Abstract
AIM: To investigate whether stress-induced visceral hypersensitivity could be alleviated by electroacupuncture (EA) and whether EA effect was mediated by endogenous opiates.
METHODS: Six to nine week-old male Sprague-Dawley rats were used in this study. Visceral hypersensitivity was induced by a 9-d heterotypic intermittent stress (HIS) protocol composed of 3 randomly stressors, which included cold restraint stress at 4 °C for 45 min, water avoidance stress for 60 min, and forced swimming stress for 20 min, in adult male rats. The extent of visceral hypersensitivity was quantified by electromyography or by abdominal withdrawal reflex (AWR) scores of colorectal distension at different distention pressures (20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg). AWR scores either 0, 1, 2, 3 or 4 were obtained by a blinded observer. EA or sham EA was performed at classical acupoint ST-36 (Zu-San-Li) or BL-43 (Gao-Huang) in both hindlimbs of rats for 30 min. Naloxone (NLX) or NLX methiodide (m-NLX) was administered intraperitoneally to HIS rats in some experiments.
RESULTS: HIS rats displayed an increased sensitivity to colorectal distention, which started from 6 h (the first measurement), maintained for 24 h, and AWR scores returned to basal levels at 48 h and 7 d after HIS compared to pre-HIS baseline at different distention pressures. The AWR scores before HIS were 0.6 ± 0.2, 1.3 ± 0.2, 1.9 ± 0.2 and 2.3 ± 0.2 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. Six hours after termination of the last stressor, the AWR scores were 2.0 ± 0.1, 2.5 ± 0.1, 2.8 ± 0.2 and 3.5 ± 0.2 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. EA given at classical acupoint ST-36 in both hindlimbs for 30 min significantly attenuated the hypersensitive responses to colorectal distention in HIS rats compared with sham EA treatment [AWRs at 20 mmHg: 2.0 ± 0.2 vs 0.7 ± 0.1, P = 4.23 711 E-4; AWRs at 40 mmHg: 2.6 ± 0.2 vs 1.5 ± 0.2, P = 0.00 163; AWRs at 60 mmHg: 3.1 ± 0.2 vs 1.9 ± 0.1, P = 0.003; AWRs at 80 mmHg: 3.6 ± 0.1 vs 2.4 ± 0.2, P = 0.0023; electromyographic (EMG) at 20 mmHg: 24 ± 4.7 vs 13.8 ± 3.5; EMG at 40 mmHg: 60.2 ± 6.6 vs 30 ± 4.9, P = 0.00 523; EMG at 60 mmHg: 83 ± 10 vs 39.8 ± 5.9, P = 0.00 029; EMG at 80 mmHg: 94.3 ± 10.8 vs 49.6 ± 5.9, P = 0.00 021]. In addition, EA at the acupuncture point BL-43 with same parameters did not alleviate visceral hypersensitivity in HIS rats. EA in healthy rats also did not have any effect on AWR scores to colorectal distention at distention pressures of 20 and 40 mmHg. The EA-mediated analgesic effect was blocked by pretreatment with NLX in HIS rats [AWR scores pretreated with NLX vs normal saline (NS) were 2.0 vs 0.70 ± 0.20, 2.80 ± 0.12 vs 1.50 ± 0.27, 3 vs 2.00 ± 0.15 and 3.60 ± 0.18 vs 2.60 ± 0.18 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg; P = 0.0087, 0.0104, 0.0117 and 0.0188 for 20, 40, 60 and 80 mmHg, respectively]. Furthermore, EA-mediated analgesic effect was completely reversed by administration of m-NLX, a peripherally restricted opioid antagonist (EMG pretreated with m-NLX vs NS were 30.84 ± 4.39 vs 13.33 ± 3.88, 74.16 ± 9.04 vs 36.28 ± 8.01, 96.45 ± 11.80 vs 50.19 ± 8.28, and 111.59 ± 13.79 vs 56.42 ± 8.43 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg; P = 0.05 026, 0.00 034, 0.00 005, 0.000 007 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg, respectively).
CONCLUSION: EA given at classical acupoint ST-36 alleviates stress-induced visceral pain, which is most likely mediated by opioid pathways in the periphery.
Keywords: Irritable bowel syndrome, Visceral pain, Electroacupuncture, Opioid pathway, Stress
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterized by chronic visceral pain and bloating in association with altered bowel movements[1-3]. Chronic visceral pain, the cardinal feature of IBS, has been difficult to treat[4-6]. Acupuncture is an ancient form of traditional Chinese medicine that can be traced back for more than 3000 years. The acupuncture procedure involves the insertion of thin needles into the skin and underlying muscle layer, which are termed acupuncture loci, or “acupoints”. In traditional acupuncture, the needles are twisted right and left at 0.5- to 1-s intervals. More recently, acupuncture needles are stimulated by electricity at various frequencies (1-100 Hz), which is termed electroacupuncture (EA). EA has been used extensively for treatment of various painful conditions and gastrointestinal diseases, including IBS, functional dyspepsia, constipation, and diarrhea[7-9]. It has been shown that EA treatment results in a significant improvement both in general conditions and in symptoms of bloating[10,11]. Combined EA at the acupuncture points ST-36 and PC-6 significantly increases the threshold of rectal sensations induced by rectal distension in IBS patients[8,12,13], suggesting that EA might be a promising method to treat visceral pain in patients with IBS. However, research into EA for chronic visceral pain is still in its infancy, and much of the limited scientific evidence surrounding it is fragmentary and often contradictory[14-16]. Thus, further investigations of EA efficacy and its mechanisms are definitely merited. Recently, we have developed a rat model of visceral hypersensitivity induced by heterotypic intermittent stress (HIS)[17]. These rats displayed no robust inflammation or injury in the colon, but a significantly higher visceromoter response (VMR) to colorectal distention (CRD) compared with age-matched controls. Thus, the animal model resembles some characteristics of IBS seen in human patients. The aim of this study was to investigate whether EA has therapeutic benefits on visceral hypersensitivity induced by HIS, and if so, what is the underlying mechanism. We found that EA treatment at acupoint ST-36 significantly attenuated abdominal withdrawal reflexes (AWRs) in HIS rats at distention pressures of 20 and 40 mmHg, and in both stressed and non-stressed rats at distention pressures of 60 and 80 mmHg. Pretreatment with naloxone (NLX), an opioid receptor antagonist, or NLX methiodide (m-NLX), a selectively peripherally acting opioid receptor antagonist, completely reversed the EA effect in HIS rats.
MATERIALS AND METHODS
Animals
Six to nine week-old male Sprague-Dawley rats (n = 94) housed at approximately 22 °C with a 12-h light/dark cycle were used in this study. Care and handling of these animals were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and at Soochow University. The animals were euthanized by decapitation at various times indicated in the Result section after the end of in vivo behavioral studies. Compared with our previous report that HIS-induced visceral hypersensitivity returned to normal level 24 h after termination of the last stressor in Wistar rats[17], HIS-induced visceral hyperalgesia lasted longer in SD rats than in Wistar rats. Therefore, SD rats were used in this experiment. Rats were grouped before experiment and were single-housed during experiments.
