Abstract
OBJECTIVE
To investigate the relationship between contact precautions and delirium among inpatients, adjusting for other factors.
DESIGN
Retrospective cohort study.
SETTING
A 662-bed tertiary care center.
PATIENTS
All nonpyschiatric adult patients admitted to a tertiary care center from 2007 through 2009.
METHODS
Generalized estimating equations were used to estimate the association between contact precautions and delirium in a retrospective cohort of 2 years of admissions to a tertiary care center.
RESULTS
During the 2-year period, 60,151 admissions occurred in 45,266 unique nonpsychiatric patients. After adjusting for comorbid conditions, age, sex, intensive care unit status, and length of hospitalization, contact precautions were significantly associated with delirium (as defined by International Classification of Diseases, Ninth Revision), medication, or restraint exposure (adjusted odds ratio [OR], 1.40 [95% confidence interval {CI}, 1.24–1.51]). The association between contact precautions and delirium was seen only in patients who were newly placed under contact precautions during the course of their stay (adjusted OR, 1.75 [95% CI, 1.60–1.92]; P < .01) and was not seen in patients who were already under contact precautions at admission (adjusted OR, 0.97 [95% CI, 0.86–1.09]; P=.60).
CONCLUSIONS
Although delirium was more common in patients who were newly placed under contact precautions during the course of their hospital admission, delirium was not associated with contact precautions started at hospital admission. Patients newly placed under contact precautions after admission but during hospitalization appear to be at a higher risk and may benefit from proven delirium-prevention strategies.
Delirium is a transient global disturbance of mental function marked by confusion or altered consciousness.1 Delirium occurs in 11%–16% of hospitalized patients, with a higher incidence in intensive care units (ICUs).2,3 Delirium is associated with adverse outcomes, including increased length of stay, morbidity, and mortality.3,4 Risk factors for delirium include decreased environmental stimuli (such as absence of family members, reading glasses, or orienting objects), immobilization, dehydration, electrolyte disorders, age, polypharmacy, substance use, and multiple medical problems.2,4
Contact precautions are recommended by the Centers for Disease Control and Prevention to prevent the spread of multidrug-resistant (MDR) bacteria in the hospital. Contact precautions require healthcare workers to use gown and gloves and to place patients in a private room.5,6 Approximately 15% of patients in the hospital are under contact precautions.7,8 Contact precautions may decrease environmental stimuli by isolating patients from healthcare workers and family members and by changing the flow of care in the hospital. Negative outcomes—including fewer healthcare worker visits, increased adverse events, electrolyte disturbances, poorer monitoring, fewer daily notes, and more symptoms of depression and anxiety—have been associated with patient isolation.9–11 Because of these changes in care, contact precautions have been hypothesized to result in more delirium.12,13 The only study to examine the relationship between contact precautions and delirium found a nonsignificant increase in delirium severity in a small subgroup of patients isolated after hospital admission.12 Patients may have contact precautions applied at the time of hospital admission or later during their hospitalization. Reasons patients would be switched to contact precautions after admission include positive surveillance culture, clinical cultures related to an active infection, longer stay in the hospital, ICU admission, or other factors associated with more severe illness.
To examine whether there is an association between contact precautions and delirium, we performed a 2-year retrospective cohort study of all nonpsychiatric hospital admissions. On the basis of the application of contact precautions after admission being associated with more severe illness and past studies,12 we hypothesized that these 2 groups of contact-precaution patients would be different populations. Patients under contact precautions were examined as an entire group as well as broken down into those newly placed under contact precautions during the course of their admission (who were exposed for only part of their hospitalization) and those exposed from the time of hospital admission.
METHODS
Eligible participants included all patients 18 years or older admitted to the University of Maryland Medical Center (UMMC) between February 1, 2007, and January 31, 2009. UMMC is a 662-bed tertiary care teaching hospital in Baltimore, Maryland. Patients with psychotic disorders or those admitted to a psychiatric service were excluded. This study received Institutional Review Board approval from the University of Maryland, Baltimore.
