Abstract
In much of the world antimicrobial drugs are sold without prescription or oversight by health-care professionals. The scale and effect of this practice is unknown. We systematically reviewed published works about non-prescription antimicrobials from 1970–2009, identifying 117 relevant articles. 35 community surveys from five continents showed that non-prescription use occurred worldwide and accounted for 19–100% of antimicrobial use outside of northern Europe and North America. Safety issues associated with non-prescription use included adverse drug reactions and masking of underlying infectious processes. Non-prescription use was common for non-bacterial disease, and antituberculosis drugs were available in many areas. Antimicrobial-resistant bacteria are common in communities with frequent non-prescription use. In a few settings, control efforts that included regulation decreased antimicrobial use and resistance. Non-prescription antimicrobial and antituberculosis use is common outside of North America and northern Europe and must be accounted for in public health efforts to reduce antimicrobial resistance.
Introduction
Antimicrobials are among the most commonly purchased drugs worldwide.1 They are essential treatments, especially in the developing world where infectious diseases are a common cause of death.2 Crucial to success of antimicrobial treatment is the use of well-tolerated drugs with activity against common pathogens. Antimicrobial resistance is shrinking the range of antimicrobial drugs and is a worldwide public health problem.3–5 Although resistance is commonly studied in Europe and North America, developing countries also face the threat of antimicrobial resistance. A substantial proportion of healthy people in develop ing countries are colonised with multidrug-resistant bacteria.6–11 Common bacterial pathogens such as Escherichia coli, Salmonella spp, or Streptococcus pneumoniae are often multidrug resistant in these settings. Mycobacterium tuberculosis, including multidrug-resistant M tuberculosis, is also common in low-income to middle-income countries, and extremely drug resistant strains have emerged.12,13 By contrast with Europe and North America, developing countries have limited access to antimicrobials, particularly to new drug classes, and drugs that are available often lack activity against these multidrug-resistant bacteria that are becoming more common.14–16
Antimicrobial resistance is a global issue.17 Resistance genes spread throughout the world, as shown by the global spread of CTX-M extended spectrum β-lactamase (ESBL), NDM-1, or Klebsiella pneumoniae carbapenemase (KPC) producing Enterobacteriaceae.18–20 Travellers might acquire resistant bacteria that can be transmitted in their home countries.20–22 Transmission between developed and developing countries is presumably two-way and is rarely identified in developing countries because of inadequate surveillance.17,19,20
Antimicrobials, when appropriately targeted to a susceptible pathogen, improve outcomes for patients.23 However, use and overuse at the population level is associated with the emergence of bacterial resistance.16,24 Behaviours that promote resistance in one individual or population can have wider consequences for the global community.25 These behaviours extend beyond human antimicrobial use to areas such as antimicrobial use in livestock production.26 Although non-prescription antimicrobial use is typically studied within a single country, the effect of widespread antimicrobial overuse is likely to be felt worldwide.15
By contrast with northern Europe and North America, where outpatient antimicrobials are largely restricted to prescription-only use, non-prescription access to antimicrobials is common in the rest of the world.9,14–16 Pharmaceutical surveillance in Spain showed that about 30% of outpatient antimicrobials purchased were not identified by reimbursement data, largely because nonprescription sales were not tracked.27 In all countries, most antimicrobial use occurs outside of hospitals.28 Interventions to preserve the effectiveness of antimicrobials have focused on hospitals or providers thereby missing non-prescription antimicrobial use.29 Previous reviews have broadly described the problem of antimicrobial resistance in developing countries. However, none have expressly addressed nonprescription antimicrobial use.14,15,30–35 To quantify the global frequency and effect of non-prescription antimicrobial use, we did a systematic review of published work.
Methods
Search strategy and selection criteria
We searched Medline, PubMed, and Google Scholar for articles published in English or Spanish between 1970–2009 using the keywords “antibiotic(s)”, “antimicrobial(s)”, “antituberculosis”, or “antitubercular” combined with “over-the-counter (OTC)”, “nonprescription”, “community pharmacy”, “pharmacies”, “developing country”, “pharmacoepidemiology”, “rational use of medicines”, and “self-medication”, and the corresponding Medical Subject Heading (MeSH) terms for the above keywords.
Data extraction and analysis
DJM and SW reviewed each paper independently. Articles that studied a community setting (as opposed to hospital) and reported if antimicrobial dispensing was prescription-based were selected. Discrepancies were resolved through discussion between the authors. We classified providers to be either physicians or specially trained nurses. We excluded antimalarials from this Review because many countries recommend home-based treatment, which often entails non-prescription use as standard practice.36
We identified the following general types of studies: community surveys of individuals reporting frequency of prescription and non-prescription antimicrobial use, simulated-client-method pharmacy studies in which actors surveyed antimicrobial dispensing practices of pharmacies, harm or potential harm related to nonprescription antimicrobial use, associations between non-prescription antimicrobial use and bacterial resistance, and non-prescription use of antituberculosis drugs.
Community surveys were assessed for quality on the basis of these criteria: appropriate design, standardised questionnaires, and response rates. Those judged to be of moderate-to-high quality were included.37 For each region, community surveys of antimicrobial use were combined to report frequency with which patients received antimicrobials. To account for different sample sizes in each survey, the percentage of patients using non-prescription antimicrobials was multiplied by the total number of survey respondents for each survey. Absolute numbers of patients who received non-prescription antimicrobials for a region were then divided by total number of survey respondents. This number is reported as weighted non-prescription antimicrobial use. Similar methods were used to report location from which non-prescription antimicrobials were obtained (when information was available).
