Abstract
AIM: To investigate the effect and mechanism of oridonin on the gastric cancer cell line HGC-27 in vitro.
METHODS: The inhibitory effect of oridonin on HGC-27 cells was detected using the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. After treatment with 10 μg/mL oridonin for 24 h and 48 h, the cells were stained with acridine orange/ethidium bromide. The morphologic changes were observed under an inverted fluorescence microscope. DNA fragmentation (a hallmark of apoptosis) and lactate dehydrogenase activity were examined using DNA ladder assay and lactate dehydrogenase-release assay. After treated with oridonin (0, 1.25, 2.5, 5 and 10 μg/mL), HGC-27 cells were collected for anexin V-phycoerythrin and 7-amino-actinomycin D double staining and tested by flow cytometric analysis, and oridonin- induced apoptosis in HGC-27 cells was detected. After treatment with oridonin for 24 h, the effects of oridonin on expression of Apaf-1, Bcl-2, Bax, caspase-3 and cytochrome c were also analyzed using reverse-transcript polymerase chain reaction (RT-PCR) and Western blotting.
RESULTS: Oridonin significantly inhibited the proliferation of HGC-27 cells in a dose- and time-dependent manner. The inhibition rates of HGC-27 treated with four different concentrations of oridonin for 24 h (1.25, 2.5, 5 and 10 μg/mL) were 1.78% ± 0.36%, 4.96% ± 1.59%, 10.35% ± 2.76% and 41.6% ± 4.29%, respectively, which showed a significant difference (P < 0.05). The inhibition rates of HGC-27 treated with oridonin at the four concentrations for 48 h were 14.77% ± 4.21%, 21.57% ± 3.75%, 30.31% ± 4.91% and 61.19% ± 5.81%, with a significant difference (P < 0.05). The inhibition rates of HGC-27 treated with oridonin for 72 h at the four concentrations were 25.77% ± 4.85%, 31.86% ± 3.86%, 48.30% ± 4.16% and 81.80% ± 6.72%, with a significant difference (P < 0.05). Cells treated with oridonin showed typical apoptotic features with acridine orange/ethidium bromide staining. After treatment with oridonin, the cells became round, shrank, and developed small buds around the nuclear membrane while forming apoptotic bodies. Lactate dehydrogenase (LDH) release assay showed that after treated with 1.25 μg/mL and 20 μg/mL oridonin for 24 h, LDH release of HGC-27 caused by apoptosis increased from 22.94% ± 3.8% to 52.68% ± 2.4% (P < 0.001). However, the change in the release of LDH caused by necrosis was insignificant, suggesting that the major cause of oridonin-induced HGC-27 cell death was apoptosis. Flow cytometric analysis also revealed that oridonin induced significant apoptosis compared with the controls (P < 0.05). And the apoptosis rates of HGC-27 induced by the four different concentrations of oridonin were 5.3% ± 1.02%, 12.8% ± 2.53%, 28.5% ± 4.23% and 49.6% ± 3.76%, which were in a dose-dependent manner (P < 0.05). After treatment for 24 h, DNA ladder showed that oridonin induced a significant increase in DNA fragmentation in a dose-dependent manner. RT-PCR revealed that mRNA expression levels were up-regulated compared with the controls in caspase-3 (0.917 ± 0.103 vs 0.357 ± 0.019, P < 0.05), cytochrome c (1.429 ± 0.111 vs 1.002 ± 0.014, P < 0.05), Apaf-1 (0.688 ± 0.101 vs 0.242 ± 0.037, P < 0.05) and Bax (0.856 ± 0.101 vs 0.278 ± 0.027, P < 0.05) (P < 0.05), whereas down-regulated in Bcl-2 (0.085 ± 0.012 vs 0.175 ± 0.030, P < 0.05). Western blotting analysis also confirmed this result.
CONCLUSION: Apoptosis of HGC-27 induced by oridonin may be associated with differential expression of Apaf-1, caspase-3 and cytochrome c, which are highly dependent upon the mitochondrial pathway.
Keywords: Oridonin, Gastric cancer, Proliferation, Apoptosis, Apaf-1/caspase-3/cytochrome C
INTRODUCTION
Gastric cancer is a common cancer of the digestive system and the second most common type of cancer worldwide. Its incidence varies among different regions and countries. There are approximately 934 000 new cases of gastric cancer worldwide each year, of which 56% occur in East Asia. Among these new cases in East Asia, 41% come from China and 11% from Japan[1,2]. Most patients present with advanced stage. Surgery plus chemotherapy remains the first-line therapy. Despite rapid advances in surgical procedures and radiotherapy/chemotherapy, few chemotherapeutic drugs for gastric cancer have shown promising results. Treatment efficacies vary from person to person, with a prevalence of cure of < 30%[3]. Therefore, it is important to search and develop new and more effective anti-gastric cancer drugs. In recent years, traditional Chinese medicine (TCM) has played an increasingly important role in the prevention and treatment of tumors. In particular, the integration of TCM with Western medicine has appreciably improved the efficacy of drug combinations and prolonged patient survival.