Heterotypic intermittent stress protocol
Rats were subjected to 9 consecutive days of a HIS protocol comprised of 3 randomly selected stressors, which included cold restraint stress (CRS) at 4 °C for 45 min, water avoidance stress (WAS) for 60 min, and forced swimming stress (FSS) for 20 min, as described previously[17] and in Figure 1A. In brief, each stressor was applied between 8 am and 11 am. For CRS, the rats were restrained in a clear plastic container (6 cm in diameter × 18 cm in length). The container had 2-cm diameter openings at each end for the rat to breathe normally. The restraining container was placed in cold room at 4 °C for 45 min. The CRS was given to rats on days 1, 4 and 9. For WAS, the rat was placed for 60 min on a brick (12 cm high × 6 cm wide × 8.5 cm long) in the middle of a plastic container (14 cm high × 36 cm wide and × 54 cm long) filled with water at room temperature (approximately 22 °C) within 1 cm from the top. The rats were subjected to WAS on days 3, 5 and 7. For FSS, the rat was forced to swim for 20 min in a plastic container (38 cm high × 24 cm wide × 32 cm long) filled to a depth of 12 cm below the top with water at room temperature (approximately 22 °C). The FSS was given to rats on days 2, 6 and 8. Age-matched control rats were brought to the laboratory and handled identically without the stress protocol.
Figure 1.
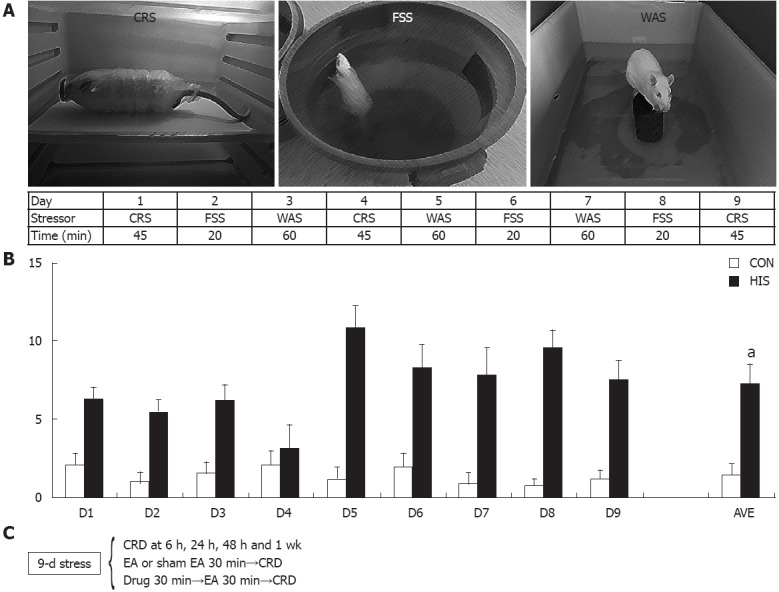
Heterotypic intermittent stress protocol and pellets count. A: Nine-day heterotypic intermittent stress (HIS) protocol comprising three different randomly arranged stressors; B: Stress accelerated colonic transit (n = 10 for control, n = 8 for HIS), which was accompanied by an increase in visceral hypersensitivity (aP < 0.05). C: A schematic representation of the various treatments: stressors, colorectal distention (CRD) and drug treatment with time sequence. CON: Control; AVE: The mean number of pellets for all 9-d stress protocol or from respective control rats; CRS: Cold restrained stressor; FSS: Forced swimming stressor; WAS: Water avoidance stressor.
Heterotypic intermittent stress was chosen because clinical findings suggest that long-term stress, rather than short-term stress, exacerbates symptoms of IBS. In addition, variable stressors are less likely to produce adaptation when compared to repeated applications of the same stressor. The number of pellets during each stress protocol was counted for each rat in order to measure colonic transit during stress protocol (Figure 1B). It is remarkable that stress accelerated defection rate when compared with controls (two sample t test, P < 0.001). The number of pellets in the stress situations is not time dependent as it is in the control situation. This is most likely due to the different stressors used. The increase in number of pellets and visceral hypersensitivity in the stress situations indicates that this model can mimic the major characteristics of patients with IBS and thus it is a suitable rat model for study of the effect and mechanisms of EA treatment.
Measurement of visceral hypersensitivity to graded colorectal distention
Electromyographic recordings. Visceral hypersensitivity was measured by electromyographic (EMG) measurements of VMR to CRD as described previously[18]. Briefly, under anesthesia with isofluorane, 2 electrodes were implanted in the external oblique muscle and externalized behind the head. Rats were allowed 1 wk to recover from the surgery. After recovery, baseline EMG measurement was recorded, and then 9-d stress protocol was applied. After termination of last stressor, the EMGs were recorded again from these rats. In some cases, EA or sham EA was applied during the stress protocol. Under anesthesia with isofluorane, a flexible balloon (5 cm) constructed from a surgical glove finger attached to a tygon tubing was inserted 8 cm into the descending colon and rectum via the anus and held in place by taping the tubing to the tail. Rats were placed in small Lucite cubicles (20 cm × 8 cm × 8 cm) (Bioengineering Department, University of Texas Medical Branch, Galveston, TX) and allowed to adapt for 30 min. CRD was performed by rapidly inflating the balloon to constant pressure. Pressure was measured using a sphygmomanometer connected to a pressure transducer. The balloon was inflated to various pressures (20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg) for a 20 s stimulation period followed by a 2 min rest. EMG was recorded continuously during the experiment on a Biopac System EMG 100 °C. The EMG signal was amplified, filtered at 300 Hz and digitized using Acknowledge software (Biopac Systems, Inc., CA, United States). The area under the curve (AUC) for EMG activities during each 20 s of distention was calculated using an in-house written computer program[18]. The net value for each distension was calculated by subtracting the baseline value derived from the AUC for the 20 s pre-distention period. EMG was measured before HIS and 6 h, 1 d, 2 d and 7 d after termination of last stressor. Each rat was tested for EMG twice for each distention pressure and the mean AUC of EMG calculated from the two repeated measurements was used for each rat for each pressure in the following statistical analysis.
Abdominal withdrawal reflex scores: Visceral hypersensitivity was also measured by grading behavioral response of rats to CRD as described previously[3,19]. In brief, under anesthesia with isofluorane, a flexible latex balloon (5 cm) attached to a tygon tube was inserted 8 cm into the descending colon and rectum via anus and held in place by taping the tube to tail. Rats were placed in small lucite cubicles and allowed to adapt for 30 min. CRD was performed by rapidly inflating the balloon to constant pressure using a sphygmomanometer. The balloon was inflated to 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg for 20 s followed by 2 min rest. Behavioral response to CRD was measured by visual observation of AWR by a blinded observer and AWR was scored either 0 (normal behavior), 1 (slight head movement), 2 (contraction of abdominal muscles), 3 (lifting of abdominal wall) or 4 (body arching and lifting of pelvic structures). AWR scores were measured before HIS and 6 h, 1 d, 2 d and 7 d after termination of last stressor. The experimenter, who assigned the AWR scores and performed the EMG analysis, was masked to the control or stressed group assignment, to the sham or EA treatment, and to the drug applied (saline or NLX/m-NLX). Each rat was tested twice for AWR score for each distention pressure and the mean AWR score from the two repeated measurements was used for each rat for each pressure in the following statistical analysis.