Data were obtained from the UMMC central data repository. The UMMC central data repository is a relational database containing patients' administrative, pharmacy, and laboratory information. The central data repository is maintained by the University of Maryland Information Technology Group. These data have been used extensively in hospital epidemiological studies.14–18 Within UMMC, active surveillance culturing is performed on targeted populations for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and MDR gram-negative bacteria.6 Targeted active surveillance for MRSA occurs at admission and entails anterior nares swab specimens being obtained from all high-risk patients, defined as those who self-reported admission to a healthcare facility in the previous 12 months, had an active skin infection at admission, or were admitted to an ICU.19 VRE active surveillance is performed for ICU patients, and MDR gram-negative active surveillance is performed for all patients transferred from another healthcare facility. VRE cultures are obtained at admission and weekly for ICU patients. MDR gram-negative surveillance is performed at admission only. Patients were placed under contact precautions when they had an electronic medical record indicator for the presence of MDR bacteria, including MRSA, VRE, and gram-negative bacteria susceptible to 2 or fewer antibiotic classes not including polymyxin or tigecycline.6 Patients are not preemptively placed under contact precautions; the electronic indicator is initiated when microbiology results come back positive. In our hospital, contact precautions entail a single room or cohorting of patients with similar organisms and use of gowns and gloves for room entry. The use of contact precautions was our primary exposure. To examine 2 subpopulations of patients under contact precautions, we separated patients newly placed under contact precautions during their stay because of new identification of a colonizing or infecting MDR bacterium from those placed under contact precautions for the entire hospitalization.
Delirium is difficult to diagnose and is severely undercoded in administrative data.20 We used a proxy measure based on unexplained antipsychotic use or physical restraints to measure delirium.21 This measure has been validated as a better means of assessing delirium in administrative data than International Classification of Diseases, Ninth Revision (ICD-9), coding alone.21 Patients were considered to have delirium if they had any previously validated ICD-9 codes for delirium or use of antipsychotics or restraints. The appendix lists the antipsychotics considered. ICD-9 codes for delirium included the following: 290.11 (presenile dementia with delirium), 290.41 (vascular dementia with delirium), 780.09 (alteration of consciousness other), 293.0× (delirium due to conditions classified elsewhere), 290.3× (senile dementia with delirium), and 293.1× (subacute delirium).20 Although previous authors have used 291.0× (alcohol-withdrawal delirium),20 we choose to exclude alcohol-related admissions from our analysis because this code is related to a diagnosis that was present before hospital admission and exposure to contact precautions. The primary exposure variable, contact precautions, was validated by chart review. Of 80 randomly selected charts, the electronic indicator for contact precautions was validated as 96% accurate (77/80) compared with paper records. Sensitivity was 97%, and specificity was 93%. The outcome of delirium was also validated by chart review. The electronic indicator for ICD-9 delirium was validated as 95% accurate (38/40), with 100% sensitivity and 87% specificity. Medication use was 95% accurate (38/40) in our database (100% sensitivity, 93% specificity), and restraint orders were 88% accurate (35/40; 100% sensitivity, 83% specificity).
Bivariable analyses were performed using the χ2 test for categorical variables and the Student t test or the Wilcoxon rank-sum test for continuous variables. Potential confounding variables included patient age, sex, length of stay, individual comorbidities, and the Charlson comorbidity index.22 Breslow-Day and interaction tests were used to test for effect modification between contact precautions and delirium by ICU status or dementia.