Rabdosia rubescens, a medicinal herb in TCM, has therapeutic actions (e.g., heat-clearing, detoxifying, anti-inflammation, antinociceptive, anti-tumor). Oridonin (molecular formula: C20H2006; relative molecular weight: 364.42) is a tetracyclic diterpenoid compound. It is a monomer component extracted from Rabdosia rubescens. In China, structural and pharmacological studies on Rabdosia rubescens started in the mid 1980s. Results indicated that oridonin is one of the most important anti-tumor components of Rabdosia rubescens[4,5]. Studies have suggested that oridonin has certain anti-tumor effects on cervical cancer, human epidermal squamous cell carcinoma, leukemia, liver cancer, malignant melanoma, colon cancer, breast cancer, and other tumors[6-12]. A study on colon cancer found that the mechanism of oridonin-induced apoptosis and aging of colon cancer cells might lie in increased histone acetylation and changes in the expressions of p16, p21, p27 and c-myc[13]. A study on laryngeal cancer indicated that oridonin can induce apoptosis through inhibiting the epidermal growth factor receptor signaling pathway and by increasing oxidative stress[14]. Oridonin can induce the apoptosis of hepatoma cells through the reactive oxygen species-mitogen-activated protein kinase-p53 pathway[15]. In another study on cervical cancer, oridonin induced the apoptosis of cervical cancer cells through the phosphatidyl inositol 30-kinase-Akt pathway[16]. In addition, some recent studies suggested that oridonin can also inhibit the proliferation of tumor cells by increasing the autophagy of tumor cells[17-19]. All of these findings suggest that oridonin has good anti-tumor effects.
Our previous studies on gastric cancer also suggested that oridonin has a notable inhibitory effect on the proliferation of gastric cancer cells[20]. However, the exact mechanism by which oridonin induces the apoptosis of gastric cancer cells remains undefined. In this study, the effect of oridonin on gastric cancer cells and its possible mechanism of action were explored.
MATERIALS AND METHODS
Experimental reagents
The human gastric cancer cell line HGC-27 as well as the instruments and equipments needed for this study were provided by the Zhejiang Provincial Key Laboratory of Gastroenterology (Zhejiang, China). Roswell Park Memorial Institute (RPMI) 1640 medium was purchased from the Shanghai Pufei Biotech (Shanghai, China). Fetal bovine serum (FBS), 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, acridine orange/ethidium bromide (AO/EB) fluorescent dye and lactate dehydrogenase (LDH) test reagents were purchased from YK Biotech (Hangzhou, China). Bcl-2, Bax, Apaf-1, caspase-3 and β-actin primers were synthesized by Invitrogen (Shanghai, China). Trziol RNA extraction reagent was purchased from Invitrogen (Carlsbad, CA, United States). Taq DNA polymerase and PrimeScript™ reverse-transcript polymerase chain reaction (RT-PCR) Kit and other PCR reagents were purchased from TaKaRa Corporation (Osaka, Japan).
Cell culture
HGC-27 cells were routinely cultivated in RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37 °C with 5% CO2. Cells were passaged at 80% confluency using 1 mmol/L ethylene diamine tetraacetic acid (EDTA)-0.025% trypsin for 3-5 min, and subcultured at a ratio of 1:3-1:5. Cells at the logarithmic growth phase were collected for experiments.
Determination of inhibition of cell growth
HGC-27 cells at the logarithmic growth phase were obtained. The cell concentration was adjusted to 4 × 104/mL. Then, 200 μL of the above-mentioned cell suspension was added into each well of several 96-well plates. When cells adhered to the wall, the stock solution of oridonin (50 mg/mL) was added into each well to obtain final concentrations of oridonin of 0, 1.25, 2.5, 5 and 10 μg/mL. Five parallel wells were established for each drug concentration. After each well was removed from the incubator after different time periods of cultivation (based on experimental design), 20 μL MTT [prepared using 5 mg/mL phosphate-buffered saline (PBS) at pH 7.4] was added. The well was removed again after 4 h of cultivation. The supernatant was aspirated carefully; 150 μL dimethyl sulfoxide was added to each well and homogenized. A Multi-Mode Microplate Reader (Infinite M200; Tecan, Geneva, Switzerland) was used to determine the absorbance (A), with a detection wavelength of 570 nm and a reference wavelength of 630 nm. The inhibition of cell growth was calculated using the following formula:
Inhibition (%) = [1 - (Atreated - Ablank)/(Acontrol - Ablank)] × 100%
Determination of LDH activity
HGC-27 cell culture media treated with oridonin (0, 1.25, 2.5, 5, 10 and 20 μg/mL) for 24 h were collected. After centrifugation of the cell culture medium at 1000 g for 5 min at 4 °C, supernatants were carefully aspirated: these were marked “LDHnecrosis”. Precipitated cells were rinsed three times in PBS (pH 7.4), and then lysed with 0.4% Triton X-100 for 30 min on ice. Cell lysate supernatants were centrifuged at 3000 g for 5 min at 4 °C: these were marked “LDHapoptosis”. Residual adherent HGC-27 cells were collected after treatment with different concentrations of oridonin using 0.4% Triton X-100 for 30 min on ice. Cell lysate supernatants were centrifuged at 3000 g for 5 min at 4 °C: these were marked “LDHlive”. LDH levels were measured in the three supernatants mentioned above (LDHnecrosis, LDHapoptosis and LDHlive) on a fully automatic biochemical analyzer (LX20; Beckman Coulter, Brea, CA, United States). LDH levels in supernatants (LDHnecrosis and LDHapoptosis) were applied for the analysis of oridonin-induced necrosis and apoptosis of HGC-27 cells. The percentages of apoptosis and necrosis were calculated using the following formulae:
Necrosis (%) = [LDHnecrosis/(LDHnecrosis + LDHapoptosis + LDHlive)] × 100%
Apoptosis (%) = [LDHapoptosis/(LDHnecrosis + LDHapoptosis + LDHlive)] × 100%
DNA fragmentation (DNA ladder) assay
HGC-27 cells (including suspended cells and adherent cells) were collected after treatment with oridonin (0, 1.25, 2.5, 5, 10 μg/mL) for 24 h. They were rinsed three times in PBS at 4 °C. This was followed by degradation using 100 μg cell lysates (1% NP-40, 20 mmol/L EDTA, 50 mmol/L Tris-HCl, pH 7.5) at 4 °C for 10 min, followed by centrifugation at 15 000 g for 20 min. RNase A (20 μg/mL) was added to the supernatant at 37 °C for 1 h. Then 20 μL 0.5 mol/L NaCl and 120 μL 50% isopropanol were added and the mixture left overnight at –20 °C. The supernatant was removed after centrifugation at 15 000 g for 15 min at 4 °C. The supernatant was allowed to dry naturally and was dissolved in TE buffer (10 mmol/L Tris-HCl pH 7.4, 10 mmol/L EDTA pH 8.0), followed by electrophoresis at 100 V for 40 min using 0.1 mg/L, 2% agarose gels. A gel imaging system (FluorChem FC2; Alpha Innotech, Palo Alto, CA, United States) was used for observation and taking photographs.
