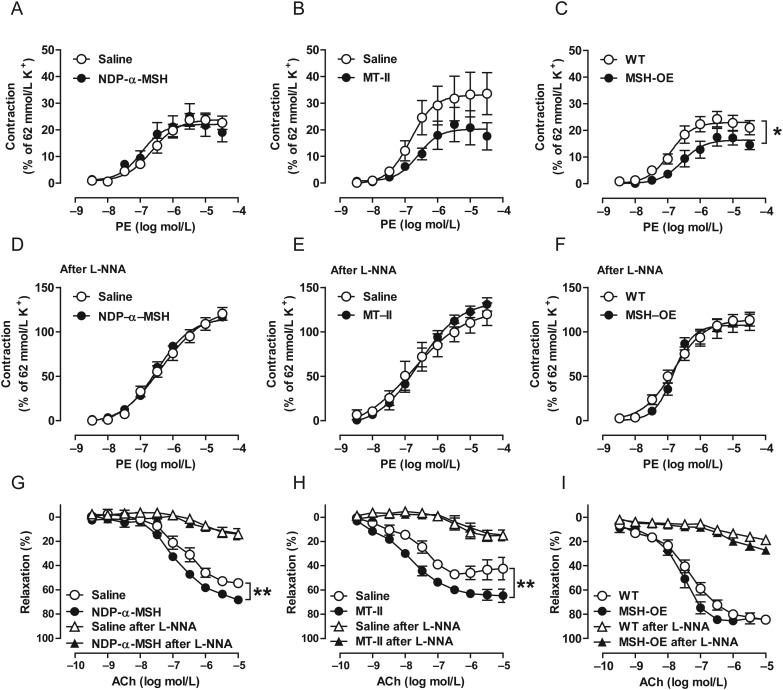
To evaluate vascular function in melanocortin-treated and MSH-OE mice, isolated rings of the aorta and mesenteric arteries were first studied for contractile responses to PE. Acute administration of NDP-α-MSH had no effect on PE-induced vasoconstriction (Figure 2A and Supplementary material online, Table S4). However, the maximal vasoconstriction induced by PE was significantly smaller in the aorta of MSH-OE mice compared with wild-type (WT) control mice (Figure 2C and Supplementary material online, Table S4), and a similar trend (P = 0.07) was observed in MT-II-treated DIO mice (Figure 2B and Supplementary material online, Table S4). In contrast, in small mesenteric arteries, the maximum contractile response was unaffected by melanocortins (see Supplementary material online, Figure S2). Pre-treatment with the NOS inhibitor L-NNA substantially augmented the aortic contractile responses and abolished the differences in the maximum response to PE between the MSH and control groups (Figure 2E and F).
Figure 2.
Melanocortins reduce vasoconstrictor influences and enhance endothelium-dependent vasodilatation primarily by augmenting NO formation. (A–C) Concentration–response curves for PE-induced contractions in the aorta of NDP-α-MSH-treated 10-month-old mice (left panel), MT-II-treated DIO mice (middle panel), and MSH-OE mice (right panel). Contraction is expressed as a percentage of the maximal KCl (62 mmol/L)-induced contraction. 10-month-old mice: saline, n = 10; NDP-α-MSH, n = 8. DIO mice: saline, n = 8; MT-II, n = 8. WT, n = 14; MSH-OE, n = 8. (D–F) Concentration–response curves for PE-induced contractions after the addition of the NOS inhibitor L-NNA. (G–I) Endothelium-dependent relaxation to ACh and the effect of NOS blockade on the corresponding responses. *P < 0.05 and **P < 0.01 vs. control mice.
Next, we monitored endothelium-dependent relaxation to ACh and observed that acute and chronic melanocortin administration improved relaxation responses to ACh in the aorta of 10-month-old mice and DIO-mice (Figure 2G and H). MSH-OE mice showed no distinct increment in the maximum response to ACh (Figure 2I). However, a tendency (P = 0.06) towards increased sensitivity (EC50) to ACh-evoked relaxation was observed in the aorta of MSH-OE mice. NOS inhibition with L-NNA significantly attenuated relaxation responses to ACh and eliminated the effect of melanocortin administration on the ACh-responses (Figure 2G and H). The enhancements in NO-driven relaxation occurred without apparent differences in eNOS protein levels (see Supplementary material online, Figure S3). In contrast to the findings in the aorta, melanocortins were again incapable of enhancing ACh-responses of small mesenteric arteries (see Supplementary material online, Figure S2).