Simulated-client-method pharmacy surveys were defined as studies in which individuals identifying themselves as patients or relatives of patients approached pharmacy staffrequesting medical assistance with a predetermined scenario.38 Surveys of pharmacy bulk purchase or exit interviews with patients, although recommended by WHO for monitoring antimicrobial use, were not included as these do not provide estimates of non-prescription use.39
Results
Frequency of non-prescription use
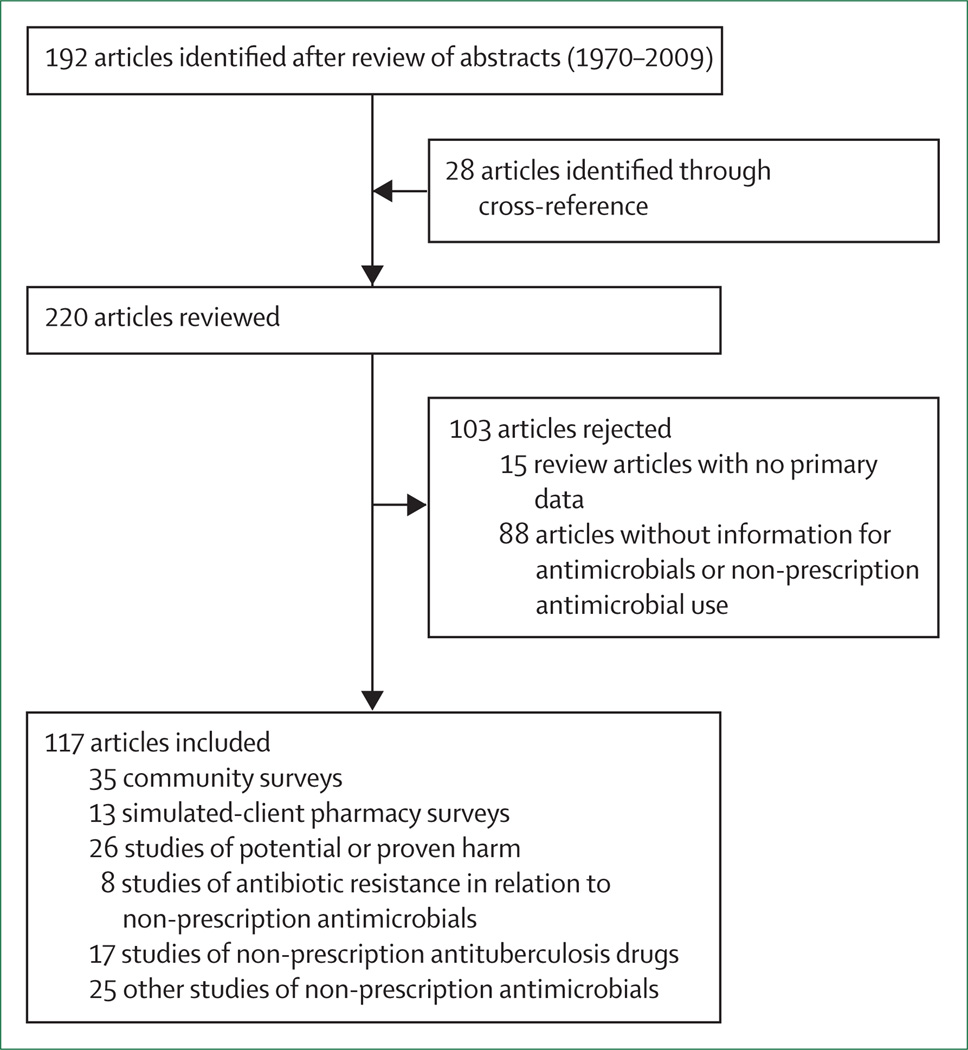
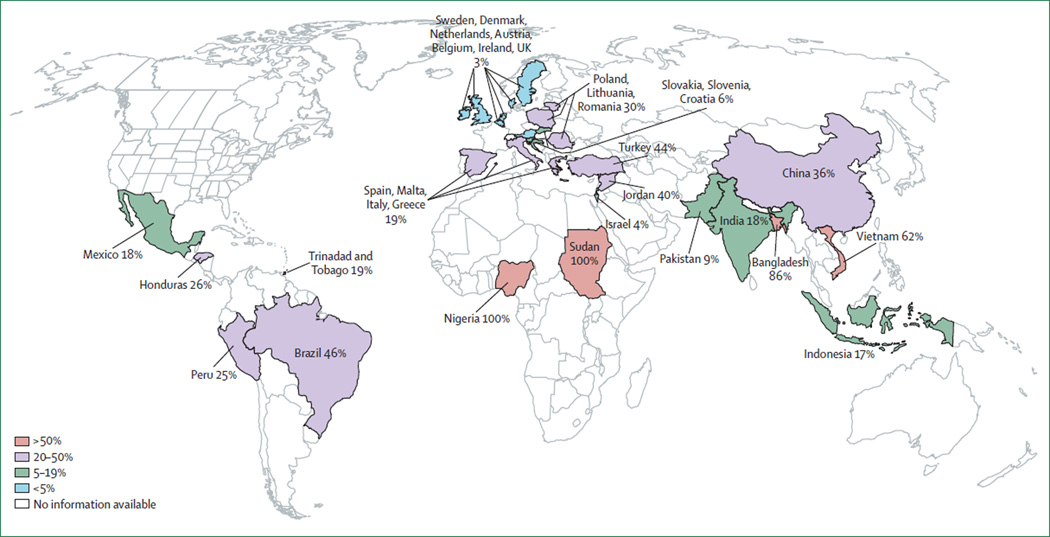
We identified 1434 potential references. 117 articles are included in this Review (figure 1). We identified 35 studies that surveyed use of antimicrobials by patients outside of hospital settings (figure 2). We identified three African surveys; two were surveys of the general population in Nigeria40 and Sudan41 and one was a survey of sex workers or soldiers who had sex with them in Nigeria.42 Both general community surveys focused on non-prescription use of anti microbials and reported frequency of antimicrobial use from 48% over 1 month to 100% lifetime use. Of the antimicrobials used, weighted nonprescription use was 100% (2250 of 2250). Of the nonprescription antimicrobials, 76% were purchased at a pharmacy and 24% were from friends, family, or home (one study enrolled participants at a pharmacy, potentially increasing the proportion of non-prescription antimicrobials purchased at a pharmacy).42
Figure 1. Flowchart of study selection.
Some articles were classified as fitting into more than one type of study (eg, simulated client pharmacy surveys and potential or proven harm).
Figure 2. Frequency of non-prescription use of antimicrobials in the general population based on published works.
In small areas, countries with similar frequency of non-prescription antimicrobial use have been grouped.
Nine surveys from Asia were identified, of which seven were studies of the general population,8,43–48 one was of sex workers,49 and one was based on a review of pharmacy records.50 Frequency of antimicrobial use varied from 4–75%, 4 weeks before each survey. Of antimicrobials used, weighted non-prescription use was 58% (7761 of 13 366). The source of non-prescription antimicrobials was not reported reliably.
Of seven European studies,51–57 one surveyed 19 countries with at least 200 respondents per country.52 Sufficient survey data were available to describe individual regions. Northern Europe, southern Europe, central Europe, and eastern Europe were described separately because of sufficient granularity of data and similarity of practice within each region.
Two surveys of the general population from northern Europe were identified,52,56 which represented one primary survey of Austria, Belgium, the Czech Republic, Denmark, Ireland, Luxembourg, the Netherlands, Sweden, and the UK.52 Frequency of antimicrobial use varied from 14–37% in the past year. Of antimicrobials used, weighted non-prescription use was 3% (330 of 9707). Of the non-prescription antimicrobials, 19% were purchased at a pharmacy and 81% were from friends, family, or home.
Four surveys of the general population from southern Europe were identified, with data for Greece, Italy, Malta, and Spain.51–55 Frequency of antimicrobial use varied from 47–73% in the past year. Of antimicrobials used, weighted non-prescription use was 19% (4113 of 21 599). Of the non-prescription antimicrobials, 47% were bought at a pharmacy and 53% were from friends, family, or home.
Two surveys of the general population from eastern Europe were identified,52,57 which had data from one primary survey of Lithuania, Poland, and Romania.52 Frequency of antimicrobial use varied from 23–51% in the past year. Of antimicrobials used, weighted nonprescription use was 30% (625 of 2112). Of the nonprescription antimicrobials, 68% were purchased at a pharmacy and 32% were from friends, family, or home.
One survey of the general population from central Europe was identified with data for Croatia, Slovakia, and Slovenia.52 Frequency of antimicrobial use varied from 31–61% in the past year. Of antimicrobials used, weighted non-prescription use was 6% (141 of 2304). Of the nonprescription antimicrobials, 68% were purchased at a pharmacy and 32% were from friends, family, or home.
Six surveys of the general population from the Middle East had data for Turkey,58,59 Jordan,60,61 and Israel.52,62 Two studies52,62 reported data from the same population, and the most exhaustive study was included.52 We identified one study of adolescent girls in Saudi Arabia.63 Antimicrobial use varied from 23–46% in the past year. Of antimicrobials used, overall non-prescription use was 39% (4782 of 12 283), although Israel was an outlier for the region with 4% non-prescription use. Of nonprescription antimicrobials, 44% were purchased at a pharmacy and 56% were from friends, family, or home.
Three surveys of the general population from South America included data for Bolivia,10 Brazil,64 and Peru.65 Frequency of antimicrobial use varied from 23%, in the past 2 weeks, to 62%, in the past 4 months. Weighted non-prescription antimicrobial use was 25% (312 of 1224). The source of non-prescription antimicrobials was not described.
From Central America and the Caribbean, studies from Honduras,66 Mexico,67,68 and Trinidad and Tobago69,70 were available. Four studies66–70 reported on the same populations and in these cases the more exhaustive study was reviewed.68,70 Frequency of antimicrobial use varied from 5–55% in the 2 weeks to 6 months before each study. Of antimicrobials used, weighted non-prescription use was 19% (1867 of 9917). Of the non-prescription antimicrobials, 86% were purchased at a pharmacy and 14% were from friends, family, or home.
No surveys of the general population in North America were identified. However, two surveys of Latin American immigrants71,72 and one survey of patients attending a sexually transmitted disease clinic,73 all in the USA, discussed non-prescription antimicrobial use. Within these groups, 14–26% reported non-prescription antimicrobial use a year before each study.
We identified no clear temporal trends in proportion of non-prescription antimicrobial use over the 40-year period examined in this Review.
13 studies were identified in which a simulated-client survey was used to assess willingness of pharmacy staffto dispense an antimicrobial in response to specific clinical scenarios (table 1). We did not include simulatedclient surveys without a predefined clinical scenario or direct surveys of pharmacy staffor general national policies, because these have been reported to be less reflective of actual practice.84 These surveys examined practices of ten to 197 pharmacies. Two studies examined many countries, and country-specific results are shown in table 1.79,81
Table 1.
