Abstract
AIM: To explore the prognostic value in the monitoring of treatment efficacy of serial α-fetoprotein (AFP) in hepatocellular carcinoma (HCC) patients.
METHODS: We searched MEDLINE, EMBASE and COCHRANE LIBRARY through April 21, 2012, to find qualifying articles. Our overall search strategy included terms for HCC, AFP, treatment response, and prognosis. Literature was limited to English-language, human studies. Studies reporting cumulative survival rates were summarized qualitatively. For the prognostic meta-analysis, we undertook a series of meta-analyses that summarised the Cox proportional hazard ratios (HRs) by assuming a random effects model. With regards to the correlation of AFP change with radiologic response, the categorical dichotomous variables were assessed using Poisson relative risks (RRs), which were incorporated into the random effects model meta-analysis of accuracy prediction. Between-study heterogeneity was estimated by use of the I² statistic. Publication bias was evaluated using the Begg funnel plot and Egger plot. Sensitivity analyses were conducted first by separating systemic treatment estimates from locoregional therapy estimates, evaluating different AFP response cut-off point effects, and exploring the impact of different study sizes.
RESULTS: Of 142 titles identified in our original search, 11 articles (12 clinical studies) met our criteria. Six studies investigated outcome in a total of 464 cases who underwent systemic treatment, and six studies investigated outcome in a total of 510 patients who received locoregional therapy. A random-effects model meta-analysis showed that AFP response was associated with an mortality HR of 0.55 (95%CI, 0.47-0.65) across HCC in overall survival (OS) and 0.50 (95%CI, 0.38-0.65) in progression-free survival. Restricting analysis to the six eligible analyses of systemic treatment, the pooled HRs were 0.64 (95%CI, 0.53-0.77) for OS. Limiting analysis to the six analyses of locoregional therapy, the pooled HRs for OS was 0.39 (95%CI, 0.29-0.53). We showed a larger pooled HR in the 50% definition studies (HR, 0.67, 95%CI, 0.55-0.83) compared with that from the 20% definition studies (HR, 0.41, 95%CI, 0.32-0.53). Restricting analysis to the four studies including over 100 patients individually, the pooled HR was 0.65 (95%CI, 0.54-0.79), with a pooled HR for OS of 0.35 (95%CI, 0.23-0.46) in the studies of less than 100 patients. As to radiological imaging, 43.1% (155/360) of the patients in the AFP response group presented with a radiological overall response, while the response rate decreased to 11.5% (36/313) in the patients from the AFP nonresponse group. The RR of having no overall response was significantly lower in the AFP response group than the AFP nonresponse group (RR, 0.67; 95%CI, 0.61-0.75). In terms of disease control rate, 86.9% (287/330) in the AFP response group and 51.0% (153/300) in the AFP nonresponse group showed successful disease control, respectively. The RR of disease control failure, similarly, was significantly lower in the AFP response group (RR, 0.37; 95%CI, 0.23-0.58). But these findings could be overestimates because of publication and reporting bias.
CONCLUSION: HCC patients presenting with an AFP response are at decreased risk of mortality. In addition, patients with an AFP response also present with a higher overall response rate and disease control rate.
Keywords: Liver cancer, α-fetoprotein, Response, Prognosis, Monitoring
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and as a result of the spread of hepatitis C virus and hepatitis B virus infection during the past century, its incidence will further increase in the future in both Asia and in western countries[1,2]. Transarterial therapy, systemic agents, and radiofrequency ablation remain as mainstays of treatment in advanced HCC[3]. Conventionally, the treatment response of HCC to systemic therapy or other nonsurgical treatment modalities is assessed by radiologic imaging using conventional criteria, such as World Health Organization (WHO) criteria or Response Evaluation Criteria in Solid Tumors[4-6]. However, despite successful clinical correlations for other solid tumors, these radiological response-based criteria have been criticized for not adequately reflecting treatment response and tumor viability for HCC. It has constantly been observed that a small subset of HCC patients could derive benefits from treatment despite the absence of radiologic response[7-9]. Thus, the sole use of radiologic criteria has underestimated the efficacy of novel treatment in early clinical trials and the search for better, alternative ways of assessing treatment response continues to be important.
While imaging is being explored, widespread efforts have been made to identify serological markers that predict survival and response to treatment[10], and among which, serum α-fetoprotein (AFP) (a glycoprotein that is expressed by HCC and secreted into the serum of approximately 70% of patients with HCC) have been widely studied[11,12]. In the half century after AFP was first described, it was extensively studied as a screening and diagnostic tool for HCC[13-15]. Although there have been some early observational studies that have suggested that AFP trend might be useful in assessing treatment response, until recently, there has been no clinical study to validate the significance of serial AFP monitoring in association with the treatment response of HCC[16,17]. In the past few years, several studies have investigated the role of AFP response to treatment in HCC, which mainly focused on the overall survival (OS) and progression-free survival (PFS), and validated its correlation with radiological response. However, estimates of the prognostic value of the AFP response between studies differed wildly. The aim of this analysis is to review published studies that investigated the correlation between AFP response and prognosis in HCC, and to use standard meta-analysis techniques to summarise the accuracy of AFP response in prediction of survival in HCC patients.