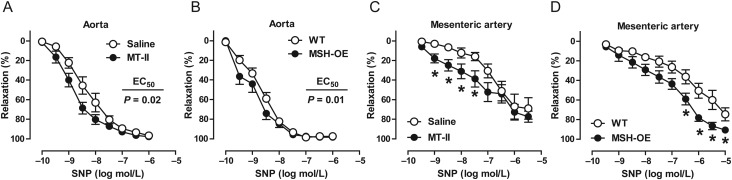
In addition to improving endothelium-dependent relaxation in the aorta, chronic MT-II treatment and MSH-OE increased endothelium-independent vasodilatation evoked by SNP. This effect was observed in the aorta and small mesenteric arteries (Figure 3A–D).
Figure 3.
α-MSH enhances endothelium-independent blood vessel relaxation. (A–D) Endothelium-independent relaxations in the aorta and mesenteric arteries of MT-II-treated DIO mice and MSH-OE mice. In mesenteric arteries, accurate estimates for EC50-values could not be obtained by nonlinear regression and therefore, differences between the groups were analysed by two-way ANOVA followed by Bonferroni post-hoc tests. *P < 0.05 vs. control group at given concentration. n = 6–8 per group in each graph.
3.3. Melanocortins contribute to coronary flow regulation
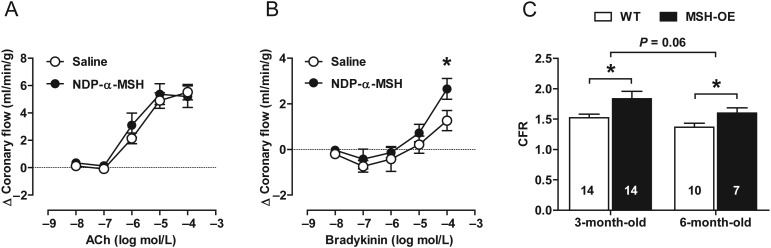
Having identified novel effects of α-MSH on the function of isolated vessels, we next investigated whether similar effects appear also in an intact coronary circulation. We assessed coronary vascular function in Langendorff-perfused hearts from saline- and NDP-α-MSH-treated mice. Vasodilator responses to endothelium-dependent agonists were studied by measuring increases in coronary outflow. Responses to ACh were comparable between the groups while bradykinin induced significantly greater vasorelaxation and consequent increase in total coronary flow in the hearts of NDP-α-MSH-treated mice (Figure 4A and B).
Figure 4.
Melanocortins improve coronary flow regulation. (A and B) Coronary flow responses to increasing doses of ACh and bradykinin in saline- and NDP-α-MSH-treated hearts. n = 8 per group. *P < 0.05 vs. saline-treated mice. (C) Coronary flow reserve (the ratio of hyperaemic to baseline flow velocity) of WT and MSH-OE mice. *P < 0.05 vs. age-matched WT mice.
To obtain complementary in vivo evidence, we evaluated the CFR of MSH-OE mice by applying Doppler echocardiography. We found that CFR was increased in 3-month-old as well as in 6-month-old MSH-OE mice compared with age-matched WT control mice (Figure 4C). These results, derived from different methods and models, together underscore that melanocortins contribute not only to vascular function of isolated peripheral arteries but also to coronary flow regulation.