Frequency of pharmacist recommendation of antimicrobial based on simulated client method surveys
| Country (year) | Cough or runny nose |
Pharyngitis (afebrile) |
Upper respiratory infection or influenza |
Acute sinusitis |
Diarrhoea | Urinary tract infection |
|---|---|---|---|---|---|---|
| Americas | ||||||
| Mexico (1994)74 | 100%* † | ·· | ·· | ·· | ·· | ·· |
| Bolivia (1992)10 | 16% | 91%‡ | 24% | ·· | 92%§ | 63% |
| Brazil (2002)75 | ·· | ·· | ·· | 74% | ·· | ·· |
| Europe | ||||||
| Spain (2007)76 | ·· | 35% | 16% | ·· | ·· | 80% |
| Greece (2000)77 | ·· | ·· | ·· | 80% | ·· | ·· |
| Middle East | ||||||
| Iran (1975)78 | ·· | 60% | ·· | ·· | 40% | ·· |
| Yemen (1985)79 | ·· | ·· | ·· | ·· | 9% | ·· |
| Africa | ||||||
| Zimbabwe (2004)80 | ·· | ·· | ·· | ·· | 9% | 8% |
| Asia | ||||||
| Sri Lanka (1985)79 | ·· | ·· | ·· | ·· | 41% | ·· |
| Bangladesh (1985)79 | ·· | ·· | ·· | ·· | 68% | ·· |
| Bangladesh (2004)43 | ·· | ·· | ·· | ·· | 40% | ·· |
| Vietnam (1999)45 | ·· | ·· | 99% | ·· | 75% | ·· |
| Vietnam (1999)81 | 98% | ·· | ·· | ·· | ·· | ·· |
| Nepal (1996)82 | ·· | ·· | ·· | ·· | 97% | 38% |
| Thailand (1999)81 | 76% | ·· | ·· | ·· | 9% | ·· |
| Thailand (2006)83 | ·· | 74% | 65% | 80% | 76% | 100%† |
Cough duration 1 month.
Rifampicin recommended.
Pharyngitis with high fever.
40% of children and 92% of adults received an antimicrobial for diarrhoea.
Wide variation was noted between studies, with some studies finding infrequent prescription of antimicrobials. Some of this variation might be ascribed to variations in the clinical scenario presented to pharmacists. However, frequency of antimicrobial dispensing was not always logical: in one study, fewer patients with high fever and sinusitis received antimicrobial than did those in the same scenario with low fever.77 No obvious temporal or geographical patterns in antimicrobial dispensing were evident. Inappropriate antimicrobial dispensing was common, including use of antituberculosis drugs for standard bacterial infections and anti tuberculosis monotherapy. 74,83,85 No studies comparing frequency of antimicrobial dispensing by pharmacists and providers in the same area were identified. Community surveys have shown that pharmacists were more likely than physicians to dispense inappropriate antimicrobials in Brazil,63 but less likely in Mexico.67
One study,81 in Vietnam and Thailand, assessed a pharmacy intervention that included enforcement of existing regulation and education in pharmacies randomly assigned to intervention or control. In Vietnam, 71% of patients with cough were given antimicrobials by pharmacies after the intervention compared with 95% without. The same intervention in Thailand resulted in no difference (88% vs 92%), showing the importance of culture.81 Finally, one study surveyed internet-based pharmaceutical sites accessed within the USA, finding that all major classes of antimicrobials were available for purchase without prescription, generally from countries that do not regulate antimicrobials.86
Safety
26 studies addressed potential or confirmed patient-safety issues related to non-prescription use of antimicrobials. No standardised method for these studies was identified. Most reported potential adverse events, although four reported actual adverse events including death, in relation to non-prescription use of antimicrobials (table 2).
Table 2.
Potential and proven adverse events associated with antimicrobials taken without prescription
| Frequency | Description | |
|---|---|---|
| No questioning by pharmacists regarding allergies76,82 | >80% | No advice or questions from pharmacy staff regarding allergies, side-effects, or drug interactions |
| No explanation of potential side-effects87,88 | About 50% | No advice or questions from pharmacy staff regarding allergies, side-effects, or drug interactions |
| Contraindicated antimicrobials | Up to 8% of antimicrobials used for children | Tetracyclines and fluoroquinolones dispensed for children8,50 |
| Parenteral antimicrobials for home use52,66,68,89–92 | Unknown | Injectable streptomycin, gentamicin, and penicillin provided |
| Inadequate treatment (of true bacterial infections) | ||
| Short course41,79,82,88,93 | Many treatment courses <1 day | ·· |
| Inadequate dose10,41,85,88 | Common | ·· |
| Inappropriate antimicrobial74,83 | Common | Inappropriate drug for indication |
| Low-quality medication30,94–96 | Unknown | ·· |
| Documented adverse events | ||
| Diarrhoea51,64 | 5–11% | As reported by patients |
| Rash51 | 4% | ·· |
| Masked diagnosis of infectious disease97 | 90% increased risk | Emergency room patients with detectable antimicrobials in urine had higher risk than those without for masked or missed diagnosis of infections |
| Renal failure98 | Case report | Non-prescribed rifampicin used for cough resulting in renal failure |
| Aplastic anaemia and death99 | Case report | Woman vacationing in Spain took chloramphenicol for upper respiratory infection |
Potential adverse events were common. Pharmacists dispensing non-prescription antimicrobials had no knowledge of patients’ allergies 83–100% of the time.76,82 Potential side-effects were discussed in about 50% of non-prescription antimicrobial purchases.81,87 Duration of antimicrobial use was shorter without than with a prescription.100 Many studies of non-prescription dispensing showed short, often single-day, courses of antimicrobials.41,48,82,85,88,94,101 Dose of antimicrobials was also commonly lower than standard doses.10,41,85,89 Parenteral antimicrobials were often available without prescription.52,66,68,89–92
An additional safety concern was substandard quality of antimicrobials available without prescription. Expired drugs or those that, as a result of degradation, have decreased bioavailability might both predispose a patient to treatment failure and promote antimicrobial resistance.30,94 Outright counterfeit antimicrobials are available in developing countries and can lead to treatment failure or direct harm;94,95 non-prescription use of substandard antimicrobials is probably more common, although low-quality antimicrobials have also been identified through official prescription sources.102
Definite adverse effects of non-prescription antimicrobials were rarely reported (table 2), probably due in part to the decentralised health-care systems in most areas with non-prescription sale of antimicrobials. In Taiwan, patients admitted to hospital through the emergency room with detectable anti microbial concentrations in urine were nearly twice as likely to have a masked or missed diagnosis of an infectious disease.97 Two case reports described severe adverse events in relation to non-prescription antimicrobials (table 2).
Adverse effects of non-prescription antimicrobials are rarely reported, but they are likely at least as common as adverse effects of prescription antimicrobials. In a study from North America, penicillin, cephalosporins, clindamycin, sulphonamides, and fluoroquinolones were the antimicrobials most commonly associated with adverse events. Of adverse events requiring emergency room attendance, 79% were allergic reactions. Antimicrobials accounted for 19% of all visits to the emergency room for adverse drug events, with some associated with a rate of serious adverse events as high as 20 per 10 000 prescriptions.103
Resistance
Eight studies assessed bacterial resistance while surveying frequency of non-prescription use of antimicrobials in human beings. Bartoloni and colleagues10 studied non-pathogenic E coli isolates from children under age 5 years in Bolivia. Overall, 40% of children harboured non-pathogenic E coli resistant to ampicillin, co-trimoxazole, tetracycline, and chloramphenicol (which were the antimicrobials most commonly used in the communities studied). In this population, antimicrobials were available without a prescription and were frequently used. Bartoloni and colleagues11 did a larger follow-up study of non-pathogenic E coli in Bolivia and Peru and reported that, 8 years after the first study, multidrug resistance had increased to 90% of isolates and that after the introduction of fluoroquinolones resistance to them had increased.11
Frequency of resistance has been examined in respiratory pathogens in Vietnamese children and Senegalese adults with urinary tract infections.8,45,104 In all studies, high rates of community resistance were reported and were associated with patients receiving antimicrobials 6 months before each study, in communities with high non-prescription antimicrobial use. The risk conferred by use of non-prescription antimicrobials in these small studies seems to be roughly equal to that reported for prescription antimicrobial use in a recent meta-analysis (odds ratio 1·4–3·7).8,104,105
At a population level, clinically important bacterial resistance including penicillin-resistant and erythromycin- resistant S pneumoniae and ciprofloxacin-resistant non-typhi salmonella has been associated with increasing non-prescription use of these antimicrobials in a Thai community.106
At least some of these shifts in antimicrobial resistance are potentially reversible: data about pharmaceutical use in Pakistan was combined with data about antimicrobial resistance to show a relation between declining use of antimicrobials and declining resistance in Salmonella enteric serotype Typhi.15 In Chile, as a result of regulation of antimicrobials, consumption decreased and E coli antimicrobial resistance seems to have decreased.6
Antituberculosis drugs
Essential antituberculosis drugs were available without prescription in many regions (panel). Non-prescription use included monotherapy with rifampicin for urinary tract infection or sexually transmitted diseases,49,83 isoniazid for general respiratory ailments,107 or other antituberculosis drugs, including streptomycin, for many non-tuberculosis indications.40,52,93,110,112
Panel: Reports of WHO first-line antituberculosis drugs available without prescription for treatment of tuberculosis and other bacterial infections.