Generalized estimating equations were used to estimate odds ratios (ORs) for delirium and 95% confidence intervals (CIs), to account for the nonindependence of repeat hospital stays and to adjust for confounding variables. Up to 4 visits for each patient were included in the model because of small sample sizes beyond 4 visits (and subsequent breakdown of generalized estimating equation models). The first 4 admissions accounted for 75% of total admissions (45,266/60,151). To properly control for confounding variables, all variables that were significantly associated with contact precautions in the bivariable analyses were added in the order that their addition was expected to change the OR between contact precautions and delirium. Those with the largest ORs in the bivariable analyses were added first. Variables were left in the model if they were significantly associated with the outcome (P < .05) or altered the regression coefficient of the primary exposure variable by greater than 10%. Analyses were performed using SAS software version 9.2 (SAS).
RESULTS
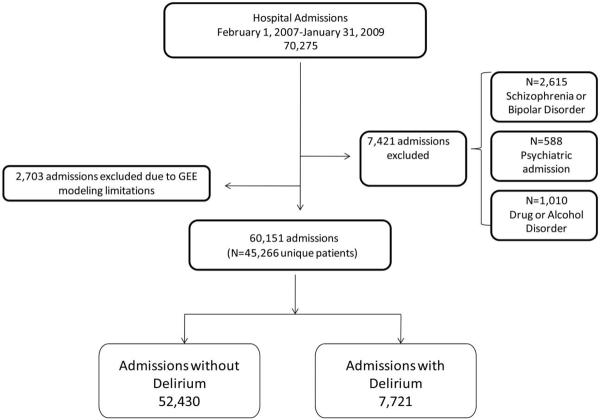
Our analysis consisted of 60,151 admissions in 45,266 unique patients; 20% (9,487/45,266) of patients had multiple admissions. Patients admitted to a psychiatric service (2,067 admissions), those with schizophrenia (772 admissions) or bipolar disorder (1,201 admissions), and those admitted for alcohol or drug abuse (811 admissions) were excluded because antipsychotics are used in these populations for reasons other than delirium. Figure 1 illustrates how we identified our study population.
FIGURE 1.
Flowchart for selection of the study population and determination of delirium using a surrogate marker. GEE, generalized estimating equation.
Fifteen percent of admissions were under contact precautions (9,684/60,151). Of these 9,684 patients, 42% were placed under contact precautions after admission (4,032/9,684), and 58% (5,652/9,684) were placed under contact precautions at admission (because of a previous indicator in the database indicating the need for contact precautions). Bivariable analyses showed that patients under contact precautions were more likely to be male and to be older than patients not under contact precautions. Patients under contact precautions had a longer length of stay and were more likely to spend time in the ICU. All comorbidities except for myocardial infarction and metastatic tumor were more common in patients under contact precautions (Table 1). Patients moved to contact precautions during their stay were older (54.4 vs 50.8 years; P < .01), had longer lengths of stay in the hospital (median, 11.7 vs 5.1 days; P < .01), were more likely to transfer to an ICU (42.6% vs 16.2%; P < .01), and were more likely to die during their stay (10.0% vs 4.2%; P < .01) than patients who were placed under contact precautions at admission. Patients moved to contact precautions were more than twice as likely to have a positive clinical culture for MRSA (28.6% vs 11.5%; P < .01).
TABLE 1.