Electroacupuncture treatment
EA was applied by a pair of stainless steel suture needles. Hook-shaped needles were used to avoid spontaneous removal of inserted acupuncture needles from rat body[20,21]. Two acupoints were used in this study: ST-36 (Zu-San-Li) or BL-43 (Gao-Huang). ST-36, equivalent to the human acupoint ST-36 (Figure 2), is located at 5 mm lateral to the anterior tubercle of the tibia and 10 mm below the knee joint[21-23]. BL-43, equivalent to the human acupoint BL-43 (Figure 2), was used as an irrelevant acupuncture point to the colon. The needles were inserted bilaterally at a depth of 5 mm into the skin and underlying muscles at acupuncture point. To compare the effect of EA at an irrelevant acupoint, the same stimulation parameters were used to stimulate the BL-43. The needles inserted into acupoints were stimulated by an EA apparatus (Model G-6805-2, Shanghai Medical Electronic Apparatus Company, China) with a constant rectangular current of alternating trains of dense-sparse frequencies (100 Hz for 1.05 s and 2 Hz for 2.85 s alternately, pulse width, 0.1 ms). This combination of dense-sparse frequency would maximally induce opioid release of met-enkephalin and dynorphin A[24]. Electrical stimulus intensity was set at the threshold for a detectable muscle twitch (approximately 1 mA). The stimulation was delivered for 30 min. For sham EA group, the needle set was inserted into the ST-36, but no electrical stimulation was applied. Behavioral tests were performed immediately after termination of EA.
Figure 2.
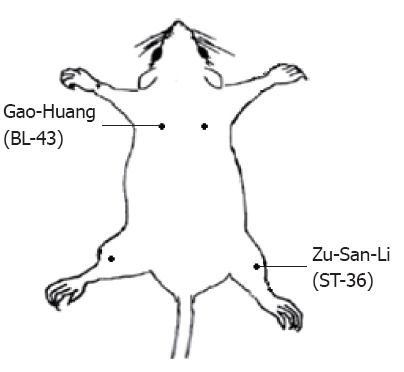
Schematic representation of electroacupuncture points. ST-36 (Zu-San-Li) is thought to be relevant to gastrointestinal tract while Gao-Huang is not.
Drug administration
After finishing the first distension series, NLX (0.1 mg/kg, Sigma) or m-NLX (1 mg/kg, Sigma) was administered intraperitoneally to HIS rats on the second day. Thirty min after the administration of NLX or m-NLX, EA at ST-36 was given for 30 min. The second distension series was performed immediately after termination of EA. The AUCs for the EMG signals or AWR scores from two distention series were calculated. m-NLX is a selectively peripherally acting opioid receptor antagonist. The time schedule of experimental protocol is shown in Figure 1C.
Statistical analysis
All data were expressed as mean ± SE. Statistical analysis were conducted using commercial software OriginPro 8 (OriginLab, United States) and Matlab (Mathworks, United States). Normality was checked for all analyses. Significance between groups was determined using two sample t test, Friedman analysis of variance (ANOVA) or two-way repeated-measures ANOVA followed by Tukey post hoc test or Mann-Whitney test where appropriate. The level of significance was set at P < 0.05.
RESULTS
HIS produced visceral hypersensitivity
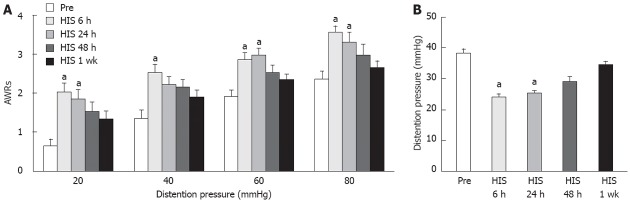
To determine whether HIS induces visceral hypersensitivity, AWR scores to CRD were measured in rats before and after the 9-d HIS protocol. The AWR scores before HIS were 0.6 ± 0.2, 1.3 ± 0.2, 1.9 ± 0.2 and 2.3 ± 0.2 for 20, 40, 60 and 80 mmHg distention pressures, respectively. Six hours after termination of the last stressor, the AWR scores were 2.0 ± 0.1, 2.5 ± 0.1, 2.8 ± 0.2 and 3.5 ± 0.2 for 20, 40, 60 and 80 mmHg distention pressures, respectively (Figure 3A). Since the first measurement was at 6 h, the time when it started was unknown. To determine the time course of stress-induced visceral hypersensitivity, AWR scores were recorded 6, 24, 48 h, and 7 d after the HIS protocol. There was clear time effect for HIS on AWR scores for all distention pressures (Friedman ANOVA; n = 8 rats for each group). The increase in AWR scores started at 6 h, maintained for 24 h, and AWR scores returned to basal levels at 48 h and 7 d after HIS compared with pre-HIS baseline at different distention pressures (P < 0.05, Tukey post hoc test following Friedman ANOVA, Figure 3A). In addition, the distention threshold was measured in these rats before and after HIS protocol. Distention threshold was the minimal distention pressure to evoke abdominal movement. The distention threshold was 38.3 ± 1.5 mmHg before HIS and was 23.9 ± 1.3, 25.3 ± 0.8, 29.0 ± 1.8, 34.7 ± 0.9 mmHg at 6, 24, 48 h and 1 wk after termination of the last stressor, respectively. There was significant time effect on the distention threshold (Friedman ANOVA, P < 0.001, n = 8 rats for each group). In agreement with the AWR scores, the distention threshold was significantly lower at 6 h and 24 h, and returned to the baseline level at 48 h and 7 d after HIS protocol compared with pre-HIS baseline (P < 0.05, Tukey post hoc test following Friedman ANOVA, Figure 3B).
Figure 3.
Heterotypic intermittent stress increased abdominal withdrawal reflex scores to colorectal distention. A: Abdominal withdrawal reflex (AWR) scores were used as a function of distention pressure (20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg). Heterotypic intermittent stress (HIS) significantly enhanced AWR scores measured at 6 h and 24 h after termination of last stressor when compared with baseline (Pre) under 20 mmHg, 60 mmHg and 80 mmHg distention pressure, while HIS significantly enhanced AWR scores measured at 6 h after termination of last stressor when compared with Pre group under 40 mmHg distention pressure [Tukey post hoc test following Friedman analysis of variance (ANOVA)]. Therefore, HIS can enhance visceral hypersensitivity in rats at 6 h and 24 h after termination of last stressor generally when compared with baseline (Pre). AWR scores returned to normal level 48 h after termination of last stressor (n = 8 rats for each group; aP < 0.05 vs Pre); B: HIS remarkably reduced distention threshold. Distention threshold was the minimal distention pressure to evoke abdominal movement. In agreement with AWR scores, distention threshold started to reduce at 6 h and maintained at a low level at 24 h and returned to normal level 48 h after termination of last stressor (Friedman ANOVA followed by Tukey post hoc test, n = 8 rats for each group; aP < 0.05 vs Pre).
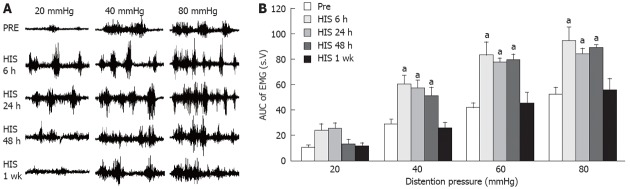
To further confirm the visceral hypersensitivity induced by HIS, EMG measurements were performed on rats before and after HIS. The AUC of EMG recordings before HIS was 10.6 ± 1.6, 29.2 ± 3.3, 41.3 ± 4.3 and 52.4 ± 5.1 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. Six h after termination of the HIS protocol, the AUCs were 24.0 ± 4.7, 60.2 ± 6.6, 83.0 ± 10.0 and 94.3 ± 10.9 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. There was significant time effect of HIS on EMG for all pressures [P < 0.001, two-way repeated measures ANOVA, with significant time × pressure interaction (P < 0.001); n = 6 rats for each group]. The AUCs were higher at 6, 24 and 48 h, and returned to baseline values one week after termination of HIS compared with pre-HIS baseline for 40, 60 and 80 mmHg, with no significant difference between AUC of EMG of different time points at 20 mmHg (P < 0.05, Tukey post hoc test following two-way repeated measures ANOVA, Figure 4A and B).