First-line drug antituberculosis drugs include rifampicin, isoniazid, pyrizinimide, ethambutol, streptomycin.12
Non-tuberculosis infections
Asia (Thailand,83 Philippines,49,107,108 India,92 Vietnam8,87)
Middle East (Saudi Arabia109)
Central America (Mexico74)
Tuberculosis
Treatment of presumed tuberculosis with nonprescription antituberculosis drugs was common—over half of antimicrobials purchased for treatment of tuberculosis in Manila, Philippines, and Nagpur, India, were dispensed without prescription.93,108 Pharmacies in Mexico often recommended non-prescription rifampicin monotherapy for tuberculosis.74 Second-line antituberculosis drugs, including moxifloxacin and aminoglycosides, have been widely available without prescription according to published works,58,93,108 although this may have improved after recent WHO recommendations.12
Discussion
The causes and consequences of non-prescription antimicrobial use are varied. Poor regulation of antimicrobials results from absent policies or, more commonly, from absent enforcement of policies, as happens in southern European countries.51–55 Community antimicrobial resistance was common in several studies that examined communities with frequent use of nonprescription antimicrobials.6–11 Antimicrobial use, whether it is prescription or non-prescription, exerts antimicrobial selection pressure.3–5 No studies have shown that non-prescription antimicrobial use is worse than equally frequent prescription antimicrobial use. However, non-prescription antimicrobials are associated with very short courses41,48,82,85,88,93,101 and inappropriate drug and dose choice.10,41,74,83,85,88 These factors make non-prescription use a greater concern than prescription use. Of note, most community surveys relied on patient reporting of antimicrobial use (with a variable degree of confirmation). Since some surveys reported that patients often do not know they were prescribed an antimicrobial, the true proportion of patients using antimicrobials is probably higher than reported.93 In support of this assumption, more widespread antimicrobial use was identified with urine sampling than with patient selfreport. 97,115–118 Expansion of internet commerce provides virtual worldwide access to non-prescription antimicrobials; those available on the internet are diverse and generally seem to originate in countries in which non-prescription antimicrobials are available.86
Non-prescription use of antimicrobials is inherently associated with little guidance regarding appropriate antimicrobial selection for individual syndromes and safe practices to minimise adverse drug effects (even when provided by a pharmacist).76,81,82,87 Appropriate antimicrobial use is complex and must be based on local susceptibility patterns. In areas where antimicrobial susceptibility data is unavailable, antimicrobial selection is difficult, even for skilled providers.14–16 Uninformed patients or undertrained pharmacy staffrarely have access to basic information regarding appropriate antimicrobial use and do not appreciate the complexity involved in decisions surrounding drug selection.64,67,76,83 As a result, financial concerns often guide antimicrobial selection resulting in short duration of treatment.15,35,119 The conflict of interest inherent in the same individual prescribing and dispensing antimicrobials is a potential target for regulatory intervention.
Despite concerns about inappropriate use, nonprescription status of antimicrobials might be an important mechanism of access to antimicrobials in resource-limited settings. Interventions to reduce inappropriate antimicrobial use, be they focused on prescription or non-prescription, must be monitored so as to not limit access to antimicrobials for those patients with true bacterial disease.120 Receipt of appropriate antimicrobials in a timely fashion is a determinant of good outcome in pneumonia and other serious bacterial illnesses.121
Clear evidence that antimicrobials obtained without prescription are used less appropriately than prescription antimicrobials does not exist. Providers, pharmacists, and patients might be equally as likely to overuse antimicrobials in any given setting.39,64,67 Studies with patients simulated by actors showed that inappropriate antimicrobial dispensing by pharmacists without a prescription occurred frequently.74–83,85 In low-income to medium-income countries, training of health-care workers as part of WHO’s Integrated Management of Childhood Illness strategy has been shown to increase appropriate use of antimicrobials and decrease inappropriate use.122–125 Non-physician health-care workers trained in integrated management of childhood illness might use antimicrobials more judiciously than physician providers. In resource-limited settings, training of nonphysician providers could increase both access to and appropriate use of antimicrobials; this is especially important in regions where inadequate numbers of physician providers could drive non-prescription antimicrobial use.126
Inappropriate antimicrobial use by patients with true bacterial infections is associated with treatment failure and masking of the underlying clinical syndrome.97 In patients treated inappropriately, either because they do not have an underlying bacterial infection or receive an inappropriate drug or suboptimum duration of treatment, patients are exposed to the risks of an antimicrobial without benefit. Although widely perceived as low-risk drugs, antimicrobials are the second most common cause of adverse drug events in the USA,103 and nonprescription antimicrobial use has been associated with severe adverse events including death.98,99
Non-prescription use of antituberculosis drugs for non-tuberculosis indications was common. Since up to a third of the world’s population is estimated to be infected with tuberculosis,13 substantial use of antituberculosis drugs in countries with a high prevalence of tuberculosis is concerning. Essential firstline antituberculosis drugs include rifampicin, isoniazid, pyrizinamide, ethambutol, and streptomycin.13 Although these drugs are generally poor antibiotics, they are available and used for tuberculosis and nontuberculosis indications in many countries with a high prevalence of tuberculosis. Previous use of antituberculosis drugs is a risk factor for development of multidrug-resistant tuberculosis. Second-line drugs including fluoroquinolones and aminoglycosides are also available without a prescription in most countries, which could be a factor in the emergence of extremely drug-resistant tuberculosis.
We have not reviewed interventions to improve nonprescription use, because solutions are often specific to a culture, country, or region.14,15,35 Many groups have described comprehensive guidelines that emphasise development of health-care systems, including a WHO report on containment of antimicrobial resistance.14,33–35,127 Chile and South Korea are notable examples of countries that improved regulation of non-prescription antimicrobial use and seem to have improved resistance profiles.128–130 However, whether or how quickly resistance can be reversed in response to a reduction in selective pressure is unknown.131,132
The contribution of non-prescription antimicrobial use to the worldwide development and spread of antimicrobial resistance genes and bacteria is not known. Hospital and community prescription use and antimicrobial use in livestock production all exert selection pressure for antimicrobial resistance.26 Countries with high levels of community antimicrobial resistance often have non-prescription antimicrobial use. Non-prescription use has been speculated to play an important role in selecting and maintaining these high levels of community antimicrobial resistance.18,20,133,134
Our Review has limitations including the combination of studies with heterogeneous populations and the analysis of studies over a broad time period. Data might not mirror current practice in countries included in our Review. Data about dispensing practices in regions were extrapolated, and although many neighbouring countries probably had similar practices, exceptions exist such as Chile in South America. As in any review, a positive publication bias could affect some findings. Our Review is not meant to be a reference for policy or enforcement of such policy in specific countries, but is meant to be a broad overview of general issues associated with non-prescription antimicrobial use. Future studies are needed that include standardised definitions and methods and sample many countries, such as those done in Europe by Grigoryan and colleagues.52 Studies that focus on individual patients or providers are important to accurately identify practice and not just policy in those regions where substantial differences might exist.83 Studies of antimicrobial use should assess the appropriateness of prescription and non-prescription use so that local interventions could be best targeted for the greatest effect.