Study Population of General Medical and Surgical Inpatients at Their First Admission, Comparing Patients under Contact Precautions (CP) with Those Not under CP
| Non-CP (n = 50,458) | CP (n = 9,684) | P | |
|---|---|---|---|
| Age, mean ± SD, years | 50.1 ± 18.8 | 52.3 ± 16.9 | <.01 |
| Male | 51.4 (25,893) | 59.1 (5,722) | <.01 |
| LOS, median (IQR), days | 2.8 (4.6) | 7.1 (15.3) | <.01 |
| CCI, median (IQR) | 1.00 (2.00) | 2.00 (3.00) | <.01 |
| AIDS | 1.5 (731) | 5.7 (558) | <.01 |
| Chronic pulmonary disease | 15.1 (7,600) | 18.0 (1,739) | <.01 |
| Diabetes | 16.1 (8,076) | 21.9 (2,118) | <.01 |
| Complications of diabetes | 2.2 (1,120) | 4.9 (475) | <.01 |
| Cerebrovascular disease | 7.6 (3,838) | 8.2 (791) | .05 |
| Heart failure | 7.4 (3,713) | 13.1 (1,265) | <.01 |
| Hemi- or paraplegia | 1.4 (711) | 4.9 (473) | <.01 |
| Malignancy | 8.8 (4,416) | 12.0 (1,164) | <.01 |
| Metastatic tumor | 3.2 (1,647) | 3.1 (300) | .40 |
| Mild liver disease | 1.4 (686) | 3.3 (320) | <.01 |
| Myocardial infarction | 6.9 (3,484) | 6.7 (647) | .44 |
| Peptic ulcer disease | 1.1 (535) | 1.6 (159) | <.01 |
| Peripheral vascular disease | 3.4 (1,689) | 4.9 (474) | <.01 |
| Renal disease | 8.0 (4,058) | 18.9 (1,831) | <.01 |
| Severe liver disease | 0.8 (416) | 2.7 (261) | <.01 |
| Rheumatologic | 1.8 (933) | 1.8 (178) | .95 |
| Intensive care unit | 14.3 (7,239) | 27.2 (2,630) | <.01 |
| Death in hospital | 2.4 (1,217) | 6.6 (635) | <.01 |
NOTE. Data are % (no.), unless otherwise indicated. CCI, Charlson comorbidity index; IQR, interquartile range; LOS, length of stay; SD, standard deviation.
Delirium was identified in 13.5% (7,721/60,151) of admissions by the proxy measure, of which 826 cases were identified by ICD-9 codes and 7,412 were identified through the unexplained use of antipsychotics or physical restraints. The prevalence of delirium in patients under contact precautions was 16.1% (1,562/9,684), compared with 7.6% (3,785/50,467) in patients not under contact precautions. Without adjusting for other factors, there was approximately a 2-fold greater odds of delirium in patients under contact precautions than in patients not under contact precautions (unadjusted OR, 2.4 [95% CI, 2.2–2.5]). The Breslow-Day test for effect modification did not identify effect modification in patients with dementia. Although the Breslow-Day test for effect modification was significant for ICU status, the association was simply stronger in the ICU patients, and an interaction term between contact precautions and ICU was not significant.
After adjusting for other variables and accounting for multiple admissions, contact precautions were associated with delirium (OR, 1.40 [95% CI, 1.24–1.51]; Table 2). There was no relationship between contact precautions and delirium in patients who had been placed under contact precautions during their entire stay (OR, 0.97 [95% CI, 0.86–1.09]; P = .60). Patients placed under contact precautions after being newly identified as colonized or infected with an MDR bacterium were 1.75 times more likely to experience delirium than patients not under contact precautions (OR, 1.75 [95% CI, 1.60–1.92]; P < .01).
TABLE 2.
Adjusted Odds of Delirium in a Study Population of General Hospital Admissions, Comparing Patients under Contact Precautions (CP) with Those Not under CP
| Adjusted OR (95% CI) | |
|---|---|
| Model 1 | |
| CP | 1.40 (1.29–1.51) |
| Male | 1.41 (1.32–1.52) |
| LOS | 1.01 (1.01–1.02) |
| CCI | 1.01 (0.99–1.02) |
| Age ≥65 years | 1.25 (1.16–1.34) |
| ICU | 11.42 (10.70–12.19) |
| Visit no. | 0.92 (0.88–0.96) |
| Model 2 | |
| New CPa | 1.75 (1.6–1.9) |
| CP at admission | 0.97 (0.9–1.1) |
| Male | 1.42 (1.33–1.52) |
| LOS | 1.01 (0.99–1.02) |
| CCI | 1.01 (1.01–1.02) |
| Age ≥65 years | 1.24 (1.15–1.33) |
| ICU | 11.05 (10.35–11.80) |
| Visit no. | 0.95 (0.91–0.99) |
NOTE. CCI, Charlson comorbidity index; CI, confidence interval; ICU, intensive care unit; LOS, length of stay; OR, odds ratio.