Flow cytometric analysis
HGC-27 cells were treated by oridonin (0, 1.25, 2.5, 5, 10 μg/mL) as describe above and the cells were collected for anexin V-phycoerythrin and 7-amino-actinomycin D double staining. Briefly, the cells were washed with PBS for three times, and stained according to the manufacturer’s instructions (Guava Nexin® Reagent kit, 4700-1140, Millipore, United States). Samples were then analyzed using Guava EasyCyte Plus (Millipore, United States) within 30 min after the staining.
Cell morphology
AO/EB apoptotic staining was used to detect the morphology of apoptotic cells. After treatment with 10 μg/mL oridonin for 24 h and 48 h, the cells were washed three times in PBS at room temperature. The 80 μL AO/EB cocktail (Solomon Bio-Sci and Tech Co, China) was added to the culture plates for 30 min prior to observation under an inverted fluorescence microscope (IX71; Olympus, Tokyo, Japan). Viable cells stained only by AO appeared bright green with intact structure, whereas cells in early apoptosis showed bright green nuclear staining. Late apoptotic cells stained by AO low and EB were red-orange with condensation of chromatin as dense orange areas. The experiment was repeated three times in each group.
Semi-quantitative RT-PCR analysis
HGC-27 cells were collected after treatment with 10 μg/mL oridonin for 24 h. Total cellular RNA was extracted using the Trizol Reagent Kit according to manufacturer’s instructions. The concentration and quality of total RNA extracted were confirmed using a Protein-Nucleic Acid Analyzer (GeneQuant Pro DNA/RNA; GE Healthcare, Piscataway, NJ, United States) and RNA electrophoresis. cDNA synthesis and PCR detection were conducted using a PCR instrument (PTC-200; Bio-Rad, Hercules, CA, United States) according to the instructions given for the PrimeScript™ RT-PCR Kit. Primers were designed using the freely available primer design software Primer Premier 5.0. The primers of β-actin, Apaf-1, Bcl-2, Bax, caspase-3 and cytochrome c are shown in Table 1.
Table 1.
Primers used in the study
| Primer (forward) | Primer (reverse) | Product size (bp) | |
| β-actin | 5-CGGGACCTGACTGACTACCTC | 5-GGACTCGTGATACTCCTGCTTG | 500 |
| Apaf-1 | 5-TTAGGAGCCAGGTGCGGT | 5-GCTTGTCTTTCTTCCCATTTTTC | 148 |
| Bcl-2 | 5-TCGCCCTGTGGATGACTG | 5-CAGGAGAAATCAAACAGAGGC | 124 |
| Caspase-3 | 5-CATCCAGTCGCTTTGTGCC | 5-TGCCCACAGATGCCTAAGTTC | 619 |
| Bax | 5-CCCGAGAGGTCTTTTTCC | 5-GCCTTGAGCACCAGTTTG | 108 |
| Cytochrome c | 5-GAGCGGGAGTGTTCGTTGT | 5-GTCTGCCCTTTCTTCCTTCT | 327 |
The reaction conditions were as follows: 94 °C for 4 min; 94 °C for 45 s, 50 °C for 45 s, and 72 °C for 45 s, for 30 cycles; and followed by extension at 72 °C for 10 min before ending. Electrophoresis (1% agarose gel, 120 V for 30 min) was carried out. The gel imaging system (FluorChem FC2; Alpha Innotech) was used for observation and taking photographs.
Western blotting verification
Gastric cancer cell samples were homogenized in lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L, 1% Triton X-100, and 100 μg/mL Phenylmethanesulfonyl fluoride). The concentration of total protein was quantitated by bicinchoninic acid method. Sixty μg total proteins from each sample were loaded on a 15% sodium dodecylsulfonate-polyacrylate gel electrophoresis gel and the proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad, United States). Blotted membranes were blocked in 5% bovine serum albumin and subsequently exposed to primary antibodies specific for Apaf-1 (1:500, sc65891, Santa Cruz, United States), Bcl-2 (1:300, sc7382, Santa Cruz, United States), Bax (1:300, sc70406, Santa Cruz, United States), caspase-3 (1:300, sc7272, Santa Cruz, United States) and cytochrome c (1:800, sc13156, Santa Cruz, United States), respectively. After incubation with the appropriate secondary antibody, the membranes were treated with electrochemiluminescence reagent (Generay, China) and exposed to autoradiographic films. Beta-actin was also detected as an internal control.