MATERIALS AND METHODS
Study identification
We searched MEDLINE, EMBASE, and COCHRANE LIBRARY from inception to April 21, 2012, for articles evaluating the AFP level and response to treatment on the prognostic outcome in HCC patients. Our overall search strategy included terms for HCC (e.g., hepatocellular carcinoma, liver carcinoma, liver cancer, liver malignant neoplasm), AFP (e.g., α-fetoprotein, alpha-fetoprotein, AFP), treatment response (e.g., change, decline, response), and prognosis (e.g., mortality, survival, recurrence). Literature was limited to English-language, human studies. We also searched references of included articles. Only published studies in peer-review journals were included. Data from review articles, case reports, abstracts, and letters were not included.
Study eligibility and selection
Studies were eligible if survival was analyzed in HCC cases stratified by AFP response or not. To be included in our meta-analysis, articles had to meet both of the following criteria: they reported a risk estimate [e.g., hazard ratio (HR) or relative risk (RR) relating AFP response to subsequent death using survival analysis regression models], and they reported an estimate of precision, such as a standard error or 95%CI. We also included articles that failed to report precision directly but from which we could reconstruct an estimate of precision using P values and other study data[18]. Correlation of AFP change with radiologic response was desirable, but it was not a must (Table 1).
Table 1.
Main characteristics and results of eligible studies
| Author | Year | Country | OS | PFS | Overall response rate | Disease control rate | ||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Chen et al[21] | 2005 | China | S | S | N/A | N/A | N/A | N/A |
| Chan et al[20] | 2009 | China | S | S | N/A | N/A | P | P |
| Riaz et al[27] | 2009 | United States | S | S | N/A | N/A | P | P |
| Vora et al[29] | 2009 | United States | N/A | NS | N/A | NS | P | P |
| Shao et al[28] | 2010 | China | N/A | S | N/A | S | P | P |
| Kim et al[23] | 2011 | South Korea | S | S | S | S | P | P |
| Yau et al[30] | 2011 | China | S | S | S | S | N/A | N/A |
| Kao et al[22] | 2012 | China | N/A | S | N/A | N/A | N/A | N/A |
| Lee et al[24] | 2012/H | South Korea | S | S | S | NS | P | P |
| Lee et al[24] | 2012/C | South Korea | S | S | S | NS | P | P |
| Memon et al[25] | 2012 | United States | S | S | N/A | N/A | C | N/A |
| Personeni et al[26] | 2012 | Italy | N/A | S | N/A | N/A | N/A | N/A |
2012/H: Hepatic arterial infusional chemotherapy study; 2012/C: Concurrent chemoradiation therapy study; S: Significant relationship between α-fetoprotein (AFP) response and survival; NS: No significant relationship between AFP response and survival; N/A: Not available or not applicable; P: Directly provided by the article; C: Need to be calculated by the data provided by the article; OS: Overall survival; PFS: Progression-free survival.
Data synthesis and statistical analysis
To avoid duplicate data, we identified articles that included the same cohort of patients by reviewing inter-study similarity in the country in which the study was done, investigators in the study, source of patients, recruitment period, and inclusion criteria. Early studies published as a series of articles from the same author or institution that contained significant overlap of patient data were excluded and only the most recently published study containing the most up-to-date data was included. If several estimates were reported in the same article, we chose the most fully adjusted estimate (i.e., multivariate regression was selected over univariate regression, which was selected over unadjusted Kaplan-Meier analysis).
For the prognostic meta-analysis, we undertook a series of meta-analysis that summarised the HRs by assuming a random effects model. With regards to the correlation of AFP change with radiologic response, the categorical dichotomous variables were assessed using the RRs, which were incorporated into the random effects model meta-analysis of accuracy prediction. Between-study heterogeneity was estimated by use of the I² statistic; typically, values above 50% are deemed to suggest large between-study heterogeneity, values below 50% are deemed to represent low heterogeneity. Publication bias was evaluated using the Begg funnel plot and Egger plot. However, these estimates can have large uncertainty, especially in the presence of few trials, and should be interpreted with caution[19].