3.4. Activation of endothelial MC1 receptors by α-MSH regulates NO availability
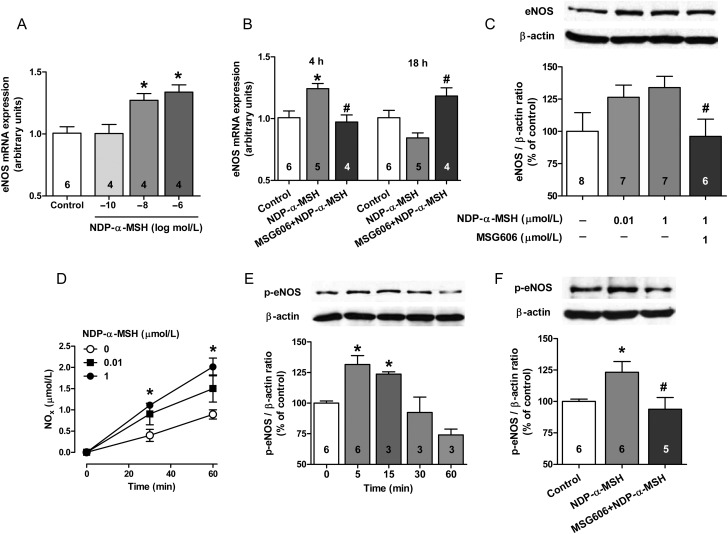
To investigate the mechanism whereby α-MSH improves endothelial function, we used cultured primary (HUVEC) and transformed (EA.hy926) human endothelial cells. First, we were able to confirm that human endothelial cells exclusively express the MC1 receptor subtype (see Supplementary material online, Table S5). MC1-R expression was also found in the mouse aorta (see Supplementary material online, Table S5). Next, we examined the effects of NDP-α-MSH on eNOS expression and phosphorylation, central factors affecting NO production. Stimulation with NDP-α-MSH for 4 h elicited a dose-dependent increase in eNOS mRNA expression (Figure 5A), which then seemed to be downregulated after 18 h stimulation (Figure 5B). These changes in mRNA expression were translated into a tendency (P = 0.07) of increased eNOS protein levels after 18 h treatment with 1 μmol/L NDP-α-MSH (Figure 5C). To address the role of the MC1-R as a mediator of these responses, we tested whether the observed effects were reversed by the selective MC1-R antagonist MSG606. Indeed, MC1-R antagonism abolished the stimulatory effects of NDP-α-MSH on eNOS expression (Figure 5B and C). Since NDP-α-MSH enhanced NO production of endothelial cells in a relatively short time scale (Figure 3D), we investigated whether it regulates post-translational activation of eNOS. NDP-α-MSH time-dependently (Figure 5E) and through the MC1-R (Figure 5F) stimulated phosphorylation of eNOS at Ser1177, which is critical for its enzymatic activity.
Figure 5.
α-MSH regulates the expression and phosphorylation of eNOS system in human endothelial cells. (A) Primary HUVECs were treated for 4 h with the indicated concentrations of NDP-α-MSH and analysed for eNOS mRNA expression. (B) Primary HUVECs were stimulated with NDP-α-MSH (1 μmol/L) for 4 h and 18 h and analysed for eNOS mRNA expression. A selective MC1-R antagonist (MSG606) was used to address the dependence of the responses on the MC1-R. (C) Transformed endothelial cells (EA.hy926) were treated with NDP-α-MSH for 18 h and analysed thereafter for eNOS protein levels by western blotting. Graph shows representative immunoblots for eNOS and β-actin (loading control) and densitometric analysis of the immunoblots. (D) NO production in EA.hy926 cells after stimulation with NDP-α-MSH. (E) EA.hy926 cells were stimulated with NDP-α-MSH (1 μmol/L) for the indicated times and quantified for the degree of phosphorylation of eNOS on Ser1177 by western blotting. (F) The inhibitory effect of MSG606 on NDP-α-MSH (1 μmol/L for 5 min) stimulated eNOS phosphorylation. In each experiment, MSG606 (1 μmol/L) was applied 15 min prior to addition of NDP-α-MSH. *P < 0.05 vs. control and #P < 0.05 vs. NDP-α-MSH.
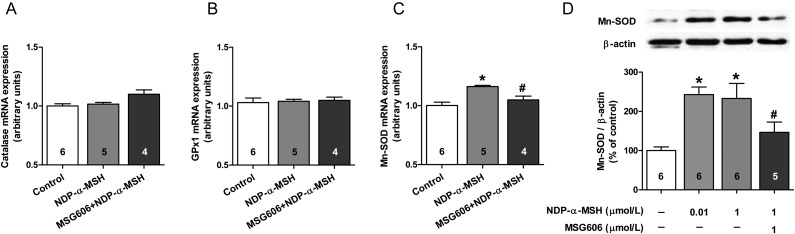
Next, we investigated the effects of NDP-α-MSH on mRNA expression of the antioxidant enzymes catalase, glutathione peroxidase (GPx1), and mitochondrial superoxide dismutase (Mn-SOD) in HUVECs (Figure 6E–G). We found that NDP-α-MSH selectively increased the expression of Mn-SOD (Figure 6G), an enzyme responsible for the neutralization of superoxide anions to oxygen and hydrogen peroxide. This effect, mediated by the MC1-R, was also observed at the protein level (Figure 6H). Taken together, our results demonstrate that α-MSH, by binding to endothelial MC1-R, regulates the eNOS system at the transcriptional and post-translational levels and increases Mn-SOD expression.