Statistical analysis
The experiments were repeated three times independently. Data were presented as the mean ± SD. Data were analyzed using SPSS software ver13.0 (SPSS, Chicago, IL, United States). If the results were distributed normally, the two independent samples t test was used for comparison. For comparisons between groups of more than two unpaired values, one-way analysis of variance (ANOVA) was used. If an ANOVA F value was significant, post-hoc comparisons were performed between groups. If results were not normally distributed, the Mann-Whitney U test was used to compare two groups of unpaired values, whereas for comparisons between groups of more than two unpaired values, the Kruskal-Wallis H test was used. P < 0.05 was considered significant.
RESULTS
Inhibitory effect of oridonin on growth of HGC-27 cells
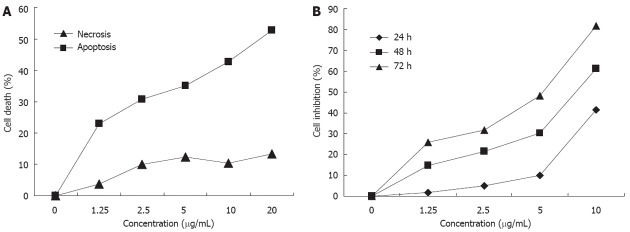
The HGC-27 cell-growth inhibition rate after treatment with different concentrations of oridonin (0, 1.25, 2.5, 5, 10 μg/mL) is shown in Figure 1A. The inhibition rates of HGC-27 treated with the four different concentrations of oridonin for 24 h (1.25, 2.5, 5, 10 μg/mL) were 1.78% ± 0.36%, 4.96% ± 1.59%, 10.35% ± 2.76% and 41.6% ± 4.29%, with a significant difference (P < 0.05). The inhibition rates of HGC-27 treated with oridonin for 48 h at the four concentrations were 14.77% ± 4.21%, 21.57% ± 3.75%, 30.31% ± 4.91% and 61.19% ± 5.81%, with a significant difference (P < 0.05). The inhibition rates of HGC-27 treated with oridonin for 72 h at the four concentrations were 25.77% ± 4.85%, 31.86% ± 3.86%, 48.30% ± 4.16% and 81.80% ± 6.72%, respectively, with a significant difference (P < 0.05). As the drug concentration increased, HGC-27 cell-growth inhibition was gradually enhanced.
Figure 1.
Inhibition of growth and lactate dehydrogenase release assay of HGC-27 cells after treatment with different concentrations of oridonin. A: Inhibition of growth HGC-27 cells; B: Lactate dehydrogenase (LDH) release assay of HGC-27 cells. The change in the release of LDH caused by apoptosis was significant.
Oridonin induced apoptosis of HGC-27 cells
HGC-27 cells were tested by LDH release assay after treated with oridonin for 24 h, and we found that apoptosis-induced LDH release increased from 22.94% ± 3.8% at 1.25 μg/mL to 52.68% ± 2.4% at 20 μg/mL) (P < 0.001). However, the change in the release of LDH caused by necrosis was insignificant (P > 0.05, Figure 1B), suggesting that the major cause of oridonin-induced HGC-27 cell death was apoptosis.
DNA of HGC-27 cells was also extracted and tested by DNA ladder analysis after treatment with oridonin for 24 h. As shown in Figure 2, oridonin induced a significant increase in DNA fragmentation in a dose-dependent manner.
Figure 2.
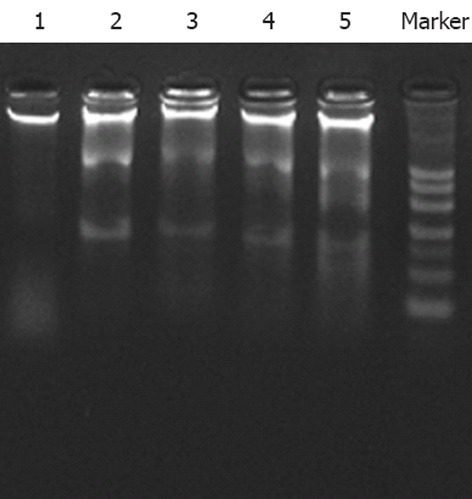
DNA ladder diagram after treatment with different concentrations of oridonin for 24 h. 1: Control; 2: 1.25 μg/mL oridonin; 3: 2.5 μg/mL oridonin; 4: 5 μg/mL oridonin; 5: 10 μg/mL oridonin.
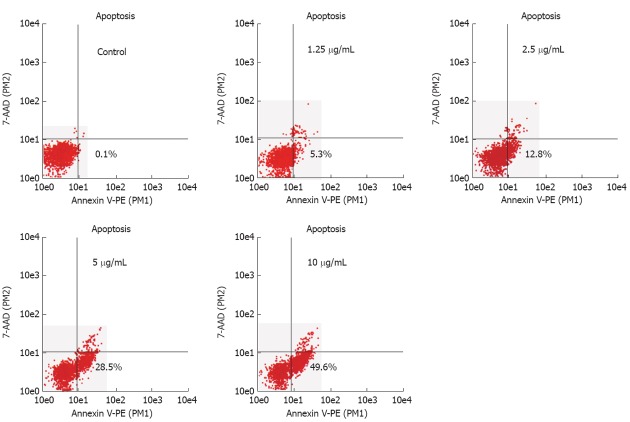
Flow cytometric analysis also revealed that oridonin could induce significant apoptosis compared with the controls (P < 0.05, Figure 3). And the apoptosis rates of HGC-27 induced by oridonin at the four concentrations were 5.3% ± 1.02%, 12.8% ± 2.53%, 28.5% ± 4.23% and 49.6% ± 3.76%, respectively, which were in a dose-dependent manner (P < 0.05).
Figure 3.