Defined as a clinical or surveillance culture being positive on the index admission.
DISCUSSION
During a 2-year study period at a tertiary care center, exposure to contact precautions was associated with delirium when a patient was moved to contact precautions during their hospitalization. No association was seen in patients placed under contact precautions from the time of admission through discharge.
Delirium has been hypothesized to be an outcome of contact precautions,12,13 but the only study to examine this was a subanalysis of a delirium severity study that included only 52 patients under contact precautions (used primarily for patients who transferred from another healthcare facility and who were placed under contact precautions for only 48–72 hours).12 In this 2001 study, McCusker et al concluded that isolation did not impact delirium index scores, independent of other environmental variables.12
In our study, delirium was associated with a change to contact precautions during hospitalization (but not with patients under contact precautions at time of admission). These patients are known to be more severely ill than those not under contact precautions.9,11 In our population, patients newly transferred to contact precautions during their admission had an increased length of stay, had more frequent MDR clinical cultures indicating likely infections, and were more likely to be admitted to an ICU or to die in the hospital than patients under contact precautions at admission. Previous studies have shown that comorbidities, preexisting cognitive decline, and alcohol and drug use or withdraw can lead to delirium.23 Despite adjusting for relevant confounding variables and comorbidities with the Charlson comorbidity index, we did not have information available to adjust for severity of illness. Unmeasured confounding due to lack of a severity-of-illness variable may be present.
We could not examine environmental components of contact precautions that may lead to delirium (such as presence of orienting objects or timing of room changes). A limitation of this retrospective data set is that we were unable to tell when delirium began in relation to the exact timing of the patient moving to contact precautions because of the use of discharge ICD-9 codes or delirium markers that were not reliably linked to a date but that only represented delirium being present at some point during the hospital stay. In addition, delirium is an underdiagnosed and potentially undercoded condition in hospital inpatients.20,24 To overcome this, we used a validated proxy measure to define delirium. This proxy method identifies only active delirium, which accounts for approximately 1 in 3 patients with delirium.21
To our knowledge, this is the largest study examining the relationship between infection control isolation practices and delirium in a hospital-wide population. Trials to assess this association are impractical, as would be a multicenter administrative study, given the lack of coding of isolation status in larger administrative databases. We found that the incidence of delirium is higher only in patients who were moved to contact precautions during their hospital stay. There was some evidence that the patients who were moved to contact precautions were a sicker group of patients. Alternatively, patients who are placed under contact precautions at admission may have had previous experience with contact precautions and, therefore, not be as affected by the institution of precautions and associated environmental changes.
In summary, delirium was more common in patients transferred to contact precautions during their stay, but no relationship was seen between contact precautions and delirium in patients who were under contact precautions for the duration of their hospitalization. New use of contact precautions marks a group of patients who are more likely to develop delirium. Independent of the reasons patients placed under contact precautions are at a heightened risk for delirium, these patients could be targeted for interventions—such as delirium screening and management—to prevent delirium.25
ACKNOWLEDGMENTS
Financial support. This work was funded by the Agency for Healthcare Research and Quality (grant 1 K08 HS18111-01 AHRQ to D.J.M.), the National Institutes of Health (grant 5K24AI079040-02 to A.D.H.), and a Veterans Affairs Health Services Research and Development Merit Investigator-Initiated Research grant (05-123 to E.N.P.).
APPENDIX
ANTIPSYCHOTICS USED IN THE ANALYSIS
Typical antipsychotics
Chlorpromazine, fluphenazine, fluphenazine decanoate, fluphenazine enanthate, fluphenazine hydrochloride, haloperidol, haloperidol decanoate, loxapine, molindone, perphenazine, prochlorperazine (not included in the analysis of antipsychotics because chart review showed that most patients were prescribed prochlorperazine for nausea), thioridazine, and trifluoperazine.