Sensitivity analysis were conducted first by separating systemic treatment estimates from locoregional therapy estimates. Second, to evaluate AFP response cut-off point effects, we calculated estimates for studies whose AFP response was defined as 20% decline of the initial level vs those with the AFP response definition of 50% decline. Third, to explore the impact of study size, we conducted sensitivity analyses by components which included over 100 patients. Finally, we evaluated the influence of each study on the overall estimate by calculating a pooled HR, omitting each estimate 1 at a time. All analysis were conducted using Stata 12 (StataCorp, College Station, Texas).
RESULTS
Eligible studies
Thirteen studies were identified that provided survival data stratified by AFP response or not[20-32]. Two studies were excluded from further analysis: one was excluded because it did not provide a definite AFP response cut-off point, and extraction of survival data for the AFP response cases and AFP nonresponse cases was not possible, and another article, which was a letter to editor, was also excluded[31,32]. Hence a total of 11 articles remained eligible for pooling risk estimates, reporting on 974 patients, of whom 463 had a positive AFP response. The main characteristics and results of eligible studies evaluating AFP response in HCC patients were summarised in Table 1.
Description of studies
Of the 11 eligible articles, all were based on retrospective analysis of survival data with quite heterogeneity. Sample sizes ranged from 42 to 149 patients with a median of 62 patients. Six eligible articles assessed survival in the systemic treatment setting, with data from a total of 464 patients available for pooling (sample size range, 42 to 107 patients)[20,21,26,28-30]. In the locoregional therapy setting, five articles were eligible, including 6 individual clinical studies, resulting in a total of 510 patients available for pooling (sample size range, 51 to 149 patients)[22-25,27]. In one of these studies, patients were treated in the locoregional setting either by hepatic artery infusion chemotherapy, or concurrent chemoradiation therapy[24]. In this study, it was possible to assess estimators of survival in the two patient cohorts separately, and each cohort was therefore considered separately for pooling purposes. In the systemic treatment studies, all patients received systemic regimens (sorafenib, thalidomide, doxorubicin, etc.). In the locoregional therapy studies, chemotherapy and radiotherapy were used. Of all the included analysis, five defined AFP response as a 50% decline from the initial level[21,23,25,27,29], and another seven proposed a definition of 20% decline[20,22,24,26,28,30]. Across the included studies that reported the number of participants with AFP response, the overall prevalence of AFP response was 47% (range, 17%-79%), with a median sample size of 38 (range, 9 to 101). Follow-up time varied widely across studies, with a median of 35.3 mo (range, 4 to 100 mo) (Table 2).
Table 2.
Characteristics and demographic information of eligible studies
| Author | Year | Treatment | HCC stage | AFP change level (%) | No. of patients | ||
| Study size (n = 974) | M/F (776/198) | AFP response (n = 463) | |||||
| Chen et al[21] | 2005 | Sys/thalidomide | I-III | 50 | 42 | 33/9 | 10 (23.8%) |
| Chan et al[20] | 2009 | Sys/doxorubicin or PIAF | I-III | 20 | 117 | 104/13 | 47 (40.2%) |
| Riaz et al[27] | 2009 | Loc/chemoembolization or radioembolization | I-IV | 50 | 125 | 91/34 | 81 (64.8%) |
| Vora et al[29] | 2009 | Sys/five systemic regimens | I-III | 50 | 107 | 78/29 | 18 (16.8%) |
| Shao et al[28] | 2010 | Sys/Antiantiogenic agents | I-II | 20 | 72 | 65/7 | 12 (16.7%) |
| Kim et al[23] | 2011 | Loc/chemoradiotherapy | III-IV | 50 | 149 | 127/22 | 101 (67.8%) |
| Yau et al[30] | 2011 | Sys/sorafenib | II-IV | 20 | 41 | 36/5 | 9 (21.9%) |
| Kao et al[22] | 2012 | Loc/radiofrequency ablation | I-III | 20 | 58 | 34/24 | 46 (79.3%) |
| Lee et al[24] | 2012/H | Loc/HAIC | III-IV | 20 | 60 | 49/11 | 25 (41.7%) |
| Lee et al[24] | 2012/C | Loc/CCRT | III-IV | 20 | 67 | 55/12 | 52 (77.6%) |
| Memon et al[25] | 2012 | Loc/transarterialtherapy | I-III | 50 | 51 | 30/21 | 30 (58.8%) |
| Personeni et al[26] | 2012 | Sys/sorafenib | I-III | 20 | 85 | 74/11 | 32 (37.6%) |
Sys: System treatment; Loc: Locoregional therapy; HAIC: Hepatic artery infusion chemotherapy; CCRT: Concurrent chemoradiation therapy. 2012/H: HAIC study of Lee et al; 2012/C: CCRT study of Lee et al; M/F: Male/female. HCC: Hepatocellular carcinoma; AFP: α-fetoprotein.