Figure 4.
Heterotypic intermittent stress enhanced electromyographic activities in response to colorectal distention. Electromyographic (EMG) activities in the external oblique muscle in response to graded colorectal distention (CRD) were measured before stress and 6 h, 24 h, 48 h and 7 d after termination of heterotypic intermittent stress (HIS). The magnitude of EMG activity was expressed as area under curve (AUC). A: Examples of EMG activities recorded from baseline (Pre), 6 h, 24 h, 48 h and 7 d after termination of HIS in rats responding to distention pressures of 20 mmHg, 40 mmHg and 80 mmHg; B: Bar graph shows the changes in average of AUC before and after HIS protocol. There was no significant difference between EMGs of different time points at 20 mmHg. The magnitude of EMG activity of 6 h, 24 h and 48 h was significantly larger than that of Pre at distention pressures of 40 mmHg, 60 mmHg and 80 mmHg (Tukey post hoc test following two-way repeated measures analysis of variance, n = 6 rats for each group; aP < 0.05 vs Pre).
EA treatment suppressed visceral hypersensitivity in HIS rats
To determine whether EA suppressed visceral hypersensitivity induced by HIS, AWR scores and AUCs of EMG recordings after EA treatment were compared with those after sham EA treatment. To define the specificity of EA-mediated analgesic effect in rats, we also examined EA effect on age-matched healthy rats (controls). Since AWR scores returned to the baseline level 48 h and EMG data returned to baseline level one week after termination of last stressor (Figures 3A and 4B), EA at acupoints ST-36 (Figure 2) was applied to control and HIS rats for 30 min within 48 h after termination of last stressor. For sham EA group, the needle set was inserted into the ST-36, but no electrical stimulation was applied. AWR scores and EMG activities were recorded immediately after termination of EA. Both distention stress and EA treatment affected AWRs (n = 8 rats for each group, two-way repeated measures ANOVA: under 20 mmHg, stress effect, P < 0.001; EA treatment effect, P < 0.01; under 40 mmHg, stress effect, P < 0.001; EA treatment effect, P < 0.05), with significant stress × EA treatment interaction for 20 mmHg and 40 mmHg pressures (P < 0.05 for 20 mmHg and 40 mmHg). HIS sham group showed a significant increase in AWR scores compared with control sham group under 20 mmHg and 40 mmHg distention pressures (HIS sham vs control sham, for 20 mmHg: 2 ± 0.2 vs 0.6 ± 0.1; for 40 mmHg, 2.6 ± 0.2 vs 1.4 ± 0.2; P < 0.05, Tukey post hoc test following two-way repeated measures ANOVA), while there was no significant difference in AWR scores between control and HIS groups after EA treatment. EA treatment at ST-36 point significantly decreased AWR scores in HIS rats (sham EA vs EA, AWRs at 20 mmHg: 2.0 ± 0.2 vs 0.7 ± 0.1; at 40 mmHg: 2.6 ± 0.2 vs 1.5 ± 0.2; at 60 mmHg: 3.1 ± 0.2 vs 1.9 ± 0.1; at 80 mmHg: 3.6 ± 0.1 vs 2.4 ± 0.2; P < 0.05, Tukey post hoc test following two-way repeated measures ANOVA, Figure 5A), but had no effect on control rats under 20 mmHg and 40 mmHg pressures. Under 60 mmHg and 80 mmHg pressures, stress × EA treatment interaction was not significant (n = 8 rats for each group, two-way repeated measures ANOVA); EA treatment significantly decreased AWR scores in both control and HIS rats under 60 mmHg and 80 mmHg pressures (P < 0.05, EA effect, two-way repeated measures ANOVA, Figure 5A).
Figure 5.
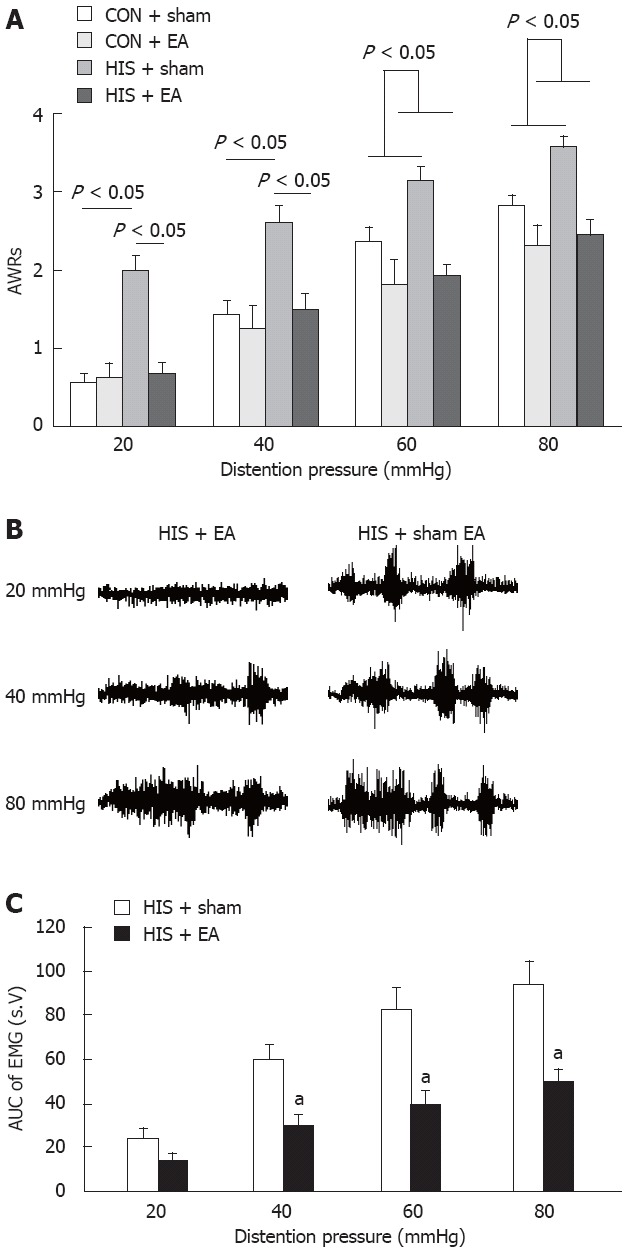
Electroacupuncture treatment attenuated the abdominal withdrawal reflex scores and electromyographic activities. Electroacupuncture (EA) treatments were delivered for 30 min within 24 h after termination of last stressor. For sham EA group, the needle set was inserted into the ST-36 but no electrical stimulation was applied. A: The effects of stress exposure and EA treatment on abdominal withdrawal reflex (AWR) scores. Heterotypic intermittent stress (HIS) sham group showed a significant increase in AWR scores compared to control sham group under 20 mmHg and 40 mmHg distention pressures, while there was no significant difference in AWR scores between control and HIS groups after EA treatment. EA treatment at ST-36 point significantly decreased AWR scores in HIS rats, but had no effect on control rats under 20 mmHg and 40 mmHg pressures [Tukey post hoc test following two-way repeated measures analysis of variance (ANOVA)]. EA treatment significantly decreased AWR scores in both control and HIS rats under 60 mmHg and 80 mmHg pressures (two-way repeated measures ANOVA, n = 8 rats for each group; P < 0.05); B: Representative electromyographic (EMG) traces recorded immediately after termination of EA (left) or sham EA (right); C: Bar graph showing effects of EA treatment and sham EA on EMG recordings. The EMG was significantly decreased by EA treatment at pressure of 40 mmHg, 60 mmHg and 80 mmHg in stressed rats compared to sham EA groups (Tukey post hoc test following two-way repeated measures ANOVA, n = 6 rats for each group; aP < 0.05 vs HIS + sham group). CON: Control.