Despite many publications describing the frequency of non-prescription use and the adverse effects of such a practice, high-quality research is scarce. In the absence of rigorous studies, interventions to improve nonprescription antimicrobial use by effective restriction of national formularies to safe antimicrobials should be used; this should include restriction of aminoglycosides and other injectable antimicrobials. Essential first-line antituberculosis drugs should be restricted to prescription-only use in all countries, and secondary antituberculosis should be restricted in countries with a high prevalence of multidrug-resistant tuberculosis. Appropriate labelling of anti microbials including common indications, treatment duration, and side-effects could improve safety. Restriction of dispensing to a set number of pills, ideally in blister packs, could aid in appropriate use when healthcare practitioners are not available. In middle-income to high-income countries with reliable access to health-care practitioners, antimicrobials should be restricted to prescription-only status.
Community antimicrobial stewardship must include a focus on non-prescription antimicrobials. Most methods of monitoring antimicrobial use including pharmacy prescription monitoring or health-care insurance billing do not reliably detect non-prescription use. Pharmacy exit interviews or community surveys more accurately identify total antimicrobial use. In countries with high levels of non-prescription use, interventions to decrease community antimicrobial use should focus on the general public. Countries that have effectively decreased non-prescription use have done so by combining regulation with expanded access to health care.
Acknowledgments
DJM is funded by The Agency for Healthcare Research and Quality (1 K08 HS18111-01). INO was supported by a Branco Weiss fellowship from the Society-in-Science, ETHZ, Switzerland. ENP is funded by VA Health Services Research and Development.
Footnotes
Search strategy and selection criteria:
These are described in detail in the Methods section.
Contributors
DJM had the original idea for the Review; RL, ENP, and SW helped develop the concept. DJM and SW searched published works. INO, RL, ENP, and SW interpreted data. DJM and INO created the figures. DJM created the tables. All authors wrote the paper.
Conflicts of interest
DM and SW have received unrestricted research grants from Merck. ENP has received unrestricted research grants from Merck and Pfizer. INO and RL declare no conflicts of interest.
References
- 1.Col NF, O’Connor RW. Estimating worldwide current antibiotic usage: report of task force 1. Rev Infect Dis. 1987;9(suppl 3):232–243. doi: 10.1093/clinids/9.supplement_3.s232. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The global burden of disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 5.Taubes G. The bacteria fi ght back. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 6.Stelling JM, Travers K, Jones RN, Turner PJ, O’Brien TF, Levy SB. Integrating Escherichia coli antimicrobial susceptibility data from multiple surveillance programs. Emerg Infect Dis. 2005;11:873–882. doi: 10.3201/eid1106.041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lester SC, del Pilar Pla M, Wang F, Perez Schael I, Jiang H, O’Brien TF. The carriage of Escherichia coli resistant to antimicrobial agents by healthy children in Boston, in Caracas, Venezuela, and in Qin Pu, China. N Engl J Med. 1990;323:285–289. doi: 10.1056/NEJM199008023230501. [DOI] [PubMed] [Google Scholar]
- 8.Larsson M, Kronvall G, Chuc NT, et al. Antibiotic medication and bacterial resistance to antibiotics: A survey of children in a Vietnamese community. Trop Med Int Health. 2000;5:711–721. doi: 10.1046/j.1365-3156.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Alliance for the Prudent Use of Antibiotics. Executive summary: select findings, conclusions, and policy recommendations. Clin Infect Dis. 2005;41(suppl 4):224–227. doi: 10.1086/430781. [DOI] [PubMed] [Google Scholar]
- 10.Bartoloni A, Cutts F, Leoni S, et al. Patterns of antimicrobial use and antimicrobial resistance among healthy children in Bolivia. Trop Med Int Health. 1998;3:116–123. doi: 10.1046/j.1365-3156.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartoloni A, Pallecchi L, Benedetti M, et al. Multidrug-resistant commensal Escherichia coli in children in Peru and Bolivia. Emerg Infect Dis. 2006;12:907–913. doi: 10.3201/eid1206.051258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Report of the second meeting of the WHO task force on XDR-TB/ Geneva: World Health Organization; 2008. [Google Scholar]
- 13.Glaziou P, Floyd K, Raviglione M. Global burden and epidemiology of tuberculosis. Clin Chest Med. 2009;30:621–636. doi: 10.1016/j.ccm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Okeke IN, Klugman KP, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis. 2005;5:568–580. doi: 10.1016/S1473-3099(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 15.Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 16.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okeke IN, Edelman R. Dissemination of antibiotic-resistant bacteria across geographic borders. Clin Infect Dis. 2001;33:364–369. doi: 10.1086/321877. [DOI] [PubMed] [Google Scholar]
- 18.Rossolini GM, D’Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008;14(suppl 1):33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 19.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 20.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray BE, Mathewson JJ, DuPont HL, Ericsson CD, Reves RR. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agents. Antimicrob Agents Chemother. 1990;34:515–518. doi: 10.1128/aac.34.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camou T, Hortal M, Tomasz A. The apparent importation of penicillin-resistant capsular type 14 Spanish/French clone of Streptococcus pneumoniae into Uruguay in the early 1990s. Microb Drug Resist. 1998;4:219–224. doi: 10.1089/mdr.1998.4.219. [DOI] [PubMed] [Google Scholar]
- 23.Pillai SK, Elioppoulos GM, Mollering RC. Principles of anti-infective therapy. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 7th edn. Philadelphia: Churchill Livingstone; 2010. pp. 267–279. [Google Scholar]
- 24.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 25.Baquero F, Campos J. The tragedy of the commons in antimicrobial chemotherapy. Rev Esp Quimioter. 2003;16:11–13. [PubMed] [Google Scholar]
- 26.Harada K, Asai T. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in Escherichia coli from food-producing animals in Japan. J Biomed Biotechnol. 2010;2010:180682. doi: 10.1155/2010/180682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos J, Ferech M, Lazaro E, et al. Surveillance of outpatient antibiotic consumption in Spain according to sales data and reimbursement data. J Antimicrob Chemother. 2007;60:698–701. doi: 10.1093/jac/dkm248. [DOI] [PubMed] [Google Scholar]
- 28.Wise R, Hart T, Cars O, et al. Antimicrobial resistance is a major threat to public health. BMJ. 1998;317:609–610. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 30.Okeke IN, Lamikanra A. Quality and bioavailability of tetracycline capsules in a Nigerian semi-urban community. Int J Antimicrob Agents. 1995;5:245–250. doi: 10.1016/0924-8579(94)00064-2. [DOI] [PubMed] [Google Scholar]
- 31.Nordberg P, Lundborg CS, Tomson G. Consumers and providers–could they make better use of antibiotics? Int J Risk Saf Med. 2005;17:117–125. [Google Scholar]
- 32.Kunin CM. Use of antimicrobial drugs in developing-countries. Int J Antimicrob Agents. 1995;5:107–113. doi: 10.1016/0924-8579(94)00039-w. [DOI] [PubMed] [Google Scholar]
- 33.Kunin CM. Resistance to antimicrobial drugs—a worldwide calamity. Ann Intern Med. 1993;118:557–561. doi: 10.7326/0003-4819-118-7-199304010-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kunin CM, Johansen KS, Worning AM, Daschner FD. Report of a symposium on use and abuse of antibiotics worldwide. Rev Infect Dis. 1990;12:12–19. doi: 10.1093/clinids/12.1.12. [DOI] [PubMed] [Google Scholar]
- 35.Kunin CM, Lipton HL, Tupasi T, et al. Social, behavioral, and practical factors aff ecting antibiotic use worldwide: report of task force 4. Rev Infect Dis. 1987;9(suppl 3):270–285. doi: 10.1093/clinids/9.supplement_3.s270. [DOI] [PubMed] [Google Scholar]
- 36.Djimde A, Plowe CV, Diop S, Dicko A, Wellems TE, Doumbo O. Use of antimalarial drugs in Mali: policy versus reality. Am J Trop Med Hyg. 1998;59:376–379. doi: 10.4269/ajtmh.1998.59.376. [DOI] [PubMed] [Google Scholar]
- 37.AAPOR. [accessed Jan 26, 2010];American Association for Public Opinion Research Best Practices. http://www.aapor.org/Best_Practices/1480.htm.