With regards to radiologic response, the evaluation criteria were based on the WHO criteria. Complete response (CR) was defined as the complete disappearance of all known lesions on radiologic grounds. Partial response (PR) was defined as a decrease of 50% or more in the product of two perpendicular diameters of the largest tumor nodule for at least 4 wk without the appearance of new lesions or progression of lesions. Stable disease (SD) was defined as a less than 50% decrease or not more than a 25% increase in the product of two perpendicular diameters of the largest tumor nodule. Progressive disease was defined as more than a 25% increase in the product of two perpendicular diameters for the largest tumor nodule or one of the measurable lesions or as the appearance of new lesions. In view of these indices, the overall response rate referred to the total rate of CR and PR, whereas the disease control rate was defined as the total rate of CR, PR and SD.
Relationship between AFP response and OS in HCC
Of the 12 clinical studies eligible for pooling of OS data, 9 provided estimates of the HR associated with AFP response and its 95%CI[20,21,23-26,28,30]. In the remaining studies, these data points were calculated from data presented in the articles[22,27,29]. Table 3 details the results of survival analyses performed ineligible studies[18], and Figure 1 displays a plot of HRs and associated 95%CIs for OS from each study. AFP response was associated with a decrease in all-cause mortality following HCC, with a pooled HR for OS across all studies of 0.55 (95%CI, 0.47-0.65), and with significant evidence of heterogeneity between the contributing studies (I2 = 65%, P = 0.001). Evidence of significant publication bias was observed according to the funnel plot of lnHRs, with smaller studies showing significant effects[18].
Table 3.
Results of survival analyses related to α-fetoprotein response
| Author | Year | Overall survival | Progression-free survival | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Chen et al[21] | 2005 | 0.242 | 0.10-0.61 | 0.003 | - | - | - |
| Chan et al[20] | 2009 | 0.412 | 0.27-0.63 | 0.0001 | - | - | - |
| Riaz et al[27] | 2009 | 0.3712 | 0.13-1.011 | 0.0002 | - | - | - |
| Vora et al[29] | 2009 | 0.952 | 0.73-1.231 | 0.88 | 0.482 | 0.15-1.451 | 0.09 |
| Shao et al[28] | 2010 | 0.362 | 0.15-0.83 | 0.017 | 0.312 | 0.14-0.67 | 0.003 |
| Kim et al[23] | 2011 | 0.432 | 0.29-0.65 | 0.001 | 0.482 | 0.33-0.70 | 0.001 |
| Yau et al[30] | 2011 | 0.32 | 0.09-1.02 | 0.05 | 0.312 | 0.13-0.76 | 0.01 |
| Kao et al[22] | 2012 | 0.1812 | 0.02-1.67 | 0.023 | - | - | - |
| Lee et al[24] | 2012/H | 0.432 | 0.23-0.81 | 0.009 | 0.672 | 0.35-1.27 | 0.22 |
| Lee et al[24] | 2012/C | 0.332 | 0.15-0.75 | 0.008 | 0.972 | 0.42-2.25 | 0.97 |
| Memon et al[25] | 2012 | 0.142 | 0.02-0.83 | 0.03 | - | - | - |
| Personeni et al[26] | 2012 | 0.522 | 0.31-0.85 | 0.009 | - | - | - |
Calculated result from data presented in article;
Multivariate result; -: Not performed; 2012/H: Hepatic arterial infusional chemotherapy study; 2012/C: Concurrent chemoradiation therapy study; HR: Hazard ratio; AFP: α-fetoprotein.
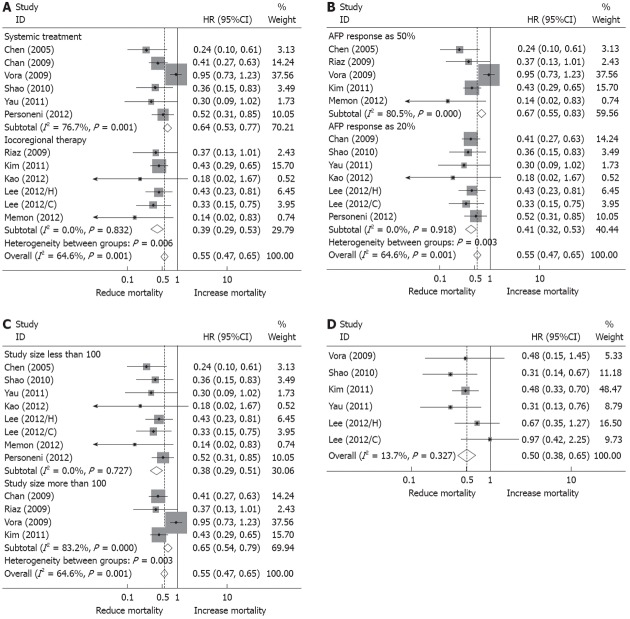
Figure 1.