To further confirm the EA effect on stressed rats, EMGs were performed before and after EA or sham EA treatment (Figure 5B and C). Both distention pressure and EA treatment affected AUCs of HIS rats significantly (n = 6 rats for each group, two-way repeated measures ANOVA: pressure effect, P < 0.001; EA treatment effect, P < 0.05), with significant pressure × EA treatment interaction (P < 0.001). Rats that received EA treatment showed a significant decrease in their AUCs compared to rats that received sham EA under 40 mmHg, 60 mmHg and 80 mmHg distention pressures 6 h after HIS (EMG for sham EA vs EA, at 20 mmHg: 24 ± 4.7 vs 13.8 ± 3.5; EMG at 40 mmHg: 60.2 ± 6.6 vs 30 ± 4.9, P = 0.00 523; EMG at 60 mmHg: 83 ± 10 vs 39.8 ± 5.9, P = 0.00 029; EMG at 80 mmHg: 94.3 ± 10.8 vs 49.6 ± 5.9, P = 0.00 021; P < 0.05, Tukey post hoc test following two-way repeated measures ANOVA, Figure 5B and C), without significant effect under 20 mmHg pressure. This demonstrates that EA suppressed visceral hypersensitivity, and is in agreement with previous studies that EA treatment attenuated chronic visceral hyperalgesia induced by neonatal colonic injection of acetic acid[23]. It is of note that results from EMG recordings at 20 mmHg were different from those of AWR scores after EA treatment.
To exclude the non-specific effect of EA treatment, EA was applied at Gao-Huang. Gao-Huang, an equivalent to the human acupoint BL-43 (Figure 2), was chosen as an irrelevant acupuncture point to the colon. EA at BL-43 for 30 min did not produce any effect on AWR scores in HIS rats [n = 7 rats for each group. Two-way repeated measures ANOVA (EA treatment effect, P > 0.05; pressure × EA interaction effect, P > 0.05), Figure 6].
Figure 6.
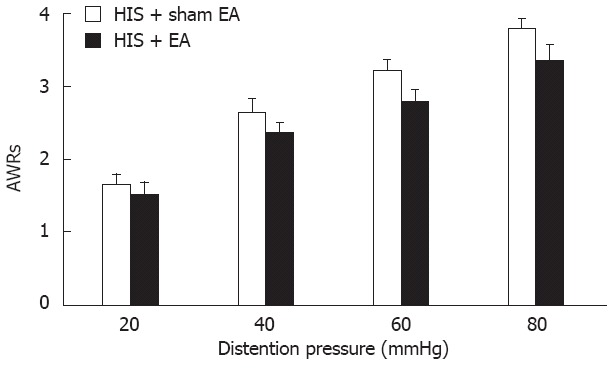
Effect of electroacupuncture at Gao-Huang. Same electroacupuncture (EA) parameters as EA at ST-36 (Zu-San-Li) were used for BL-43 (Gao-Huang) treatment in heterotypic intermittent stress (HIS) rats. Although the abdominal withdrawal reflexes (AWRs) to colorectal distention heavily depend on the pressure level (two-way repeated measures analysis of variance, pressure effect, P < 0.001), EA treatment at BL-43 point on HIS rats showed no significant effect on pain perception; n = 7 rats for each group.
NLX reversed EA-induced analgesic effects
To explore the possible involvement of endogenous opioid system in EA-induced antihyperalgesia, we then examined the effect of systemically injected NLX on EA-induced analgesia. After the 9-d HIS protocol, rats received intraperitoneal injection of 0.1 mg/kg of NLX in 1 mL 30 min before the beginning of EA at ST-36 points. Equal volume of normal saline (NS) was used as control. Immediately after EA termination, the AWR scores to CRD were determined (Figure 7A). The AWR scores from HIS rats pretreated with NS were 0.70 ± 0.20, 1.50 ± 0.27, 2.00 ± 0.15 and 2.60 ± 0.18 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. The AWR scores in HIS rats pretreated with NLX were 2.0, 2.80 ± 0.12, 3 and 3.60 ± 0.18 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. Pretreatment of NLX significantly reduced the EA-induced suppression of AWR scores of HIS rats under all distention pressures when compared with NS group [n = 5 rats for each group, Friedman ANOVA, NLX effect, P < 0.001; P < 0.05, Mann-Whitney test following Friedman ANOVA, Figure 7A]. This indicates the involvement of endogenous opioid system in mediating EA analgesic effects on HIS-induced visceral hyperalgesia. To exclude the non-specific effect of NLX, we further determined whether NLX itself produced any effect on AWR scores in control rats without EA treatment (Figure 7B). The AWR scores before 0.1 mg/kg of NLX in 1 mL treatment in control rats were 1.00 ± 0.16, 1.90 ± 0.10, 2.50 ± 0.22 and 2.70 ± 0.20 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. The AWR scores after 0.1 mg/kg of NLX in 1 mL treatment were 1.10 ± 0.19, 1.90 ± 0.24, 2.60 ± 0.24 and 2.80 ± 0.20 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. This suggests that NLX itself did not produce any effect on AWR scores in control rats (n = 5 rats for each group, Friedman ANOVA, NLX effect, P > 0.05). Similarly, we did not observe any effect of NLX on AWR scores in HIS rats without EA treatment (data not shown). These data suggest that the inhibitory effect of NLX on visceral analgesia is associated with EA treatment.
Figure 7.
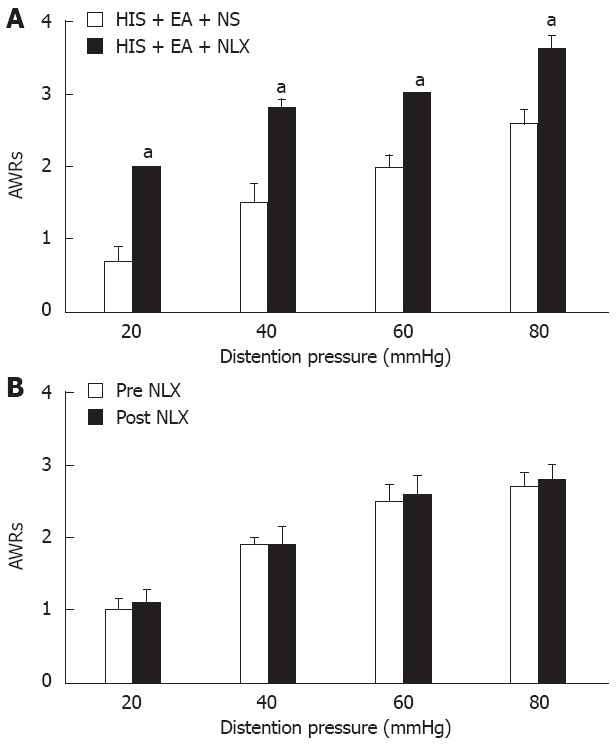
Reversal of electroacupuncture-mediated analgesic effect by naloxone. A: The opioid receptor antagonist, naloxone (NLX) (0.1 mg/kg body weight, n = 5), or normal saline (NS) was administrated intraperitoneally 30 min before electroacupuncture (EA) application. Abdominal withdrawal reflex (AWR) scores were recorded immediately after EA termination in rats pretreated with NLX or NS. Both pressure and NLX injection significantly affected the AWRs to colorectal distention in rats [Friedman analysis of variance (ANOVA), P < 0.001 for pressure effect and for NLX injection effect]. Bar graph showed that NLX (0.1 mg/kg body weight) completely blocked the EA-induced analgesic effect when compared with NS treatment at all pressures (Mann-Whitney test following Friedman ANOVA). n = 5 rats for each group. aP < 0.05 vs NS-EA group; B: Same dose of NLX treatment did not produce any effect on AWR scores in control rats without EA treatment (Friedman ANOVA). AWRs were only significantly affected by different pressure levels (P < 0.001). n = 5 rats for each group. Control rats were not exposed to heterotypic intermittent stress (HIS).