Analysis of apoptosis in HGC-27 cells. Flow cytometric analysis showed that oridonin induced the apoptosis of HGC-27 cells in a dose dependent manner. The x-axis indicates the Annexin V-positive populations and the y-axis indicates the 7-AAD-positive populations. The lower right was the early apoptotic cells.
Apoptotic morphology of HGC-27 cells after treated with oridonin
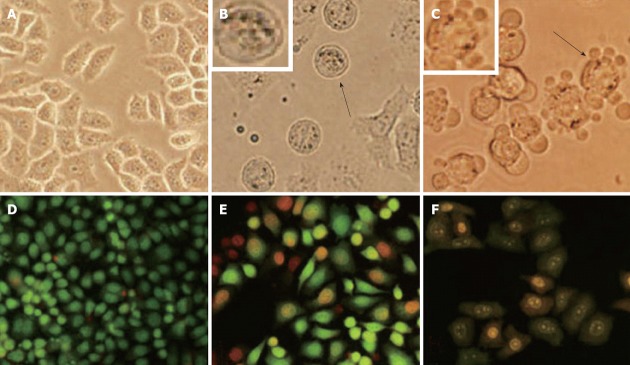
Apoptotic morphology was further observed under light microscopy and we found that, in the negative control group, HGC-27 cells were closely connected and uniform in size (Figure 4A). After treatment with oridonin, the cells became round (Figure 4B, black arrow), shrank, and developed small buds around the nuclear membrane while forming apoptotic bodies (Figure 4C, black arrow). AO/EB staining showed that, in the negative control group, cells were closely connected, uniform in size, and green in color (Figure 4D). After treatment with oridonin, the typical features of cells at different phases of apoptosis could be seen. Live cytoplasm and nuclei appeared all green. Early apoptotic nuclei were yellowish-green and pyknotic, and the cytoplasm was stained green. In the mid-phase and late apoptosis, nuclei were pyknotic, and nuclei and cytoplasm were yellowish-green. Dying cells were pyknotic and red-orange (Figure 4E and F).
Figure 4.
Morphological changes in HGC-27 cells after treatment with 10 μg/mL oridonin for 24 h and 48 h. A: Negative; B: Treatment for 24 h; C: Treatment for 48 h; D: Acridine orange/ethidium bromide (AO/EB) staining negative; E: Treatment for 24 h and AO/EB staining; F: Treatment for 48 h and AO/EB staining. Magnification ×200 in all images.
Effect of oridonin on HGC-27-induced gene expression
After treatment with oridonin for 24 h, gray ratio analyses of RT-PCR revealed that mRNA expression was up-regulated compared with control in caspase-3 (0.917 ± 0.103 vs 0.357 ± 0.019, P < 0.05), cytochrome c (1.429 ± 0.111 vs 1.002 ± 0.014, P < 0.05), Apaf-1 (0.688 ± 0.101 vs 0.242 ± 0.037, P < 0.05) and Bax (0.856 ± 0.101 vs 0.278 ± 0.027, P < 0.05), whereas down-regulated in Bcl-2 (0.085 ± 0.012 vs 0.175 ± 0.030, P < 0.05). These findings suggested that oridonin-induced apoptosis of HGC-27 cells was correlated with changes in the expression of caspase-3, cytochrome c, Apaf-1, Bcl-2 and Bax. Agarose gel electrophoresis of the RT-PCR products is shown in Figure 5A, and the ratios between the indicators for the three PCR analyses and optical density of β-actin are shown in Figure 5B. Protein levels analyzed by Western blotting also confirmed this result (Figure 5C).
Figure 5.
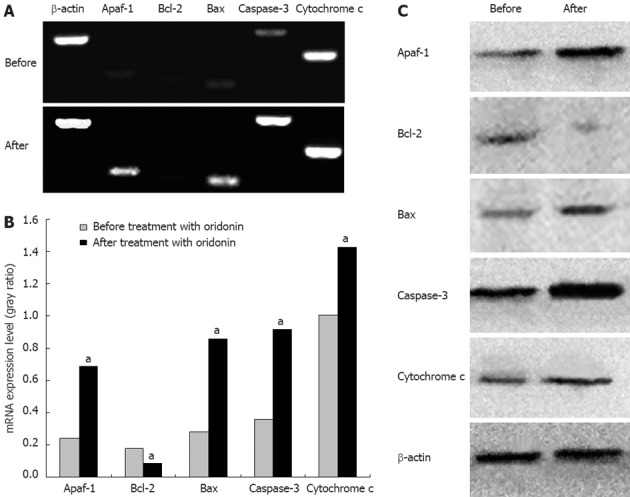
Changes in gene expression after treatment with 10 μg/mL oridonin for 24 h. After treatment with oridonin for 24 h, the expression of caspase-3, cytochrome c, Apaf-1 and Bax was up-regulated, whereas that of Bcl-2 was down-regulated. A: Agarose gel electrophoresis of the reverse-transcript polymerase chain reaction products; B: Results of optical density analyses of Apaf-1/β-actin, Bcl-2/β-actin, Bax/β-actin, caspase-3/β-actin, and cytochrome c/β-actin before and after treatment with oridonin (paired t test, aP < 0.05 vs before treatment with oridonin); C: Western blotting analysis.