Forest plots representing hazard ratios of overall survival and progression free survival in hepatocellular carcinoma patients associated with α-fetoprotein response. A: Subgroup analysis according to systemic treatment and locoregional therapy; B: Subgroup analysis according to α-fetoprotein (AFP) response definition of 20% decline of its initial level and 50% decline; C: Subgroup analysis according to study size larger than 100 patients and less than 100 patients; D: Hazard ratios (HRs) of progression free survival in hepatocellular carcinoma patients associated with AFP response.
Because the investigators’ approaches to adjustment for confounding factors varied widely by study and type of treatment, we conducted a sensitivity analysis to confirm robustness. Restricting analysis to the six eligible analysis in which patients received systemic treatment, the pooled HR was 0.64 (95%CI, 0.53-0.77) (I2 = 77%, P = 0.001) for OS[20,21,26,28-30]. Limiting analysis to the six analysis in which patients under went locoregional therapy, the pooled HR for OS was 0.39 (95%CI, 0.29-0.53) (I2 = 0%, P = 0.83)[22-25,27] (Figure 1A).
To assess the effect of cut-off point of AFP response, HRs were pooled from particular studies that defined AFP response as either 50% decline of initial level[21,23,25,27,29] or 20% decline, respectively[20,22,24,26,28,30]. This demonstrated a larger pooled HR in the 50% definition studies (HR, 0.67, 95%CI, 0.55-0.83) compared with that from the 20% definition studies (HR, 0.41, 95%CI, 0.32-0.53). In the 50% group, there was significant evidence of study heterogeneity (I2 = 81%, P < 0.001) (Figure 1B). This observed increased HR of mortality in the 50% definition studies contradicts our supposition that greater AFP response in the patient would predict better overall survival.
Restricting analysis to the four studies including over 100 patients individually, the pooled HR was 0.65 (95%CI, 0.54-0.79), again with evidence of study heterogeneity (I2 = 83%, P = 0.003)[21,22,24-26,28,30]. The remaining eight studies included less than 100 patients individually, and the pooled HR for OS was 0.35 (95%CI, 0.23-0.46), with no evidence of heterogeneity (I2 = 0, P < 0.001)[20,23,27,29] (Figure 1C).
Finally, we excluded individual study estimates 1 at a time to examine the influence of each study on the overall HR. It turned out that heterogeneity was mainly from the study by the Vora et al[29] study, that only the omission of this study appreciably changed the pooled HR, with the HR decreasing to 0.40 (95%CI, 0.33-0.49) and eliminating the heterogeneity across all studies (I2 = 0, P = 0.91).
Relationship between AFP response and PFS in HCC
Only 6 of the 12 eligible analyses presented data evaluable for assessment of PFS, and the pooled HR was 0.50 (95%CI, 0.38-0.65), with no obvious hetereogeneity (I2 = 13%, P = 0.33)[23,24,28-30]. This result should, however, be interpreted with caution because of the small number of contributing studies (Figure 1D).
AFP response in association with radiological response
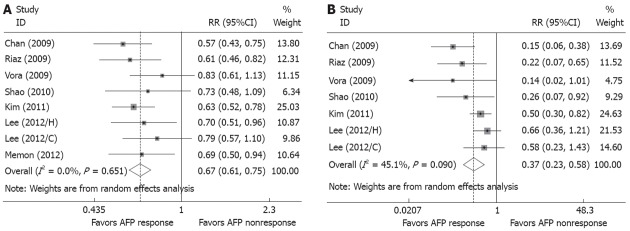
Of the 12 eligible clinical studies, 8 provided data evaluable for assessment of radiological overall response rate[20,23-25,27-29] and 7 reported on disease control rate[20,23,24,27-29]. A radiological response summary is presented in Table 4. Overall, 43.1% (155/360) of the patients in the AFP response group presented the radi logical overall response, while the response rate declined to 11.5% (36/313) in the patients from the AFP nonresponse group. The RR of having no overall response rate (Figure 2A) was significantly lower in the AFP response group than the AFP nonresponse group (RR, 0.67; 95%CI, 0.61-0.75) with no heterogeneity (P = 0.65, I2 = 0%). In terms of disease control rate, 86.9% (287/330) in the AFP response group and 51.0% (153/300) in the AFP nonresponse group showed successful disease control, respectively. The RR of disease control failure, similarly, was significantly lower in the AFP response group (RR, 0.37; 95%CI, 0.23-0.58) (Figure 2B). No significant heterogeneity of studies was found on these parameters (P = 0.09, I2 = 45%).
Table 4.