m-NLX inhibited EA-induced analgesic effects
To further determine whether peripheral opioid system is involved in the EA-induced analgesic effect, m-NLX was administrated prior to EA treatment. m-NLX is an opioid receptor antagonist that can not cross the blood-brain barrier, thereby only acting at peripheral nervous system. After the 9-d HIS protocol, rats received intraperitoneal injections of 1 mg/kg of m-NLX in 1 mL 30 min before the beginning of EA at ST-36 points. Equal volume of NS was used as control. EMG recordings were measured immediately after EA termination. The AUCs of EMG activities from HIS rats pretreated with NS were 13.33 ± 3.88, 36.28 ± 8.01, 50.19 ± 8.28 and 56.42 ± 8.43 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. The AUCs of EMG activities in HIS rats pretreated with m-NLX were 30.84 ± 4.39, 74.16 ± 9.04, 96.45 ± 11.80 and 111.59 ± 13.79 for 20 mmHg, 40 mmHg, 60 mmHg and 80 mmHg distention pressures, respectively. Pretreatment of m-NLX (1 mg/kg, i.p.) significantly reduced the analgesic effects induced by EA in a pressure-dependent manner (n = 6 rats for each group, two-way repeated measures ANOVA, NLX effect, P < 0.01; pressure × NLX interaction, P < 0.01). The effect of pretreatment of m-NLX was significant under 40 mmHg, 60 mmHg and 80 mmHg, but not under 20 mmHg (P < 0.001, Tukey post hoc test following two-way repeated measures ANOVA, Figure 8). To further determine the specificity of m-NLX, a low dose of m-NLX (0.1 mg/kg) was injected intraperitoneally. Pretreatment with a low dose of m-NLX did not affect the EA-induced suppression of EMG activities in HIS rats (data not shown). In addition, systemic injection of m-NLX (1 mg/kg, i.p.) did not produce any effect on the AUCs of EMG activities in normal or HIS rats without EA treatment (data not shown). These data suggest that EA may affect the peripheral opioid system to induce analgesia in HIS rats.
Figure 8.
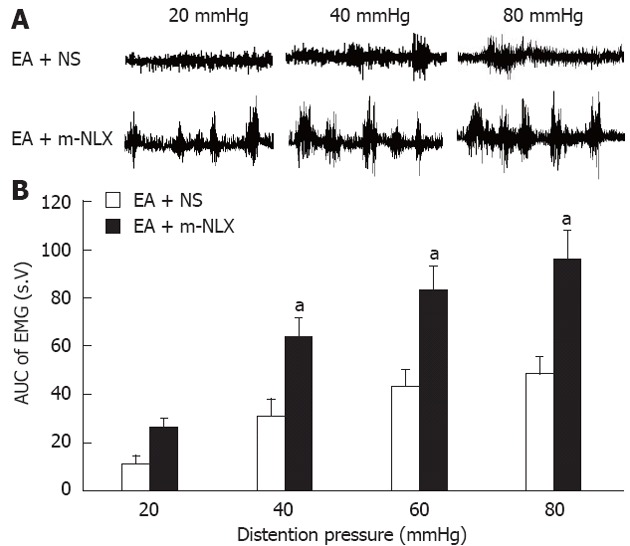
Reversal of electroacupuncture-mediated analgesic effect by naloxone methiodide. The opioid receptor antagonist, naloxone methiodide (m-NLX), or normal saline (NS) was administrated intraperitoneally (5 mg/kg body weight) 30 min before electroacupuncture (EA) application. Electromyographic (EMG) activities were recorded immediately after EA from rats pretreated with m-NLX and NS. A: Examples of EA effects on EMG activities pretreated with NS (top) or m-NLX (bottom); B: Bar graph showing that m-NLX completely blocked the EA-induced analgesic effect. The magnitude of EMG activity was significantly increased by m-NLX treatment at pressure of 40 mmHg, 60 mmHg and 80 mmHg (Tukey post hoc test following two-way repeated measures analysis of variance, n = 6 rats for each group; aP < 0.05 vs EA + NS group).
DISCUSSION
The present study examined the mechanisms involved in EA-induced analgesia in a rat model of visceral hypersensitivity induced by HIS. EA treatment significantly reduced AWR scores (Figure 5A) and suppressed EMG responses (Figure 5C) to colorectal distention in the stressed rats, but not in non-stressed rats, at the pressures of 20 mmHg and 40 mmHg, indicating that EA had an analgesic effect in this model. Although EA has been used clinically for alleviation of various types of pain[25,26], there is no enough scientific validation for the use of EA in visceral pain. Together with our previous report that EA attenuated visceral pain induced by neonatal acetic acid infusion[23], we have provided additional evidence for EA treatment for visceral pain in different models.
Acupuncture is being increasingly accepted by practitioners and patients, especially during the last three decades. EA is a modification of this technique that stimulates acupoints (or called acupuncture points) with electrical current instead of manual manipulations. The EA procedures may stimulate the somatic afferent nerves innervating the skin and muscles of the body, which was different from transcutaneous electrical nerve stimulation (TENS). EA typically involves penetration of the skin by fine, solid metallic needles, which are manipulated by electrical stimulation. TENS is a treatment that has been shown to be effective for pain relief in a variety of conditions. Electrodes for TENS are placed on the skin. Electric current applied at different pulse rates (frequencies) and intensities is used to stimulate these areas so as to provide pain relief. In this study, we focused on the EA-mediated effect in stressed animal models. We showed that EA at ST-36, but not at BL-43, significantly suppressed the visceral motor responses to CRD (Figure 6). Koo et al[27] reported that ankle sprain pain was relieved by EA at SI-6, but not at nearby LI-4. More recently, they reported that EA-induced analgesic effects in capsaicin-induced hyperalgesia are produced only by stimulation at SI-3/TE-8 of the forelimb, but not at nearby points (LI-3/LI-6) or several other points (GB-30/GB34, BL-40/BL-60, GV-2/GV-6)[28]. Kim et al[29] demonstrated that acupuncture at HT-7, but not at nearby point TE-8, inhibited dopamine release in the nucleus accumbens and suppressed behavioral hyperactivity in the morphine addiction model, thus suggesting the acupoint-specificity. However, the acupoint specificity of EA effect in visceral pain remains unknown. In the present study, two acupoints, ST-36 and BL-43, were selected. The acupoint ST-36 has been used empirically for the treatment of gastrointestinal diseases for many years, while acupoint BL-43 is not thought to be related to the GI tract (Figure 2). EA at ST-36, but not at BL-43, significantly suppressed the visceral motor responses to CRD (Figure 6), suggesting that the EA effect is not a non-specific effect and it may be associated with acupoint and its related afferent fibers. This finding is consistent with a previous study which found that EA at ST-36, but not at BL-21, significantly reduced the increase in mean arterial blood pressure in response to rectal distension in dogs[30]. Since the parameter used for EA treatment at BL-43 is the same as at ST-36, further experiments are needed to investigate whether different stimulation parameters used to stimulate BL-43 produce the analgesic effect in this model. In addition, it is worth noting that our results showed that EA produced a significant analgesic effect only on the HIS-induced visceral hyperalgesia, but not on the age-matched healthy rats, at the distention pressures of 20 mmHg and 40 mmHg (Figure 5A). This further indicates that EA-produced analgesic effect is not a non-specific effect and that it may be disease-related under low stimulation intensity. The reason why EA did not produce inhibitory effect under low distention pressure remains unknown. It is most likely that low distention pressure produced the low responses that are not sensitive to EA treatment. However, there has been a certain degree of skepticism about acupoint and disease specificity. There was no statistical difference between acupoint and nonacupoint acupuncture in an experimental human pain model, thus suggesting no acupoint specificity. Rong et al[31] reported that manual acupuncture at ST-36 produced anti-nociceptive effect on CRD in healthy rats. This discrepancy may be due to the application of different methods of acupuncture and distention pressure as well. Therefore, the acupoint and disease specificity of EA treatment is still a controversial issue and is a subject of further study of pain.