- 38.Madden JM, Quick JD, Ross-Degnan D, Kafl e KK. Undercover careseekers: Simulated clients in the study of health provider behavior in developing countries. Soc Sci Med. 1997;45:1465–1482. doi: 10.1016/s0277-9536(97)00076-2. [DOI] [PubMed] [Google Scholar]
- 39.Holloway KA, Mathai E, Sorensen T, Gray A. Community-based surveillance of antimicrobial use and resistance in resource-constrained settings: Report on five pilot projects. Geneva: World Health Organization; 2009. [Google Scholar]
- 40.Esimone CO, Nworu CS, Udeogaranya OP. Utilization of antimicrobial agents with and without prescription by out-patients in selected pharmacies in south-eastern Nigeria. Pharm World Sci. 2007;29:655–660. doi: 10.1007/s11096-007-9124-0. [DOI] [PubMed] [Google Scholar]
- 41.Awad A, Eltayeb I, Matowe L, Thalib L. Self-medication with antibiotics and antimalarials in the community of Khartoum state, Sudan. J Pharm Pharm Sci. 2005;8:326–331. [PubMed] [Google Scholar]
- 42.Fakunle YM, Watkins B. Infl uence of self-medication on prevalence and antibiotic sensitivity of N. gonorrhoeae in Zaria (Nigeria) East Afr Med J. 1976;53:693–696. [PubMed] [Google Scholar]
- 43.Larson CP, Saha UR, Islam R, Roy N. Childhood diarrhoea management practices in Bangladesh: private sector dominance and continued inequities in care. Int J Epidemiol. 2006;35:1430–1439. doi: 10.1093/ije/dyl167. [DOI] [PubMed] [Google Scholar]
- 44.Bi P, Tong S, Parton KA. Family self-medication and antibiotics abuse for children and juveniles in a Chinese city. Soc Sci Med. 2000;50:1445–1450. doi: 10.1016/s0277-9536(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 45.Quagliarello AB, Parry CM, Hien TT, Farrar JJ. Factors associated with carriage of penicillin-resistant Streptococcus pneumoniae among Vietnamese children: a rural-urban divide. J Health Popul Nutr. 2003;21:316–324. [PubMed] [Google Scholar]
- 46.Saradamma RD, Higginbotham N, Nichter M. Social factors infl uencing the acquisition of antibiotics without prescription in Kerala state, south India. Soc Sci Med. 2000;50:891–903. doi: 10.1016/s0277-9536(99)00380-9. [DOI] [PubMed] [Google Scholar]
- 47.Sturm AW, van der Pol R, Smits AJ, et al. Over-the-counter availability of antimicrobial agents, self-medication and patterns of resistance in Karachi, Pakistan. J Antimicrob Chemother. 1997;39:543–547. doi: 10.1093/jac/39.4.543. [DOI] [PubMed] [Google Scholar]
- 48.Hadi U, Duerink DO, Lestari ES, et al. Survey of antibiotic use of individuals visiting public healthcare facilities in Indonesia. Int J Infect Dis. 2008;12:622–629. doi: 10.1016/j.ijid.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Abellanosa I, Nichter M. Antibiotic prophylaxis among commercial sex workers in Cebu City, Philippines; patterns of use and perceptions of effi cacy. Sex Transm Dis. 1996;23:407–412. doi: 10.1097/00007435-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Hossain MM, Glass RI, Khan MR. Antibiotic use in a rural community in Bangladesh. Int J Epidemiol. 1982;11:402–405. doi: 10.1093/ije/11.4.402. [DOI] [PubMed] [Google Scholar]
- 51.Vaananen MH, Pietila K, Airaksinen M. Self-medication with antibiotics–does it really happen in Europe? Health Policy. 2006;77:166–171. doi: 10.1016/j.healthpol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Grigoryan L, Haaijer-Rysjamp FM, Burgerhof JG, et al. Self-medication with antimicrobial drugs in Europe. Emerg Infect Dis. 2006;12:452–459. doi: 10.3201/eid1203.050992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsi G, Jelastopulu E, Basiaris H, Skoutelis A, Gogos C. Patterns of antibiotic use among adults and parents in the community: a questionnaire-based survey in a Greek urban population. Int J Antimicrob Agents. 2005;25:439–443. doi: 10.1016/j.ijantimicag.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Borg MA, Scicluna EA. Over-the-counter acquisition of antibiotics in the Maltese general population. Int J Antimicrob Agents. 2002;20:253–257. doi: 10.1016/s0924-8579(02)00194-2. [DOI] [PubMed] [Google Scholar]
- 55.Carrasco-Garrido P, Jimenez-Garcia R, Barrera VH, Gil de Miguel A. Predictive factors of self-medicated drug use among the Spanish adult population. Pharmacoepidemiol Drug Saf. 2008;17:193–199. doi: 10.1002/pds.1455. [DOI] [PubMed] [Google Scholar]
- 56.Muscat M, Monnet DL, Klemmensen T, et al. Patterns of antibiotic use in the community in Denmark. Scand J Infect Dis. 2006;38:597–603. doi: 10.1080/00365540600606507. [DOI] [PubMed] [Google Scholar]
- 57.Berzanskyte A, Valinteliene R, Haaijer-Ruskamp FM, Gurevicius R, Grigoryan L. Self-medication with antibiotics in Lithuania. Int J Occup Med Environ Health. 2006;19:246–253. doi: 10.2478/v10001-006-0030-9. [DOI] [PubMed] [Google Scholar]
- 58.Buke C, Hosgor-Limoncu M, Ermertcan S, et al. Irrational use of antibiotics among university students. J Infect. 2005;51:135–139. doi: 10.1016/j.jinf.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Cagri Buke A, Ermertcan S, Hosgor-Limoncu M, Ciceklioglu M, Eren S. Rational antibiotic use and academic staff. Int J Antimicrob Agents. 2003;21:63–66. doi: 10.1016/s0924-8579(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 60.Al-Azzam SI, Al-Husein BA, Alzoubi F, Masadeh MM, Al-Horani MA. Self-medication with antibiotics in Jordanian population. Int J Occup Med Environ Health. 2007;20:373–380. doi: 10.2478/v10001-007-0038-9. [DOI] [PubMed] [Google Scholar]
- 61.Sawair FA, Baqain ZH, Abu Karaky A, Abu Eid R. Assessment of self-medication of antibiotics in a Jordanian population. Med Princ Pract. 2009;18:21–25. doi: 10.1159/000163041. [DOI] [PubMed] [Google Scholar]
- 62.Raz R, Edelstein H, Grigoryan L, Haaijer-Ruskamp FM. Self-medication with antibiotics by a population in Northern Israel. Isr Med Assoc J. 2005;7:722–725. [PubMed] [Google Scholar]
- 63.Abahussain NA, Taha AZ. Knowledge and attitudes of female school students on medications in eastern Saudi Arabia. Saudi Med J. 2007;28:1723–1727. [PubMed] [Google Scholar]
- 64.Schorling JB, De Souza MA, Guerrant RL. Antibiotic use among children in an urban Brazilian slum: a risk factor for diarrhea? Am J Public Health. 1991;81:99–100. doi: 10.2105/ajph.81.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kristiansson C, Reilly M, Gotuzzo E, et al. Antibiotic use and health-seeking behaviour in an underprivileged area of Peru. Trop Med Int Health. 2008;13:434–441. doi: 10.1111/j.1365-3156.2008.02019.x. [DOI] [PubMed] [Google Scholar]
- 66.Crigger NJ, Holcomb L, Grogan RL, et al. Development of the choices and acquisition of antibiotics model from a descriptive study of a lay Honduran population. Int J Nurs Stud. 2004;41:745–753. doi: 10.1016/j.ijnurstu.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Bojalil R, Calva JJ. Antibiotic misuse in diarrhea. A household survey in a Mexican community. J Clin Epidemiol. 1994;47:147–156. doi: 10.1016/0895-4356(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 68.Calva J, Bojalil R. Antibiotic use in a periurban community in Mexico: A household and drugstore survey. Soc Sci Med. 1996;42:1121–1128. doi: 10.1016/0277-9536(95)00385-1. [DOI] [PubMed] [Google Scholar]