Atypical antipsychotics
Aripiprazole, clozapine, olanzapine, olanzapine ODT (orodispersible tablet), paliperidone ER (extended release), quetiapine, risperidone, risperidone ODT, and ziprasidone.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
REFERENCES
- 1.American Psychiatric Association Task Force on DSM-IV . DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Arlington, VA: 1994. [Google Scholar]
- 2.Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB, editors. Psychiatric Care of the Medical Patient. 2nd ed Oxford University Press; New York: 2000. pp. 581–596. [Google Scholar]
- 3.Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24:657–722. doi: 10.1016/j.ccc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Trzepacz PT. Delirium advances in diagnosis, pathophysiology, and treatment. Psychiatr Clin North Am. 1996;19:429–448. doi: 10.1016/s0193-953x(05)70299-9. [DOI] [PubMed] [Google Scholar]
- 5.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory Committee 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day HR, Perencevich EN, Harris AD, et al. Do contact precautions cause depression? a two-year study at a tertiary care medical centre. J Hosp Infect. 2011;79:103–107. doi: 10.1016/j.jhin.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37:85–93. doi: 10.1016/j.ajic.2008.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? a brief report. Am J Infect Control. 2003;31:354–356. doi: 10.1016/s0196-6553(02)48250-8. [DOI] [PubMed] [Google Scholar]
- 11.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 12.McCusker J, Cole M, Abrahamowicz M, Han L, Podoba JE, Ramman-Haddad L. Environmental risk factors for delirium in hospitalized older people. J Am Geriatr Soc. 2001;49:1327–1334. doi: 10.1046/j.1532-5415.2001.49260.x. [DOI] [PubMed] [Google Scholar]
- 13.Mody L. Infection control issues in older adults. Clin Geriatr Med. 2007;23:499–514. doi: 10.1016/j.cger.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGregor JC, Kim PW, Perencevich EN, et al. Utility of the chronic disease score and Charlson comorbidity index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol. 2005;161:483–493. doi: 10.1093/aje/kwi068. [DOI] [PubMed] [Google Scholar]
- 15.Osih RB, McGregor JC, Rich SE, et al. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2007;51:839–844. doi: 10.1128/AAC.00901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor JC, Perencevich EN, Furuno JP, et al. Comorbidity risk-adjustment measures were developed and validated for studies of antibiotic-resistant infections. J Clin Epidemiol. 2006;59:1266–1273. doi: 10.1016/j.jclinepi.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Furuno JP, Harris AD, Wright MO, et al. Value of performing active surveillance cultures on intensive care unit discharge for detection of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:666–670. doi: 10.1086/518348. [DOI] [PubMed] [Google Scholar]
- 18.Furuno JP, Perencevich EN, Johnson JA, et al. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization. Emerg Infect Dis. 2005;11:1539–1544. doi: 10.3201/eid1110.050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AD, Furuno JP, Roghmann MC, et al. Targeted surveillance of methicillin-resistant Staphylococcus aureus and its potential use to guide empiric antibiotic therapy. Antimicrob Agents Chemother. 2010;54:3143–3148. doi: 10.1128/AAC.01590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubin FH, Williams JT, Lescisin DA, Mook WJ, Hassan S, Inouye SK. Replicating the hospital elder life program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc. 2006;54:969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Brown S, Fitzgerald M, Walsh K. Delirium dichotomy: a review of recent literature. Contemp Nurse. 2007;26:238–247. doi: 10.5172/conu.2007.26.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method—a new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.Hempenius L, van Leeuwen BL, van Asselt DZB, et al. Structured analyses of interventions to prevent delirium. Int J Geriatr Psychiatry. 2011;26:441–450. doi: 10.1002/gps.2560. [DOI] [PubMed] [Google Scholar]