Figure 6.
α-MSH increases the expression of Mn-SOD in human endothelial cells. (A–C) The effect of 4 h stimulation with NDP-α-MSH (1 μmol/L) on mRNA expression of catalase, GPx1, and Mn-SOD in HUVECs. (D) Analysis of Mn-SOD protein expression in EA.hy926 cells after 18 h treatment with NDP-α-MSH (1 μmol/L). *P < 0.05 vs. control and #P < 0.05 vs. NDP-α-MSH.
4. Discussion
Our study uncovers a peripheral melanocortin signalling pathway that contributes to the regulation of blood vessel tone in a favourable manner, thus promoting vascular health. First, we show that α-MSH augments vascular relaxation via enhancing NO-cGMP-dependent responses. Secondly, our results establish a mechanistic link between the endothelial MC1 receptor and α-MSH-mediated regulation of NO availability. Owing to the beneficial effects in vascular physiology, endothelial MC1 receptors might represent a new therapeutic target for vascular protection in cardiovascular disease such as hypertension and atherosclerosis.
To the best of our knowledge, this is the first study demonstrating a peripheral site of action for α-MSH in circulatory regulation. Haemodynamic effects of peripherally administered MSH peptides have been widely investigated, but their effects have been linked to central mechanisms and to γ-MSH instead of α-MSH.28,29 Interestingly, two early studies reported that i.v. injections of α-MSH increased tissue blood flow in cats and sheep, but the mechanisms whereby α-MSH exerted this action remained unknown.30,31 Consistent with these early findings, our results suggest that α-MSH could increase tissue blood flow by affecting the local control of vascular tone. Although α-MSH had no immediate effects on vascular tone, we observed that it improves endothelial function via augmenting NO availability. This, in turn, has indirect effects on vascular tone as it blunts vasoconstrictor influences and enhances endothelium-dependent vasodilatation.
We noted that α-MSH elicited vessel-size-dependent improvements in endothelium-mediated vasodilatation through enhanced NO formation. The endothelial NOS system has variable significance in different parts of the circulatory system, since vascular tone of large arteries is known to be more strongly dependent on the function of eNOS than that of small arteries.32,33 Accordingly, the relative importance of NO for ACh-induced relaxation increases as the vessel size increases, thereby explaining why the α-MSH-stimulated effects on endothelium-dependent relaxation were confined to large arteries. Furthermore, α-MSH attenuated responses to PE in the aorta and this effect was eliminated by inhibiting the activity of NOS, indicating that α-MSH blunts vasoconstrictor influences through enhanced NO release. The beneficial influence of α-MSH appeared not only in endothelial capacity for NO formation but also in vascular sensitivity to SNP-induced vasodilatation. This effect was apparent in the aorta and small mesenteric arteries alike, indicating improved signalling downstream of NO, regardless of the vessel size. However, the underlying mechanism for this improvement remains largely unclear. Based on the finding of improved endothelium-independent vasodilatation, it will be intriguing to investigate whether MC receptors are also expressed in vascular smooth muscle cells and if so, whether they could regulate downstream effectors of NO such as guanylyl cyclase.
In addition to improving the function of peripheral arteries, melanocortins showed promising effects on coronary flow regulation. In vivo treatment with NDP-α-MSH increased coronary flow response to bradykinin in the isolated heart. This observation could be attributed to increased NO production, because in mice, responses to bradykinin in the intact coronary circulation are predominantly NO-dependent.34 The finding that ACh-induced coronary flow response was unaffected by NDP-α-MSH-treatment supports this notion, since ACh increases coronary flow in the mouse heart primarily via endothelial prostacyclin formation.35 Furthermore, long-term melanocortin activation in MSH-OE mice was associated with improved flow reserve of epicardial coronary arteries. Taken together, the present findings provide conclusive evidence that α-MSH has wide-ranging effects in promoting endothelium-dependent and -independent blood vessel relaxation and is able to ameliorate endothelial dysfunction associated with ageing and diet-induced obesity independent of its well-established effects on metabolic regulation.