Results of overall response rate and disease control rate analysis
| Author | Year | Overall response rate (%) | Disease control rate (%) | ||||
| AFP responders | AFP nonresponders | RR1 | AFP responders | AFP nonresponders | RR1 | ||
| Chen et al[21] | 2005 | - | - | - | - | - | - |
| Chan et al[20] | 2009 | 46.8 | 7.1 | 0.57 | 91.5 | 41.4 | 0.15 |
| Riaz et al[27] | 2009 | 43.5 | 31.6 | 0.61 | 91.3 | 68.4 | 0.22 |
| Vora et al[29] | 2009 | 25.0 | 10.0 | 0.83 | 93.8 | 56.7 | 0.14 |
| Shao et al[28] | 2010 | 33.0 | 8.0 | 0.73 | 83.0 | 35.0 | 0.26 |
| Kim et al[23] | 2011 | 44.5 | 12.5 | 0.63 | 87.1 | 58.3 | 0.50 |
| Yau et al[30] | 2011 | - | - | - | - | - | - |
| Kao et al[22] | 2012 | - | - | - | - | - | - |
| Lee et al[24] | 2012/H | 36.0 | 8.6 | 0.70 | 64.0 | 45.7 | 0.66 |
| Lee et al[24] | 2012/C | 36.5 | 20.0 | 0.79 | 80.8 | 66.7 | 0.58 |
| Memon et al[25] | 2012 | 36.7 | 7.7 | 0.69 | - | - | - |
| Personeni et al[26] | 2012 | - | - | - | - | - | - |
Calculated result from data presented in article; 2012/H: Hepatic arterial infusional chemotherapy study; 2012/C: Concurrent chemoradiation therapy study; -: Not performed; AFP: α-fetoprotein; RR: Relative risk.
Figure 2.
Forest plots representing the correlation between α-fetoprotein response and radiological response. A: Risks of no radiological response; B: Risks of disease control failure. AFP: α-fetoprotein; RR: Relative risk.
DISCUSSION
Treatment response in HCC patients has been heterogeneous, with some patients showing impressive treatment effects, but others showing limited or no evidence of response[33,34]. The hypothesis that AFP response to treatment is a determinant of prognosis in HCC is an attractive mechanism for explaining any inter-individual variation in clinical outcome[35]. For patients with elevated AFP at baseline, the AFP trend was shown to be static or rising during the course of the disease, and a number of patients with undetectable AFP at baseline were found to have detectable and rising trends of AFP value upon disease progression[17,36]. For patients who underwent partial hepatectomy, the AFP level fell rapidly and remarkably after removal of tumors, but this rose at the time of recurrence[37,38]. These findings suggested that AFP response could be potentially useful in predicting survival and the efficacy of treatment.
The present systematic review of the literature and meta-analysis was done to assess the impact of AFP response on HCC prognosis. Twelve eligible studies were identified that investigated the relationship between AFP response and prognosis in HCC. Strengths of the study included a comprehensive, systematic review of the literature by a multidisciplinary team including specialists in cancer, hepatology, and epidemiologic methods, thereby avoiding selection bias on the basis of study quality.
Using these studies, pooled estimates of outcome of HCCs expressing AFP were derived. Although our results showed that estimates of the significance of AFP response varied substantially between studies, these results support the notion that in the HCC patients who underwent systemic treatment and locoregional therapy, AFP response is predictive for better overall survival, with a pooled HR of 0.55 (95%CI, 0.47-0.65). In the HCC patients, AFP response had prognostic significance, whether it was defined by 50% decline of its initial level or 20% decline. This latter result, however, contradicted our supposition that greater AFP response should be associated with better survival, which was probably due to the individual differences from different clinical trials, and the small number of contributing studies. AFP response also seems to predict better PFS, with a pooled HR of 0.50 (95%CI, 0.38-0.65). However, this result should be interpreted with caution considering the small number of contributing studies. Our findings suggest that AFP response has a strong correlation with radiological response. When compared with the AFP nonresponse group, there were significant trends toward higher overall response rates (RR, 0.67; 95%CI, 0.61-0.75) and disease control rates (RR, 0.37; 95%CI, 0.23-0.58) in the AFP response group.
Although our results showed that HCCs expressing AFP response to treatment seem to be associated with a better prognosis, one caveat to this conclusion is publication bias, which is a major concern in all forms of meta-analysis, as smaller studies showing no statistically significant effects are more likely to remain unpublished[39,40]. Indeed, it is not unusual for small, early studies to report a positive relationship or large effect that subsequent much larger studies fail to replicate. In the present study, there was some evidence for publication bias in the AFP response, and the risks calculated in our meta-analysis could be overestimates as a result of publication and reporting bias[41]. Furthermore, advanced tumors were over-represented in the studies, given that they constituted approximately 64% of all HCC.