- 69.Parimi N, Pinto Pereira LM, Prabhakar P. Caregivers’ practices, knowledge and beliefs of antibiotics in paediatric upper respiratory tract infections in Trinidad and Tobago: a cross-sectional study. BMC Fam Pract. 2004;5:28. doi: 10.1186/1471-2296-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parimi N, Pinto Pereira LM, Prabhakar P. The general public’s perceptions and use of antimicrobials in Trinidad and Tobago. Rev Panam Salud Publica. 2002;12:11–18. doi: 10.1590/s1020-49892002000700003. [DOI] [PubMed] [Google Scholar]
- 71.Mainous AG, 3rd, Cheng AY, Garr RC, Tilley BC, Everett CJ, McKee MD. Nonprescribed antimicrobial drugs in the Latino community, South Carolina. Emerg Infect Dis. 2005;11:883–888. doi: 10.3201/eid1106.040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKee MD, Mills L, Mainous AG., 3rd Antibiotic use for the treatment of upper respiratory infections in a diverse community. J Fam Pract. 1999;48:993–996. [PubMed] [Google Scholar]
- 73.Gordon SM, Mosure DJ, Lewis J, Brown S, McNagny SE, Schmid GP. Prevalence of self-medication with antibiotics among patients attending a clinic for treatment of sexually transmitted diseases. Clin Infect Dis. 1993;17:462–465. doi: 10.1093/clinids/17.3.462. [DOI] [PubMed] [Google Scholar]
- 74.Gellert GA, Pyle NG. Pharmacy practice and antibiotic-resistant tuberculosis along the US-Mexico border. JAMA. 1994;271:1577–1578. doi: 10.1001/jama.271.20.1577a. [DOI] [PubMed] [Google Scholar]
- 75.Volpato DE, de Souza BV, Dalla Rosa LG, Melo LH, Daudt CA, Deboni L. Use of antibiotics without medical prescription. Braz J Infect Dis. 2005;9:288–291. doi: 10.1590/s1413-86702005000400004. [DOI] [PubMed] [Google Scholar]
- 76.Llor C, Cots JM. The sale of antibiotics without prescription in pharmacies in Catalonia, Spain. Clin Infect Dis. 2009;48:1345–1349. doi: 10.1086/598183. [DOI] [PubMed] [Google Scholar]
- 77.Contopoulos-Ioannidis DG, Koliofoti ID, Koutroumpa IC, Giannakakis IA, Ioannidis JP. Pathways for inappropriate dispensing of antibiotics for rhinosinusitis: a randomized trial. Clin Infect Dis. 2001;33:76–82. doi: 10.1086/320888. [DOI] [PubMed] [Google Scholar]
- 78.Amidi S, Ajamee G, Sadeghi HR, Yourshalmi P, Gharehjeh AM. Dispensing drugs without prescription and treating patients by pharmacy attendants in Shiraz, Iran. Am J Public Health. 1978;68:495–497. doi: 10.2105/ajph.68.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomson G, Sterky G. Self-prescribing by way of pharmacies in three Asian developing countries. Lancet. 1986;2:620–622. doi: 10.1016/s0140-6736(86)92438-4. [DOI] [PubMed] [Google Scholar]
- 80.Nyazema N, Viberg N, Khoza S, et al. Low sale of antibiotics without prescription: a cross-sectional study in Zimbabwean private pharmacies. J Antimicrob Chemother. 2007;59:718–726. doi: 10.1093/jac/dkm013. [DOI] [PubMed] [Google Scholar]
- 81.Chalker J, Ratanawijitrasin S, Chuc NT, Petzold M, Tomson G. Eff ectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand—a randomized controlled trial. Soc Sci Med. 2005;60:131–141. doi: 10.1016/j.socscimed.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 82.Wachter DA, Joshi MP, Rimal B. Antibiotic dispensing by drug retailers in Kathmandu, Nepal. Trop Med Int Health. 1999;4:782–788. doi: 10.1046/j.1365-3156.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 83.Apisarnthanarak A, Tunpornchai J, Tanawitt K, Mundy LM. Nonjudicious dispensing of antibiotics by drug stores in Pratumthani, Thailand. Infect Control Hosp Epidemiol. 2008;29:572–575. doi: 10.1086/587496. [DOI] [PubMed] [Google Scholar]
- 84.Apisarnthanarak A, Mundy LM. Comparison of methods of measuring pharmacy sales of antibiotics without prescriptions in Pratumthani, Thailand. Infect Control Hosp Epidemiol. 2009;30:1130–1132. doi: 10.1086/647980. [DOI] [PubMed] [Google Scholar]
- 85.Thamlikitkul V. Antibiotic dispensing by drug store personnel in Bangkok, Thailand. J Antimicrob Chemother. 1988;21:125–131. doi: 10.1093/jac/21.1.125. [DOI] [PubMed] [Google Scholar]
- 86.Mainous AG, 3rd, Everett CJ, Post RE, Diaz VA, Hueston WJ. Availability of antibiotics for purchase without a prescription on the internet. Ann Fam Med. 2009;7:431–435. doi: 10.1370/afm.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Guzman GC, Khaleghi M, Riff enberg RH, Clark RF. A survey of the use of foreign-purchased medications in a border community emergency department patient population. J Emerg Med. 2007;33:213–221. doi: 10.1016/j.jemermed.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 88.Chalker J, Chuc NT, Falkenberg T, Do NT, Tomson G. STD management by private pharmacies in Hanoi: practice and knowledge of drug sellers. Sex Transm Infect. 2000;76:299–302. doi: 10.1136/sti.76.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lafferty J. Self-injection and needle sharing among migrant farmworkers. Am J Public Health. 1991;81:221. doi: 10.2105/ajph.81.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McVea KL. Lay injection practices among migrant farmworkers in the age of AIDS: evolution of a biomedical folk practice. Soc Sci Med. 1997;45:91–98. doi: 10.1016/s0277-9536(96)00318-8. [DOI] [PubMed] [Google Scholar]
- 91.Stratchounski LS, Andreeva IV, Ratchina SA, et al. The inventory of antibiotics in Russian home medicine cabinets. Clin Infect Dis. 2003;37:498–505. doi: 10.1086/376905. [DOI] [PubMed] [Google Scholar]
- 92.Birungi H, Whyte SR. Injections in Uganda–cause for concern. Essent Drugs Monit. 1994;18:11–12. [PubMed] [Google Scholar]
- 93.Dua V, Kunin CM, White LV. The use of antimicrobial drugs in Nagpur, India—a window on medical-care in a developing-country. Soc Sci Med. 1994;38:717–724. doi: 10.1016/0277-9536(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 94.Taylor RB, Shakoor O, Behrens RH. Drug quality, a contributor to drug resistance? Lancet. 1995;346:122. doi: 10.1016/s0140-6736(95)92145-1. [DOI] [PubMed] [Google Scholar]
- 95.Land T. Combating counterfeit drugs. Nature. 1992;355:192. doi: 10.1038/355192a0. [DOI] [PubMed] [Google Scholar]
- 96.Alubo SO. Death for sale: a study of drug poisoning and deaths in Nigeria. Soc Sci Med. 1994;38:97–103. doi: 10.1016/0277-9536(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 97.Liu YC, Huang WK, Huang TS, Kunin CM. Inappropriate use of antibiotics and the risk for delayed admission and masked diagnosis of infectious diseases—A lesson from Taiwan. Arch Intern Med. 2001;161:2366–2370. doi: 10.1001/archinte.161.19.2366. [DOI] [PubMed] [Google Scholar]
- 98.Feinfeld DA, Ansari N, Nuovo M, Hussain A, Mir R. Tubulointerstitial nephritis associated with minimal self reexposure to rifampin. Am J Kidney Dis. 1999;33:e3. doi: 10.1016/s0272-6386(99)70443-9. [DOI] [PubMed] [Google Scholar]
- 99.Ryrie DR, Fletcher J, Landman MJ, Daniels HE. Chloramphenicol over the counter. Lancet. 1973;1:150. doi: 10.1016/s0140-6736(73)90220-1. [DOI] [PubMed] [Google Scholar]
- 100.Schorling JB, Desouza MA, Guerrant RL. Patterns of antibiotic use among children in an urban Brazilian slum. Int J Epidemiol. 1991;20:293–299. doi: 10.1093/ije/20.1.293. [DOI] [PubMed] [Google Scholar]
- 101.Van Duong D, Binns CW, Van Le T. Availability of antibiotics as over-the-counter drugs in pharmacies: A threat to public health in Vietnam. Trop Med Int Health. 1997;2:1133–1139. doi: 10.1046/j.1365-3156.1997.d01-213.x. [DOI] [PubMed] [Google Scholar]
