Abstract
AIM: To evaluate the safety and efficacy of CO2 insufflation compared with air insufflation in the endoscopic submucosal excavation (ESE) of gastrointestinal stromal tumors.
METHODS: Sixty patients were randomized to undergo endoscopic submucosal excavation, with the CO2 group (n = 30) and the air group (n = 30) undergoing CO2 insufflation and air insufflation in the ESE, respectively. The end-tidal CO2 level (pETCO2) was observed at 4 time points: at the beginning of ESE, at total removal of the tumors, at completed wound management, and 10 min after ESE. Additionally, the patients’ experience of pain at 1, 3, 6 and 24 h after the examination was registered using a visual analog scale (VAS).
RESULTS: Both the CO2 group and air group were similar in mean age, sex, body mass index (all P > 0.05). There were no significant differences in PetCO2 values before and after the procedure (P > 0.05). However, the pain scores after the ESE at different time points in the CO2 group decreased significantly compared with the air group (1 h: 21.2 ± 3.4 vs 61.5 ± 1.7; 3 h: 8.5 ± 0.7 vs 42.9 ± 1.3; 6 h: 4.4 ± 1.6 vs 27.6 ± 1.2; 24 h: 2.3 ± 0.4 vs 21.4 ± 0.7, P < 0.05). Meanwhile, the percentage of VAS scores of 0 in the CO2 group after 1, 3, 6 and 24 h was significantly higher than that in the air group (60.7 ± 1.4 vs 18.9 ± 1.5, 81.5 ± 2.3 vs 20.6 ± 1.2, 89.2 ± 0.7 vs 36.8 ± 0.9, 91.3 ± 0.8 vs 63.8 ± 1.3, respectively, P < 0.05). Moreover, the condition of the CO2 group was better than that of the air group with respect to anal exsufflation.
CONCLUSION: Insufflation of CO2 in the ESE of gastrointestinal stromal tumors will not cause CO2 retention and it may significantly reduce the level of pain, thus it is safe and effective.
Keywords: Carbon dioxide insufflation, Endoscopic submucosal excavation, Gastrointestinal tract, Stromal tumor, Treatment
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most com-mon tumors of mesenchymal tissue in the digestive system, with a property of non-directional differentiation. They are formed as a result of the overproliferation of immature spindle cells or epithelioid cells[1], and characterized by overexpression of CD117 and CD34 based on the pathology. Thus, they can be distinguished from other mesenchymal tissue tumors such as leiomyoma, leiomyosarcomas, Schwann tumors and neurofibroma, etc.
GISTs appear unexpectedly, and the significance of EUS in the diagnosis and differentiation of GISTs has now been defined[2]. However, GISTs should be considered as tumors inclined to recur and metastasize because their potential malignancy is difficult to predict. GISTs which are derived from the mucous layer and submucosa in the digestive tract are usually referred to endoscopic treatment, but lesions originating from the muscularis propria are difficult to completely resection; furthermore, multiple complications such as acute perforation can occur.
In recent years, with rapid advances in endoscopic submucosal excavation (ESE) to treat GISTs, ESE has been the preferred choice instead of surgical excision and follow-up observation as in the past. However, abdominal pain and abdominal discomfort after operation are apparent in patients undergoing ESE with the insufflation of air. Using CO2 as a replacement for air could mean a remarkable improvement, because CO2 can be absorbed by the gastrointestinal mucosa rapidly and be discharged from the body by respiration.
Foreign experts and scholars have begun investigating the application of CO2 in endoscopic submucosal dissection[3], endoscopic retrograde cholangiopancreatography[4], as well as in double-balloon enteroscopy[5], etc. So far, however, there has no report about the effect of endoscopic submucosal excavation with the insufflation of CO2. In this study, we evaluate the safety and efficacy of CO2 insufflation in ESE compared with the insufflation of air as control. We conducted this work as a prospective, comparative pilot study.
MATERIALS AND METHODS
Patients
From March 2011 to February 2012, 61 patients diagnosed as having submucosal lesions of the digestive tract derived from muscularis propria distinguishable by endoscopic ultrasound (EUS) and computed tomography (CT) (26 were transferred from other hospitals) were enrolled in this study. One patient diagnosed with rectal malignant mesenchymoma with liver metastasis was not in conformity with the inclusion criteria. Sixty patients signed the medical informed consent form before the ESE. All 60 patients gave their consent to be randomized to undergo endoscopic submucosal excavation with insufflation of air (n = 30) or CO2 (n = 30) by means of random numbers generated from the computer. The 60 patients were clinically manifested by abdominal discomfort, abdominal mass and hematochezia without intestinal obstruction; 13 of the 60 patients did not experience any noticeable symptoms.
The exclusion criteria were as follows: malignant GISTs with metastasis; intestinal obstruction; vascular invasion; large lesion (> 10 cm) that failed complete resection; young age (< 14 years) and incapable of finishing the relevant questionnaire; chronic obstructive pulmonary disease patients with the retention of CO2; acute digestive tract hemorrhage or variation from normal; shock with various causes; severe cardiopulmonary cerebral diseases; inability to tolerate the preoperative preparation; allergic to propofol; pregnancy, breast-feeding, etc.