DISCUSSION
Apoptosis plays a key part in the evolution of organisms, homeostasis, and development of multiple systems, including cancer. Tumorigenesis occurs when a series of oncogenes and proto-oncogenes are activated and overexpressed within tumor cells. The genes and their products are important regulators of apoptosis. Their abnormal expression blocks the apoptotic process of tumor cells, increasing the number of tumor cells, thereby promoting tumor growth. The typical morphological features of apoptotic cells are: cell shrinkage; in some organelles, ribosomes and nuclear debris are “wrapped” by the cell membrane into apoptotic bodies, which bud off from the cell surface and are finally “swallowed” by macrophages, epithelial cells and other phagocytic cells; phosphatidylserine eversion; condensation and marginalization of nuclear chromatin; and DNA fragmentation. Important molecules involved in apoptosis are: apoptosis-promoting molecules such as cysteine containing the aspartate-specific protease (caspase) family, and cytochrome c; and apoptosis-inhibitory molecules such as the Bcl-2 family. Apoptotic signaling pathways mainly involve the cell membrane receptor pathway [e.g., Fas/Fas ligand and tumor necrosis factors (TNF)/TNF receptor] or mitochondrial pathway conduction, which activates the caspases (caspase-8 or caspase-9) and key molecules of the downstream signal transduction pathway in succession to initiate caspase (caspase-3) and start apoptosis. In addition, apoptosis can also start in a caspase-independent manner[21,22].
The Bcl-2 family contains molecules that can regulate apoptosis[23]. These can be divided into two main categories: anti-apoptotic genes (particularly Bcl-2) and pro-apoptotic genes (particularly Bax). Bcl-2 can form protein dimers with the pro-apoptotic protein Bax and plays a decisive role in the apoptosis signaling pathway. If the Bcl-2/Bax ratio is decreased, apoptosis occurs[24]. Changes in the expression of the Bcl-2 protein family lead to increased permeability of the outer membrane of mitochondria, thus triggering mitochondrial release of cytochrome c. Apaf-1 can activate caspase-3. If cytochrome c is released into the cytoplasm, it forms the Apaf-1/cytochrome c complex with Apaf-1. After the Apaf-1/cytochrome c complex binds with ATP/dATP, Apaf-1 can “call” caspase-9 through its CARD domain to form apoptotic bodies, activate caspase-3, and start the caspase cascade reaction, thereby leading to apoptosis[25].
Recent studies have shown that the anti-tumor activity of oridonin is related to its induction of apoptosis[16,26-28]. In the present study, the MTT assay initially confirmed that oridonin can have a notable inhibitory effect on the proliferation of HGC-27 cells in a dose- and time-dependent manner. Our previous studies also suggested that oridonin has a notable inhibitory effect on the proliferation of gastric cancer[20]. Previous studies also found the growth-inhibitory activity of oridonin on cancer cells[29,30]. Light microscopy and AO/EB staining revealed that, after treatment with oridonin, HGC-27 cells became round, shrank, developed pyknosis, and formed small buds around the nuclear membrane as well as apoptotic bodies. The LDH release assay showed that oridonin could induce the death of gastric cancer cells mainly through its induction of apoptosis, and that the apoptotic effect was enhanced significantly as the concentration increased. These results were consistent with previous reports[31-34]. DNA ladder analyses demonstrated that, after treatment with different concentrations of oridonin, obvious DNA fragmentation could be seen. This finding indicated that oridonin could inhibit the proliferation of HGC-27 cells and induce their apoptosis. To further study the molecular mechanism of oridonin-induced apoptosis, semi-quantitative RT-PCR and Western blotting analysis were conducted to observe changes in expression of caspase-3, cytochrome c, Apaf-1, Bax and Bcl-2 mRNA after treatment with oridonin. Expression of caspase-3, cytochrome c, Apaf-1 and Bax was up-regulated, whereas that of Bcl-2 was down-regulated. The results showed that oridonin could inhibit the proliferation of HGC-27 cells, and that this effect was related to its induction of tumor-cell apoptosis. Liu et al[35] also found that oridonin induced a decrease in Bcl-2/Bax ratio and activation of caspase-3. Zhang et al[36] reported that regulation of the Bcl-2 and MAPK families may be the effector mechanisms of oridonin-induced L929 cell death, independent of the caspase pathway. So we speculated that oridonin may change expression of Bcl-2 and Bax, and then trigger the release of cytochrome c through the mitochondrial pathway to further activate the caspase cascade reaction and induce HGC-27 apoptosis (Figure 6).
Figure 6.
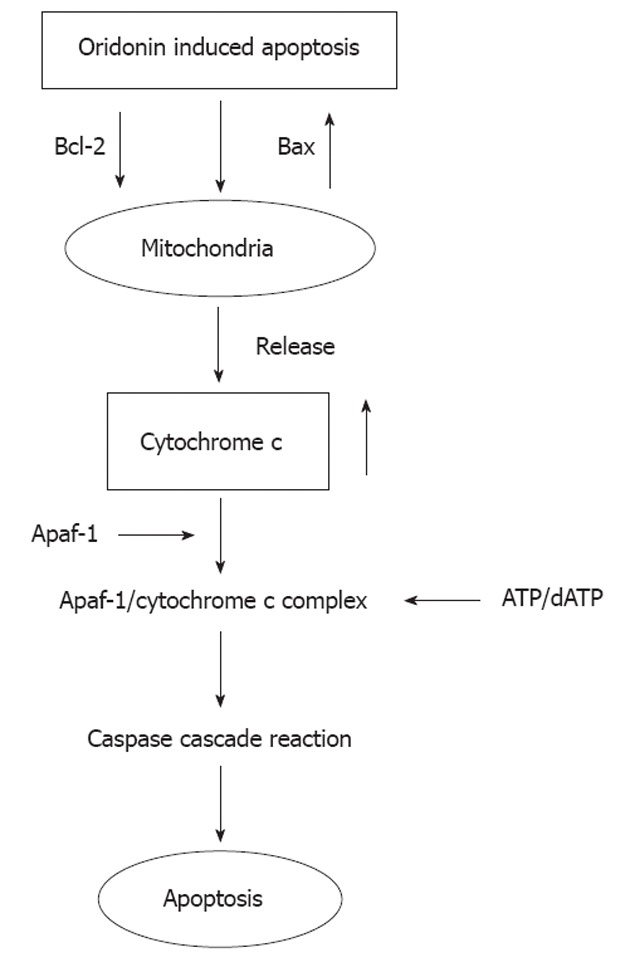
Possible mechanism by which oridonin induces the apoptosis of HGC-27 cells.