To shed light on the underlying mechanisms, we used cultured human endothelial cells and were able to show that α-MSH regulates NO availability through endothelial MC1 receptors. NO availability can be increased by enhancing NO production via transcriptional and post-translational activation of eNOS, and by reducing ROS-mediated breakdown of NO.36–38 We demonstrated that analogues of α-MSH have potential, at least in vitro, to engage all of these mechanisms to increase NO availability. α-MSH activated eNOS through transcriptional regulation and post-translational phosphorylation. Furthermore, endothelial MC1 receptors have the capacity to regulate Mn-SOD expression. All of these effects, separately and in concert with each other, are likely to enhance the local availability of NO in blood vessels, supporting the findings from vascular function measurements. Our in vitro findings are in good agreement with a recent study showing that NDP-α-MSH administration increased the expression of eNOS and Mn-SOD in the basilar artery of the rat brain.39 However, although the present results show that α-MSH increased eNOS expression in cultured endothelial cells, in vivo treatments promoted NO-dependent relaxations without changes in eNOS expression, indicating that increased NO availability is primarily attributed to other mechanisms such as eNOS phosphorylation. Beyond these molecular underpinnings, the present observations merit further research to dissect the involved intracellular signalling pathways and the correspondence of the molecular-level effects with physiological functions.
In conclusion, our results show that α-MSH has significant effects on the control of blood vessel tone by enhancing NO-cGMP-dependent relaxation responses through endothelial MC1 receptors. Vascular dysfunction and particularly impairment in endothelium-dependent vasodilation are early markers of many cardiovascular disease states. Endothelial dysfunction is characterized by reductions in eNOS activity and vascular NO availability. Furthermore, inflammation plays a key role and is likely to contribute to the development of endothelial dysfunction. In these respects, α-MSH analogues could have therapeutic value in cardiovascular disease through their ability to promote endothelial function combined with their known anti-inflammatory actions in the vasculature. Now, considering that there is unmet medical need to develop novel therapies to restore endothelial function, it would be worthwhile to further investigate whether α-MSH-based treatments could translate into therapeutic efficacy in severe pathological conditions such as atherosclerosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the Academy of Finland, the Finnish Funding Agency for Technology and Innovation, the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the U.S. Public Health Service, and the National Institutes of Health.
Supplementary Material
Acknowledgements
We thank Professor S. Wardlaw (Columbia University, New York) for providing the MSH-OE mice, S. Yang in Hruby's group for the synthesis of MSG606, and R. Kaartosalmi for technical assistance.
Conflict of interest: none declared.
References
- 1.Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- 2.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Mountjoy K, Robbins L, Mortrud M, Cone R. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 4.Lerner AB, McGuire JS. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- 5.Hadley ME. The Melanotropic Peptides. Boca Raton, FL: CRC Press Inc.; 1988. [Google Scholar]
- 6.Bhardwaj R, Becher E, Mahnke K, Hartmeyer M, Schwarz T, Scholzen T, et al. Evidence for the differential expression of the functional alpha-melanocyte-stimulating hormone receptor MC-1 on human monocytes. J Immunol. 1997;158:3378–3384. [PubMed] [Google Scholar]
- 7.Adachi S, Nakano T, Vliagoftis H, Metcalfe DD. Receptor-mediated modulation of murine mast cell function by alpha-melanocyte stimulating hormone. J Immunol. 1999;163:3363–3368. [PubMed] [Google Scholar]
- 8.Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin MC(1) receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010;160:171–180. doi: 10.1111/j.1476-5381.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmeyer M, Scholzen T, Becher E, Bhardwaj RS, Schwarz T, Luger TA. Human dermal microvascular endothelial cells express the melanocortin receptor type 1 and produce increased levels of IL-8 upon stimulation with alpha-melanocyte-stimulating hormone. J Immunol. 1997;159:1930–1937. [PubMed] [Google Scholar]