- 102.Bate R, Tren R, Mooney L, et al. Pilot study of essential drug quality in two major cities in India. PLoS One. 2009;4:e6003. doi: 10.1371/journal.pone.0006003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735–743. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 104.Dromigny JA, Nabeth P, Juergens-Behr A, Perrier-Gros-Claude JD. Risk factors for antibiotic-resistant Escherichia coli isolated from community-acquired urinary tract infections in Dakar, Senegal. J Antimicrob Chemother. 2005;56:236–239. doi: 10.1093/jac/dki158. [DOI] [PubMed] [Google Scholar]
- 105.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Eff ect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 106.Apisarnthanarak A, Mundy LM. Correlation of antibiotic use and antimicrobial resistance in Pratumthani, Thailand, 2000 to 2006. Am J Infect Control. 2008;36:681–682. doi: 10.1016/j.ajic.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 107.Nichter M. Illness semantics and international health: the weak lungs/TB complex in the Philippines. Soc Sci Med. 1994;38:649–663. doi: 10.1016/0277-9536(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 108.Lansang MA, Lucas-Aquino R, Tupasi TE, et al. Purchase of antibiotics without prescription in manila, the Philippines; inappropriate choices and doses. J Clin Epidemiol. 1990;43:61–67. doi: 10.1016/0895-4356(90)90057-v. [DOI] [PubMed] [Google Scholar]
- 109.Ellis ME, al-Hajjar S, Bokhari H, Hussein Qadri SM. High proportion of multi-drug resistant mycobacterium tuberculosis in Saudi Arabia. Scand J Infect Dis. 1996;28:591–595. doi: 10.3109/00365549609037966. [DOI] [PubMed] [Google Scholar]
- 110.Lambert ML, Delgado R, Michaux G, Volz A, Van der Stuyft P. Tuberculosis control and the private health sector in Bolivia: a survey of pharmacies. Int J Tuberc Lung Dis. 2004;8:1325–1329. [PubMed] [Google Scholar]
- 111.Balabanova Y, Fedorin I, Kuznetsov S, et al. Antimicrobial prescribing patterns for respiratory diseases including tuberculosis in Russia: a possible role in drug resistance? J Antimicrob Chemother. 2004;54:673–679. doi: 10.1093/jac/dkh383. [DOI] [PubMed] [Google Scholar]
- 112.Kobaidze K, Salakaia A, Blumberg HM. Over the counter availability of antituberculosis drugs in Tbilisi, Georgia in the setting of a high prevalence of MDR-TB. Interdiscip Perspect Infect Dis. 2009;2009:513609. doi: 10.1155/2009/513609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hurtig AK, Pande SB, Baral SC, Porter JD, Bam DS. Anti-tuberculosis treatment in private pharmacies, Kathmandu valley, Nepal. Int J Tuberc Lung Dis. 2000;4:730–736. [PubMed] [Google Scholar]
- 114.Lonnroth K, Lambregts K, Nhien DT, Quy HT, Diwan VK. Private pharmacies and tuberculosis control: A survey of case detection skills and reported anti-tuberculosis drug dispensing in private pharmacies in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2000;4:1052–1059. [PubMed] [Google Scholar]
- 115.Catalano M, Almiron MA, Romeo AM, Caruso E, Murtagh P, Harisiadi J. Comparison between parental report and results of microbiologic agar assay for presence of antibiotic in urine of Argentinian children with acute lower respiratory tract infection. Rev Infect Dis. 1990;12:998–1000. doi: 10.1093/clinids/12.supplement_8.s998. [DOI] [PubMed] [Google Scholar]
- 116.Liu YC, Huang WK, Huang TS, Kunin CM. Extent of antibiotic use in Taiwan shown by antimicrobial activity in urine. Lancet. 1999;354:1360. doi: 10.1016/S0140-6736(99)07446-2. [DOI] [PubMed] [Google Scholar]
- 117.Liu YC, Huang WK, Huang TS, Kunin CM. Detection of antimicrobial activity in urine for epidemiologic studies of antibiotic use. J Clin Epidemiol. 1999;52:539–545. doi: 10.1016/s0895-4356(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 118.Murdoch DR, Woods CW, Zimmerman MD, et al. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- 119.Liss RH, Batchelor FR. Economic evaluations of antibiotic use and resistance—a perspective: report of task force 6. Rev Infect Dis. 1987;9(suppl 3):297–312. doi: 10.1093/clinids/9.supplement_3.s297. [DOI] [PubMed] [Google Scholar]
- 120.Simoes EAF, Cherian T, Chow J, Shahid-Salles S, Laxminarayan R, John TJ. Acute respiratory infections in children. In: Disease Control Priorities in Developing Countries. 2nd edn. Washington: The World Bank; 2006. pp. 483–499. [Google Scholar]
- 121.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–644. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 122.Ahmed HM, Mitchell M, Hedt B. National implementation of integrated management of childhood illness (IMCI): policy constraints and strategies. Health Policy. 2010;96:128–133. doi: 10.1016/j.healthpol.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 123.Chopra M, Patel S, Cloete K, Sanders D, Peterson S. Eff ect of an IMCI intervention on quality of care across four districts in Cape Town, South Africa. Arch Dis Child. 2005;90:397–401. doi: 10.1136/adc.2004.059147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naimoli JF, Rowe AK, Lyaghfouri A, Larbi R, Lamrani LA. Eff ect of the integrated management of childhood illness strategy on health care quality in Morocco. Int J Qual Health Care. 2006;18:134–144. doi: 10.1093/intqhc/mzi097. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y, Dai Y, Zhang S. Impact of implementation of integrated management of childhood illness on improvement of health system in China. J Paediatr Child Health. 2007;43:681–685. doi: 10.1111/j.1440-1754.2007.01187.x. [DOI] [PubMed] [Google Scholar]
- 126.Kinfu Y, Dal Poz MR, Mercer H, Evans DB. The health worker shortage in Africa: are enough physicians and nurses being trained? Bull World Health Organ. 2009;87:225–230. doi: 10.2471/BLT.08.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.WHO. WHO global strategy for containment of antimicrobial resistance. Geneva: World Health Organization; 2001. [Google Scholar]
- 128.Bavestrello L, Cabello A, Casanova D. Impact of regulatory measures in the trends of community consumption of antibiotics in Chile. Rev Med Chil. 2002;130:1265–1272. [PubMed] [Google Scholar]
- 129.Harbarth S, Samore MH. Antimicrobial resistance determinants and future control. Emerg Infect Dis. 2005;11:794–801. doi: 10.3201/eid1106.050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park S, Soumerai SB, Adams AS, Finkelstein JA, Jang S, Ross-Degnan D. Antibiotic use following a Korean national policy to prohibit medication dispensing by physicians. Health Policy Plan. 2005;20:302–309. doi: 10.1093/heapol/czi033. [DOI] [PubMed] [Google Scholar]
- 131.Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010;8:260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 132.Sundqvist M, Geli P, Andersson DI, et al. Little evidence for reversibility of trimethoprim resistance after a drastic reduction in trimethoprim use. J Antimicrob Chemother. 2010;65:350–360. doi: 10.1093/jac/dkp387. [DOI] [PubMed] [Google Scholar]
- 133.Hawkey PM. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008;14(suppl 1):159–165. doi: 10.1111/j.1469-0691.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 134.Tangden T, Cars O, Melhus A, Lowdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–3568. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]