In conclusion, oridonin significantly inhibited the proliferation and promoted apoptosis of gastric cancer cell line HGC-27. And the apoptosis of HGC-27 induced by oridonin may be associated with differential expression of Apaf-1, caspase-3 and cytochrome c, which are highly dependent upon the mitochondrial pathway.
COMMENTS
Background
Gastric cancer is a common cancer of the digestive system and the second most common type of cancer worldwide. It is important to seek and develop new and more effective anti-gastric cancer drugs. Rabdosia rubescens, a medicinal herb, has therapeutic actions. Studies have suggested that oridonin has certain anti-tumor effects in many kinds of tumors. However, the exact mechanism by which oridonin induces the apoptosis of gastric cancer cells remains undefined. In this study, the effect of oridonin on gastric cancer cells and its possible mechanism of action were explored.
Research frontiers
Studies have suggested that oridonin has certain anti-tumor effects on cervical cancer, human epidermal squamous cell carcinoma, leukemia, liver cancer, malignant melanoma, colon cancer, breast cancer, and other tumors. It has been found that the mechanism of oridonin-induced apoptosis and aging of colon cancer cells might lie in increased histone acetylation and changes in the expressions of p16, p21, p27 and c-myc. Oridonin can induce the apoptosis of hepatoma cells through the reactive oxygen species-mitogen-activated protein kinase-p53 pathway. In addition, some recent studies suggested that oridonin can also inhibit the proliferation of tumor cells by increasing the autophagy of tumor cells. All of these findings suggest that oridonin has good anti-tumor effects.
Innovations and breakthroughs
In the present study, the authors found that oridonin significantly inhibited the proliferation and promoted apoptosis of gastric cancer cell line HGC-27. And the apoptosis of HGC-27 induced by oridonin may be associated with differential expression of Apaf-1, caspase-3 and cytochrome c. The authors speculated that oridonin may change expression of Bcl-2 and Bax, and then trigger the release of cytochrome c through the mitochondrial pathway to further activate the caspase cascade reaction and induce HGC-27 apoptosis.
Applications
Oridonin possesses potent anti-gastric cancer activities associated with inhibition of proliferation and regulation of pathways critical for maintaining apoptosis induction. These results may lay the groundwork for further studies to establish the causal relationship between oridonin anti-tumor activity and specific genetic pathways and to identify molecular markers that will guide the development of future clinical therapies. Therefore, oridonin may represent a novel therapeutic option for gastric cancer.
Terminology
Gastric cancer is still the second leading cause of cancer-related death worldwide, particularly in Asian countries. Traditional Chinese medicine (TCM) has played an increasingly important part in the prevention and treatment of tumors. In particular, integration of TCM with Western medicine has appreciably improved the efficacy of drug combinations and prolonged patient survival. Oridonin (molecular formula: C20H2006; relative molecular weight: 364.42) is a tetracyclic diterpenoid compound, which was extracted from Rabdosia rubescens.
Peer review
The authors demonstrated the effect and mechanism of oridonin on HGC-27 gastric cancer cell line and suggested that oridonin may represent a novel therapeutic option for gastric cancer.
Footnotes
Supported by Medical and Health Research Foundation of Zhejiang Province, No. 2009B019
Peer reviewers: Takaaki Arigami, MD, PhD, Department of Surgical Oncology and Digestive Surgery, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 891-0175, Japan; Liang-Shun Wang, MD, Professor, Vice-superintendent, Shuang-Ho Hospital, Taipei Medical University, No.291, Jhongjheng Rd., Jhonghe City, New Taipei City 237, Taiwan, China; B Mittal, PhD, Professor, Department of Genetics, Sanjay Gandhi Medical Institute, Lucknow 226014, India; Tomoyuki Shibata, MD, PhD, Associate Professor, Department of Gastroenterology, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukake-cho, Toyoake 470-1192, Aichi, Japan
S- Editor Gou SX L- Editor Ma JY E- Editor Li JY
References
- 1.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez RJ, Mansfield PF. Adjuvant and neoadjuvant therapy for gastric cancer. Surg Clin North Am. 2005;85:1033–1051, viii. doi: 10.1016/j.suc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Treasure J. Herbal medicine and cancer: an introductory overview. Semin Oncol Nurs. 2005;21:177–183. doi: 10.1016/j.soncn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Oridonin induced autophagy in human cervical carcinoma HeLa cells through Ras, JNK, and P38 regulation. J Pharmacol Sci. 2007;105:317–325. doi: 10.1254/jphs.fp0070336. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces human epidermoid carcinoma A431 cell apoptosis through tyrosine kinase and mitochondrial pathway. J Asian Nat Prod Res. 2008;10:77–87. doi: 10.1080/10286020701273866. [DOI] [PubMed] [Google Scholar]
- 8.Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8; 21) leukemia in vitro and in vivo. Blood. 2007;109:3441–3450. doi: 10.1182/blood-2006-06-032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong DW, Yu ZL. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep. 2010;24:647–651. [PubMed] [Google Scholar]