- 10.Scholzen TE, Brzoska T, Kalden DH, Hartmeyer M, Fastrich M, Luger TA, et al. Expression of functional melanocortin receptors and proopiomelanocortin peptides by human dermal microvascular endothelial cells. Ann NY Acad Sci. 1999;885:239–253. doi: 10.1111/j.1749-6632.1999.tb08681.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindskog A, Ebefors K, Johansson ME, Stefánsson B, Granqvist A, Arnadottir M, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 13.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 14.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, et al. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minder EI. Afamelanotide, an agonistic analog of α-melanocyte-stimulating hormone, in dermal phototoxicity of erythropoietic protoporphyria. Expert Opin Investig Drugs. 2010;19:1591–1602. doi: 10.1517/13543784.2010.535515. [DOI] [PubMed] [Google Scholar]
- 19.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. J Med Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 20.Savontaus E, Breen T, Kim A, Yang L, Chua SJ, Wardlaw S. Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice. Endocrinology. 2004;145:3881–3891. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- 21.Peiper U, Schmidt E, Laven R, Griebel L. Length-tension relationships in resting and activated vascular smooth muscle fibres. Pflugers Arch. 1973;340:113–122. doi: 10.1007/BF00588170. [DOI] [PubMed] [Google Scholar]
- 22.Delaey C, Boussery K, Van de Voorde J. Contractility studies on isolated bovine choroidal small arteries: determination of the active and passive wall tension-internal circumference relation. Exp Eye Res. 2002;75:243–248. [PubMed] [Google Scholar]
- 23.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saraste A, Kytö V, Saraste M, Vuorinen T, Hartiala J, Saukko P. Coronary flow reserve and heart failure in experimental coxsackievirus myocarditis. A transthoracic Doppler echocardiography study. Am J Physiol Heart Circ Physiol. 2006;291:H871–875. doi: 10.1152/ajpheart.01375.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hartley C, Reddy A, Madala S, Michael L, Entman M, Taffet G. Effects of isoflurane on coronary blood flow velocity in young, old and ApoE(-/-) mice measured by Doppler ultrasound. Ultrasound Med Biol. 2007;33:512–521. doi: 10.1016/j.ultrasmedbio.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallio J, Pesonen U, Kaipio K, Karvonen MK, Jaakkola U, Heinonen OJ, et al. Altered intracellular processing and release of neuropeptide Y due to leucine 7 to proline 7 polymorphism in the signal peptide of preproneuropeptide Y in humans. FASEB J. 2001;15:1242–1244. [PubMed] [Google Scholar]
- 27.Juni A, Cai M, Stankova M, Waxman AR, Arout C, Klein G, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology. 2010;112:181–188. doi: 10.1097/ALN.0b013e3181c53849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni X, Butler A, Cone R, Humphreys M. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 29.Versteeg D, Van Bergen P, Adan R, De Wildt D. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- 30.Kadowitz PJ, Chapnick BM, Kastin AJ. Comparison of alpha-MSH and several vasoactive substances on vascular resistance in the feline mesenteric vascular bed. Pharmacol Biochem Behav. 1976;5:219–221. doi: 10.1016/0091-3057(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 31.Llanos AJ, Seron-Ferre M, Ramachandran J, Creasy RK, Heymann MA, Rudolph AM. Cardiovascular responses to alpha-melanocyte stimulating hormone during the perinatal period in sheep. Pediatr Res. 1983;17:903–908. doi: 10.1203/00006450-198311000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Parsons SJ, Hill A, Waldron GJ, Plane F, Garland CJ. The relative importance of nitric oxide and nitric oxide-independent mechanisms in acetylcholine-evoked dilatation of the rat mesenteric bed. Br J Pharmacol. 1994;113:1275–1280. doi: 10.1111/j.1476-5381.1994.tb17136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gödecke A, Ziegler M, Ding Z, Schrader J. Endothelial dysfunction of coronary resistance vessels in apoE-/- mice involves NO but not prostacyclin-dependent mechanisms. Cardiovasc Res. 2002;53:253–262. doi: 10.1016/s0008-6363(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 35.Gwóźdź P, Drelicharz L, Kozlovski VI, Chlopicki S. Prostacyclin, but not nitric oxide, is the major mediator of acetylcholine-induced vasodilatation in the isolated mouse heart. Pharmacol Rep. 2007;59:545–552. [PubMed] [Google Scholar]
- 36.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 37.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatti S, Lonati C, Acerbi F, Sordi A, Leonardi P, Carlin A, et al. Protective action of NDP-MSH in experimental subarachnoid hemorrhage. Exp Neurol. 2012;234:230–238. doi: 10.1016/j.expneurol.2011.12.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.