Heterogeneity between studies may represent a further potential source of bias in our analysis. The importance of heterogeneity between studies on summary estimates of HRs was carefully assessed. Although no attempt was made in our meta-analysis to quality-score reports[42,43], it is clear that the design of some studies is not optimal. The larger study by Vora et al[29] showed less of an effect of AFP response on prognosis, probably due to the great heterogeneity produced by the HCC patients selected from the different clinical trials. Through sensitivity analysis, we found that this study was the main source of the heteregeneity in this meta-analysis, and after the exclusion of this study, the HR estimate decreased to 0.40 (95%CI, 0.33-0.49), as did heterogeneity (I2 = 0, P = 0.91).
Here we describe substantial inconsistency of results on the effectiveness of AFP response to predict survival of HCC. The main factors we identified as responsible for these findings are: long periods of patient recruitment, which leads to heterogeneity within a study because treatment of HCC has evolved over the course of the study; selection bias of patients because of different treatment clinical trials included, suggesting that AFP might be sensitive to some kinds of therapies but not to others; and the absence of a uniform definition of positive AFP response, leading to different results when using different cut-off points. Moreover, variability in the length of follow-up used to detect the events of interest also hampers comparability between studies since risk of survival is time-dependent. Inconsistency in the inclusion of clinical and pathological factors predicting survival mortality as covariables in multivariate analysis could be an additional contributing factor[41].
Notwithstanding this, the studies pooled in this meta-analysis provide data on 974 HCC cases and support the opinion that AFP response to treatment is a determinant of prognosis in HCC. However, multicenter prospective studies that are better designed, with assessors blinded to the clinical data, and homogeneous HCC cohorts analyzed prospectively, are required to unequivocally assess the precise prognostic effect of AFP response in the HCC. In addition, most studies have empirically defined a 50% drop or 20% drop as an AFP response, while it has to be validated critically in future prospective series.
ACKNOWLEDGMENTS
Ji-Chao Wei, Lei Zhou, Fan-Di Meng, Rui-Tao Wang, Zhi-Xin Wang, Jin-Yao Zhang, Jia Xu and Lin-Qiang Zhang from The First Affiliated Hospital of Xi’an Jiaotong University contributed a lot and gave many helpful suggestions for the design of this research.
COMMENTS
Background
Conventionally, the treatment response of hepatocellular carcinoma (HCC) to systemic therapy or other nonsurgical treatment modalities is assessed by radiologic imaging using conventional criteria, such as World Health Organization criteria or Response Evaluation Criteria in Solid Tumors. However, despite successful clinical correlations for other solid tumors, these radiological response-based criteria have been criticized for not adequately reflecting treatment response and tumor viability for HCC.
Research frontiers
In the half century after α-fetoprotein (AFP) was first described, it was extensively studied as a screening and diagnostic tool for HCC. Although there have been some early observational studies that suggested that AFP trend might be useful in assessing treatment response, until recently, there has been no clinical study to validate the significance of serial AFP monitoring in association with treatment response of HCC.
Innovations and breakthroughs
This is the first attempt to systemically review the prognostic value of AFP response world wide. A total of 11 articles and 974 cases of HCC patients were collected from the international literature and evaluated for clinical and biochemical features such as AFP response. This study results revealed that AFP response was associated with a decreased mortality across HCC in overall survival and progression-free survival, respectively. As to radiological imaging, when compared with the AFP nonresponse patients, there were significant trends toward higher overall response rates and disease control rates in the AFP response patients.
Applications
The study results suggest that the AFP is a potentially monitoring serum index and the AFP response to treatment could be used for monitoring treatment efficacy and determining the prognosis of HCC patients. In addition, the results demonstrate that AFP could also make up for the drawbacks of imaging and is able to aid radiological tools in the monitoring of prognosis and treatment.
Terminology
AFP, which is a glycoprotein that is secreted into the serum of approximately 70% of patients with HCC, has potential values in monitoring of prognosis and treatment for HCC. AFP response, that AFP level would reduce during treatment of HCC patients, was defined as 20% or 50% decline of the initial level by most studies.
Peer review
The authors provide results of a meta-analysis, with this manuscript, on level changes of circulating AFP in HCC patients receiving local and systemic therapies, and gave some reasonable comments and proposals for further validation of the results from literature. The analysis is of importance for prognosis assessment and evaluation of different therapies. A multi-center study is needed for further confirmation.