- 10.Wang HJ, Li D, Yang FY, Tashiro S, Onodera S, Ikejima T. Oridonin induces human melanoma A375-S2 cell death partially through inhibiting insulin-like growth factor 1 receptor signaling. J Asian Nat Prod Res. 2008;10:787–798. doi: 10.1080/10286020802030918. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Xie L, Chen G, Wang H, Zhang R. Effects of oridonin on proliferation of HT29 human colon carcinoma cell lines both in vitro and in vivo in mice. Pharmazie. 2007;62:439–444. [PubMed] [Google Scholar]
- 12.Cui Q, Yu JH, Wu JN, Tashiro S, Onodera S, Minami M, Ikejima T. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol Sin. 2007;28:1057–1066. doi: 10.1111/j.1745-7254.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao FH, Hu XH, Li W, Liu H, Zhang YJ, Guo ZY, Xu MH, Wang ST, Jiang B, Liu F, et al. Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-myc. BMC Cancer. 2010;10:610. doi: 10.1186/1471-2407-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang N, Zhang JH, Qiu F, Tashiro S, Onodera S, Ikejima T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Lett. 2010;294:147–158. doi: 10.1016/j.canlet.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370–379. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- 16.Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z, Li XM, Shen HM, Zeng HT. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol Sin. 2007;28:1819–1826. doi: 10.1111/j.1745-7254.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 17.Zang L, Xu Q, Ye Y, Li X, Liu Y, Tashiro S, Onodera S, Ikejima T. Autophagy enhanced phagocytosis of apoptotic cells by oridonin-treated human histocytic lymphoma U937 cells. Arch Biochem Biophys. 2012;518:31–41. doi: 10.1016/j.abb.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Cui Q, Chen SG, Wu LJ, Tashiro S, Onodera S, Ikejima T. Inactivation of ras and changes of mitochondrial membrane potential contribute to oridonin-induced autophagy in a431 cells. J Pharmacol Sci. 2007;105:22–33. doi: 10.1254/jphs.fpj06022x. [DOI] [PubMed] [Google Scholar]
- 20.He XJ, Wang HJ, Xia YJ, Ye ZY, Tao HQ. Empirical study of oridonin-induced gastric cancer cells MKN45 apoptosis. Zhonghua Weichang Waike Zazhi. 2009;12:607–610. [PubMed] [Google Scholar]
- 21.Schmitz I, Kirchhoff S, Krammer PH. Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol. 2000;32:1123–1136. doi: 10.1016/s1357-2725(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 22.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 23.García-Sáez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19:1733–1740. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin XM, Oltvai ZN, Veis-Novack DJ, Linette GP, Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cold Spring Harb Symp Quant Biol. 1994;59:387–393. doi: 10.1101/sqb.1994.059.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Pinkoski MJ, Waterhouse NJ, Heibein JA, Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC, Green DR. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem. 2001;276:12060–12067. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- 26.Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–864. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol Res. 2006;35:104–110. doi: 10.1016/j.hepres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces apoptosis of HeLa cells via altering expression of Bcl-2/Bax and activating caspase-3/ICAD pathway. Acta Pharmacol Sin. 2004;25:691–698. [PubMed] [Google Scholar]
- 29.Feng FF, Zhang DR, Tian KL, Lou HY, Qi XL, Wang YC, Duan CX, Jia LJ, Wang FH, Liu Y, et al. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by oridonin nanosuspension. Drug Deliv. 2011;18:265–271. doi: 10.3109/10717544.2010.536271. [DOI] [PubMed] [Google Scholar]
- 30.Wu JN, Huang J, Yang J, Tashiro S, Onodera S, Ikejima T. Caspase inhibition augmented oridonin-induced cell death in murine fibrosarcoma l929 by enhancing reactive oxygen species generation. J Pharmacol Sci. 2008;108:32–39. doi: 10.1254/jphs.fp0072079. [DOI] [PubMed] [Google Scholar]
- 31.Ji Z, Tang Q, Zhang J, Yang Y, Liu Y, Pan Y. Oridonin-induced apoptosis in SW620 human colorectal adenocarcinoma cells. Oncol Lett. 2011;2:1303–1307. doi: 10.3892/ol.2011.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye YC, Wang HJ, Xu L, Liu WW, Liu BB, Tashiro S, Onodera S, Ikejima T. Oridonin induces apoptosis and autophagy in murine fibrosarcoma L929 cells partly via NO-ERK-p53 positive-feedback loop signaling pathway. Acta Pharmacol Sin. 2012;33:1055–1061. doi: 10.1038/aps.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Li X, Wang J, Ye Z, Li JC. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Sci. 2012;8:901–912. doi: 10.7150/ijbs.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao FH, Liu F, Wei W, Liu LB, Xu MH, Guo ZY, Li W, Jiang B, Wu YL. Oridonin induces apoptosis and senescence by increasing hydrogen peroxide and glutathione depletion in colorectal cancer cells. Int J Mol Med. 2012;29:649–655. doi: 10.3892/ijmm.2012.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Shi QF, Qi M, Tashiro S, Onodera S, Ikejima T. Interruption of hepatocyte growth factor signaling augmented oridonin-induced death in human non-small cell lung cancer A549 cells via c-met-nuclear factor-κB-cyclooxygenase-2 and c-Met-Bcl-2-caspase-3 pathways. Biol Pharm Bull. 2012;35:1150–1158. doi: 10.1248/bpb.b12-00197. [DOI] [PubMed] [Google Scholar]
- 36.Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces a caspase-independent but mitochondria- and MAPK-dependent cell death in the murine fibrosarcoma cell line L929. Biol Pharm Bull. 2004;27:1527–1531. doi: 10.1248/bpb.27.1527. [DOI] [PubMed] [Google Scholar]