EA analgesic effect in various conditions may be mediated by different mechanisms. These include opioid and non-opioid mechanisms[32-35]. The involvement of the endogenous opioid system is a well-established hypothesis for explanation of EA effects. The involvement of non-opioid mechanisms in EA analgesia was confirmed by experiments in which administration of 5-HT or catecholamine or adrenoceptor antagonists or depletion of cellular monoamine content blocked the EA-induced analgesic effect[36,37]. It appears that the underlying mechanisms of EA analgesic effect in various conditions may depend on the specific conditions[28]. In this study, the systemic application of opioid receptor antagonist NLX completely blocked the EA-mediated analgesic effects, indicating that endogenous opioid pathways were involved in EA-mediated analgesia in the rat model of visceral pain. This was consistent with the reports of different animal models of visceral pain[22,38]. In the present study, we focused on the role of opioid system because previous studies indicate that opioid system was sensitive to environmental factors, and changes in its expression attenuated the pain sensitivity in different models[23,39-43]. However, we cannot rule out that other systems may also be affected by EA treatment. The net result of regulatory changes in cell signaling proteins induced by EA, however, is to attenuate visceral hypersensitivity.
The processing of pain information occurs at central (spinal and supraspinal) and peripheral sites, and thus modification of pain levels can be achieved through interventions at multiple sites. Although the exact locations where EA modifies pain are not clearly identified, EA is thought to activate the ascending sensory pathways such as spinal dorsal horn and thalamus, or the descending pain inhibitory mechanisms, such as opioid, adrenergic and serotonergic pathways. Most of previous studies have resolved only the central mechanism of EA analgesia, and only a few reports have investigated the peripheral mechanism of EA effect. Somatic inputs (i.e., EA-activated input) and noxious visceral signals (i.e., CRD) might converge and interact in the neurons at spinal dorsal horn level. Their interactions were manifested as that when the cutaneous stimuli were applied, the neuronal response to CRD was reduced in most cases[31]. Kim et al[28] showed that EA suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism, thus indicating EA modulation of pain through a central, but not peripheral, mechanism. The anti-nociceptive effect of EA at ST-36 was abolished by pretreatment with NLX but not by m-NLX by observation of mean arterial blood pressure in response to rectal distension in dogs[30]. These results suggest that EA at ST-36 may reduce visceral pain via central opioid pathway. In this study, we demonstrated that EA-mediated analgesia was completely blocked by m-NLX. m-NLX is unable to cross the blood-brain barrier and blocks only peripheral opioid receptors[30,44], thus indicating that EA analgesia is likely mediated by peripheral opioid receptors. Recent studies showed that peripheral opioid receptors were activated by EA at the inflammatory site on the CFA model[45]. NMDA receptor expression in primary sensory neurons is inhibited by EA[46], suggesting the peripheral effect of EA analgesia. Again, this discrepancy points out the fact that EA-mediated analgesic effect may be disease and/or organ-specific. Since previous studies have shown that stimulation frequencies of EA determine the types of opioid peptides released in the nervous system[24], further experiments are needed to explore which subtypes of opioid receptors are involved in mediating EA antihyperalgesic effects and to identify the location from which the opioids are released, e.g., the peripheral nerve terminals vs immunocytes, the mucosa/submucosa vs muscle layer of the colon.
In conclusion, together with our previous report[23], the present study demonstrated that EA treatment given at classical acupoint ST-36 produces an analgesic effect on visceral hyperalgesia in a rat model of IBS. The EA effect is mediated by endogenous opioid pathways, possibly at peripheral sites, thus providing scientific evidence for the treatment of visceral pain in functional gastrointestinal disorders using EA.
COMMENTS
Background
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterized by chronic visceral pain and bloating in association with altered bowel movements. Acupuncture is an ancient form of traditional Chinese medicine that has been used to treat diseases. Recently, acupuncture needles are stimulated by electricity at various frequencies, which is termed electroacupuncture (EA). However, the mechanism underlying EA-induced analgesia in visceral pain remains unknown.
Research frontiers
The present study used EA to treat visceral hypersensitivity of rats which was induced by a heterotypic intermittent stress (HIS) protocol. EA at acupoint Zu-San-Li significantly decreased visceral hypersensitivity of rats. EA-mediated analgesic effect was completely reversed by administration of naloxone (NLX) methiodide, a peripherally restricted opioid antagonist. The mechanism underlying the effect of EA on visceral hypersensitivity of rats induced by HIS which is a rat model of IBS appears to involve the mediation of peripheral opioid system.
Innovations and breakthroughs
This is the first study to indicate that EA treatment produces an analgesic effect on visceral hyperalgesia in a rat model of IBS induced by heterotypical intermittent stress. The EA effect is mediated by endogenous opioid pathways, possibly at peripheral sites.
Applications
The present study demonstrated that EA treatment produces an analgesic effect on visceral hyperalgesia in a rat model of IBS. The EA effect is mediated by endogenous opioid pathways, possibly at peripheral sites, thus providing scientific evidence for the treatment of visceral pain in functional gastrointestinal disorders using EA.
Terminology
IBS is a common gastrointestinal disorder characterized by chronic visceral pain and bloating in association with altered bowel movements. EA, acupuncture needles are stimulated by electricity at various frequencies (1-100 Hz); this method is developed from acupuncture, an ancient form of traditional Chinese medicine. NLX methiodide, a selectively peripherally acting opioid receptor antagonist.
Peer review
This paper describes positive effects of EA on responses to bowel extension in stressed rats. It is reported that EA diminished the number of pellets produced, the subjectively scored abdominal reflexes and the power of the electromyography of the abdominal muscles in response to colon extension, in stressed rats compared to control rats. Moreover, the effect of EA was antagonized by both a central and peripheral acting opiate antagonist. The effects presented are significant, and the antagonizing effect with NLX was convincing. The results presented are important for clinicians and for the fundamental scientific community as well.