Footnotes
Supported by National Natural Science Foundation of China, No. 30872482 and No. 81072051
Peer reviewer: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Jia F L- Editor O’Neill M E- Editor Li JY
References
- 1.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2010;26:189–195. doi: 10.1097/MOG.0b013e3283383ca5. [DOI] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol. 2001;74:983–986. doi: 10.1259/bjr.74.887.740983. [DOI] [PubMed] [Google Scholar]
- 6.Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, Park YS, Im YH, Kang WK, Lee MH, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–537. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459–462. doi: 10.1136/gut.51.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 10.Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272–1282. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- 11.Kew M. Alpha-fetoprotein in primary liver cancer and other diseases. Gut. 1974;15:814–821. doi: 10.1136/gut.15.10.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Conor GT, Tatarinov YS, Abelev GI, Uriel J. A collaborative study for the evaluation of a serologic test for primary liver cancer. Cancer. 1970;25:1091–1098. doi: 10.1002/1097-0142(197005)25:5<1091::aid-cncr2820250514>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies? J Clin Gastroenterol. 2001;32:383–389. doi: 10.1097/00004836-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–115. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PJ, Williams R. Serum alpha-fetoprotein estimations and doubling time in hepatocellular carcinoma: influence of therapy and possible value in early detection. J Natl Cancer Inst. 1980;64:1329–1332. doi: 10.1093/jnci/64.6.1329. [DOI] [PubMed] [Google Scholar]
- 17.McIntire KR, Vogel CL, Princler GL, Patel IR. Serum alpha-fetoprotein as a biochemical marker for hepatocellular carcinoma. Cancer Res. 1972;32:1941–1946. [PubMed] [Google Scholar]
- 18.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, Lam KC, Chan AT, Mok TS, Yeo W. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 21.Chen LT, Liu TW, Chao Y, Shiah HS, Chang JY, Juang SH, Chen SC, Chuang TR, Chin YH, Whang-Peng J. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2005;22:217–226. doi: 10.1111/j.1365-2036.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 22.Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, Wu JC, Huo TI, Huang YH, Wu WC, Lin HC, et al. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy. Clin Radiol. 2012;67:429–436. doi: 10.1016/j.crad.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim BK, Ahn SH, Seong JS, Park JY, Kim do Y, Kim JK, Lee do Y, Lee KH, Han KH. Early α-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int. 2011;31:369–376. doi: 10.1111/j.1478-3231.2010.02368.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee MH, Kim SU, Kim do Y, Ahn SH, Choi EH, Lee KH, Lee do Y, Seong J, Han KH, Chon CY, et al. Early on-treatment predictions of clinical outcomes using alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:313–322. doi: 10.1111/j.1440-1746.2011.06867.x. [DOI] [PubMed] [Google Scholar]
- 25.Memon K, Kulik L, Lewandowski RJ, Wang E, Ryu RK, Riaz A, Nikolaidis P, Miller FH, Yaghmai V, Baker T, et al. Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol. 2012;56:1112–1120. doi: 10.1016/j.jhep.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 28.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 29.Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717–725. doi: 10.1634/theoncologist.2009-0038. [DOI] [PubMed] [Google Scholar]
- 30.Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan ST, Poon RT. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011;16:1270–1279. doi: 10.1634/theoncologist.2011-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LT, Shiah HS, Chao Y, Chang JY, Cheng LT, Whang-Peng J. Alpha-fetoprotein response in advanced hepatocellular carcinoma receiving cytostatic agent. J Clin Oncol. 2009;27:e271; author reply e272. doi: 10.1200/JCO.2009.23.8311. [DOI] [PubMed] [Google Scholar]
- 32.Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, Tamaki S, Kato T, Yasui Y, Hosokawa T, et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251–258. doi: 10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 34.Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]
- 35.Chan SL, Chan AT, Yeo W. Role of alpha-fetoprotein in hepatocellular carcinoma: prognostication, treatment monitoring or both? Future Oncol. 2009;5:889–899. doi: 10.2217/fon.09.64. [DOI] [PubMed] [Google Scholar]
- 36.McIntire KR, Vogel CL, Primack A, Waldmann TA, Kyalwazi SK. Effect of surgical and chemotherapeutic treatment on alpha-fetoprotein levels in patients with hepatocellular carcinoma. Cancer. 1976;37:677–683. doi: 10.1002/1097-0142(197602)37:2<677::aid-cncr2820370211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Nagasue N, Inokuchi K, Kobayashi M, Kanashima R. Serum alpha-fetoprotein in patients following partial hepatectomy. Dig Dis Sci. 1980;25:243–247. doi: 10.1007/BF01308512. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, Kogevinas M, Real FX. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005;6:678–686. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 42.Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316:450–455. doi: 10.1056/NEJM198702193160806. [DOI] [PubMed] [Google Scholar]
- 43.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. doi: 10.1093/oxfordjournals.epirev.a036084. [DOI] [PubMed] [Google Scholar]