Footnotes
Supported by An NIH grant, No. AT005158, to Xu GY; National Natural Science Foundation of China, No. 81070884; a grant from Jiangsu Province, China, No. SR21500111
Peer reviewer: Yvette Taché, PhD, Digestive Diseases Research Center and Center for Neurovisceral Sciences and Women’s Health, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, University of California, Los Angeles and VA Greater Los Angeles Healthcare System, 11301 Wilshire Boulevard, CURE Building 115, Room 117, Los Angeles, CA 90073, United States
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
References
- 1.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- 3.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 4.Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642–649. doi: 10.1016/s1471-4892(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831–841. doi: 10.1111/j.1365-2036.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T. Acupuncture for functional gastrointestinal disorders. J Gastroenterol. 2006;41:408–417. doi: 10.1007/s00535-006-1773-6. [DOI] [PubMed] [Google Scholar]
- 9.Schneider A, Streitberger K, Joos S. Acupuncture treatment in gastrointestinal diseases: a systematic review. World J Gastroenterol. 2007;13:3417–3424. doi: 10.3748/wjg.v13.i25.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J, Carr I, Mayberry JF. The role of acupuncture in the treatment of irritable bowel syndrome: a pilot study. Hepatogastroenterology. 1997;44:1328–1330. [PubMed] [Google Scholar]
- 11.Fukazawa Y, Maeda T, Kishioka S. The pharmacological mechanisms of electroacupuncture. Curr Opin Investig Drugs. 2009;10:62–69. [PubMed] [Google Scholar]
- 12.Xing J, Larive B, Mekhail N, Soffer E. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: a pilot study. Altern Ther Health Med. 2004;10:38–42. [PubMed] [Google Scholar]
- 13.Xiao WB, Liu YL. Rectal hypersensitivity reduced by acupoint TENS in patients with diarrhea-predominant irritable bowel syndrome: a pilot study. Dig Dis Sci. 2004;49:312–319. doi: 10.1023/b:ddas.0000017458.55517.33. [DOI] [PubMed] [Google Scholar]
- 14.Schneider A, Enck P, Streitberger K, Weiland C, Bagheri S, Witte S, Friederich HC, Herzog W, Zipfel S. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649–654. doi: 10.1136/gut.2005.074518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Weiland C, Enck P, Joos S, Streitberger K, Maser-Gluth C, Zipfel S, Bagheri S, Herzog W, Friederich HC. Neuroendocrinological effects of acupuncture treatment in patients with irritable bowel syndrome. Complement Ther Med. 2007;15:255–263. doi: 10.1016/j.ctim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds JA, Bland JM, MacPherson H. Acupuncture for irritable bowel syndrome an exploratory randomised controlled trial. Acupunct Med. 2008;26:8–16. doi: 10.1136/aim.26.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304.e3. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 20.Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G285–G292. doi: 10.1152/ajpgi.00068.2005. [DOI] [PubMed] [Google Scholar]
- 21.Imai K, Ariga H, Chen C, Mantyh C, Pappas TN, Takahashi T. Effects of electroacupuncture on gastric motility and heart rate variability in conscious rats. Auton Neurosci. 2008;138:91–98. doi: 10.1016/j.autneu.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Cui KM, Li WM, Gao X, Chung K, Chung JM, Wu GC. Electro-acupuncture relieves chronic visceral hyperalgesia in rats. Neurosci Lett. 2005;376:20–23. doi: 10.1016/j.neulet.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Xu GY, Winston JH, Chen JD. Electroacupuncture attenuates visceral hyperalgesia and inhibits the enhanced excitability of colon specific sensory neurons in a rat model of irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:1302–e125. doi: 10.1111/j.1365-2982.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 24.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Cao H, Han M, Li TT, Zhao ZQ, Zhang YQ. Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res Bull. 2008;75:83–93. doi: 10.1016/j.brainresbull.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Kim HN, Park JH, Kim SK, Sun B, Koo S, Choi SM, Bae H, Min BI. Electroacupuncture potentiates the antiallodynic effect of intrathecal neostigmine in a rat model of neuropathic pain. J Physiol Sci. 2008;58:357–360. doi: 10.2170/physiolsci.SC008308. [DOI] [PubMed] [Google Scholar]
- 27.Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain. 2009;145:332–340. doi: 10.1016/j.pain.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–436. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Iwa M, Strickland C, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture reduces rectal distension-induced blood pressure changes in conscious dogs. Dig Dis Sci. 2005;50:1264–1270. doi: 10.1007/s10620-005-2770-y. [DOI] [PubMed] [Google Scholar]
- 31.Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. Acupuncture inhibition on neuronal activity of spinal dorsal horn induced by noxious colorectal distention in rat. World J Gastroenterol. 2005;11:1011–1017. doi: 10.3748/wjg.v11.i7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han JS, Terenius L. Neurochemical basis of acupuncture analgesia. Annu Rev Pharmacol Toxicol. 1982;22:193–220. doi: 10.1146/annurev.pa.22.040182.001205. [DOI] [PubMed] [Google Scholar]
- 33.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms of actions. Am J Chin Med. 2008;36:635–645. doi: 10.1142/S0192415X08006107. [DOI] [PubMed] [Google Scholar]
- 36.Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain. 2008;135:11–19. doi: 10.1016/j.pain.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Kim HY, Chung K, Chung JM. Electroacupuncture reduces the evoked responses of the spinal dorsal horn neurons in ankle-sprained rats. J Neurophysiol. 2011;105:2050–2057. doi: 10.1152/jn.00853.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sci. 2008;83:356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Xu GY, Duanmu ZX, Yin QZ. [Reversal of the inhibitory effect of electroacupuncture on the nociceptive response of neurons in parafascicular nucleus by naloxone and atropine in acute arthritic rats] Shengli Xuebao. 1994;46:427–434. [PubMed] [Google Scholar]
- 40.Fu X, Wang YQ, Wang J, Yu J, Wu GC. Changes in expression of nociceptin/orphanin FQ and its receptor in spinal dorsal horn during electroacupuncture treatment for peripheral inflammatory pain in rats. Peptides. 2007;28:1220–1228. doi: 10.1016/j.peptides.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Zhang RX, Li A, Liu B, Wang L, Xin J, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12:870–878. doi: 10.1016/j.ejpain.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taguchi T, Taguchi R. Effect of varying frequency and duration of electroacupuncture stimulation on carrageenan-induced hyperalgesia. Acupunct Med. 2007;25:80–86. doi: 10.1136/aim.25.3.80. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Meng X, Li A, Xin J, Berman BM, Lao L, Tan M, Ren K, Zhang RX. Acupuncture alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neurochem Res. 2011;36:2104–2110. doi: 10.1007/s11064-011-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber TP, Meissner A, Stypmann J, Hartlage MG, Van Aken H, Rolf N. Naloxone improves splanchnic perfusion in conscious dogs through effects on the central nervous system. Anesthesiology. 2002;96:438–441. doi: 10.1097/00000542-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 45.Zhang GG, Yu C, Lee W, Lao L, Ren K, Berman BM. Involvement of peripheral opioid mechanisms in electroacupuncture analgesia. Explore (NY) 2005;1:365–371. doi: 10.1016/j.explore.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Zhang Y, Dai J, Yang J, Gang S. Electroacupuncture (EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res. 2006;1120:46–53. doi: 10.1016/j.brainres.2006.08.077. [DOI] [PubMed] [Google Scholar]