Experimental equipment
The main instruments used included a gastroscope (GIF-Q260J, Olympus, Japan), enteroscope (CF-Q260AI, Olympus, Japan), ultrasound gastroscope (UE-260, Olympus, Japan), CO2 air-insufflation equipment (UCR, Olympus, Japan), needle (INJ1-A1-07.160, Medwork, Germany), hot biopsy forceps (FD-1U-1, Olympus, Japan), hemoclips (HX-610-135 and HX-610-090, Olympus, Japan), needle knife (3281, Boston, America), TT knife (KD-640L, Olympus, Japan), snare (99052012225MW, MTW/Edoscopic, Germany), high-frequency electric knife (ICC-200, ERBE, Germany) and argon plasma coagulation (APC300, ERBE, Germany). A transparent cap (D-201-11802/D-201-13404/D-201-10704, Olympus, Japan) was added to the tip of the endoscope during the endoscopic submucosal excavation.
Experimental methods
Intravenous anesthesia was executed by an anesthetist, who used propofol (AstraZeneca) to maintain general anesthesia. Routine oxygen treatment was performed (2-3 mL/min); meanwhile, heart rate, respiration, blood pressure, blood oxygen saturation and PetCO2 were continuously monitored under anesthesia. Propofol was started as normal with 1.5-2.5 mg/kg, observing the change of life signs. Routine endoscopy was performed as soon as the absence of consciousness was seen, while respiration, heart rate, blood oxygen saturation, etc., were essentially normal. Additional propofol at 0.3-0.5 mg/kg was given to sustain proper sedation in the event that the patient had a reaction.
No eating or drinking for 8 h prior to surgery was allowed. EUS was performed to ensure the stage and identification of the main lesions. CT examination was also used to observe the composition of the tumor and the relationship with the surrounding organs and vessels, in order for it to be distinguished from other lesions. Like GISTs, advanced gastric cancer or gastric lymphoma can also grow outward, while GIST always showed the uneven thickening of stomach wall, obvious local invasion as well as the swelling of perigastric, hilar and abdominal lymph nodes, with an evenly enhanced mass. Patients with GISTs originating from the muscularis propria without metastasis to other regions were treated by endoscopic submucosal excavation under anesthesia.
The standard operating procedures were as follows: (1) Marking: it is recommended to carry out electrical coagulation at the edge of the distinguished lesion marked by use of an argon knife; (2) Submucosal insufflation: multiple parts of the submucosa outside the marker points were irrigated with normal saline (including methylene blue and epinephrine); (3) Incising mucosa at the edge of lesion: a needle knife was employed to spot-incise up till the submucosa, and a TT knife was used to incise the mucosa along the lateral margin of the marking point; (4) Excision: a snare was used to enclose the mucosa and submucosa of the lesions and expose the muscularis propria, then the lesion was excised along the edge with a TT knife. If lesions which were clinging to the serosa layer could not be excised completely, it was recommended to perform full-thickness isolation and carry out a perforation initiative. The wound was then observed carefully to see if there were tumors remaining. No remaining tumors existed under endoscopy. Subsequently all the removed tissues were sent for pathological examination to rule out positive margins, which proved that the tumor was excavated completely; and (5) Wound management: for the small blood vessels which were visible in the wound, electrocoagulation hemostasis was recommended by use of argon knife, and if need be, the wound was closed by suture with a metallic hemostatic clamp, as well as by spraying tissue glue on the wound to prevent hemorrhaging.
In order to ensure the double-blind nature of the trial, both the endoscope and the valve of the CO2 insufflation equipment were covered by black cloth. Someone was put in charge of the valve of the insufflation equipment and the switch of the gas pump; both the operator and the patient did not know what type of gas had been used.
Measuring of PetCO2
Studies have shown that the partial pressure of end-tidal carbon dioxide (PetCO2) and the arterial partial pressure of carbon dioxide [p(CO2)] of a normal adult are very similar and close to each other, so that [p(CO2)] is usually replaced by PetCO2 because of its noninvasive characteristic[6]. In this trial, we use the portable CO2 analyzer (ULT-1, Datex-Ohmeda, Finland) to measure PetCO2. The PetCO2 was measured by nurses randomly at the following four time-points: at beginning of ESE, at total removal of the tumors, after completed wound management, and 10 min after ESE.
Grading of abdominal pain
The 100 mm visual analog scale (VAS) was applied to grade pain according to the varying degrees of severity[7]. The spectrum of VAS is 0-100; the minimal point is 0 which means no pain, the maximal one is 100 which means unbearable agony. Patients’ abdominal pain was assessed at 1, 3, 6 and 24 h after ESE. Consequently, questionnaires were collected at the endoscopy center in our hospital.
Statistical analysis
Statistical analysis was performed by using the software SPSS13.0 (SPSS Inc, Chicago, IL, United States). The results were expressed as mean ± SD, variables in the two groups were analyzed with a Student’s t test. The comparison of mean VAS at all time points was analyzed with the nonparametric, rank-sum test for two independent samples (Wilcoxon, 1945). Percentage of pain scores of 0 at each time point between the two groups were preceded with chi-square test. The value of PetCO2 at each time point was analyzed through the repeated measures of analysis of variance. P value < 0.05 was considered statistically significant.
RESULTS
Comparison of baseline characteristics between the two groups
All 60 patients completed the study protocol. Thirty patients were enrolled in the CO2 group (17 males, 13 females, mean age 52.1 years) and 30 patients in the air group (16 males, 14 females, mean age 50.9 years). The body type was indicated by body mass index; in the CO2 group the value was 21.63 kg/m2, while in the air group this was 21.79 kg/m2, there was no statistical difference between the two groups (P > 0.05). The actual clinical data are shown in Table 1.
Table 1.
Patient characteristics
| Group | CO2 insufflation | Air insufflation | P value1 |
| (n = 30) | (n = 30) | ||
| Age, yr, mean ± SD | 52.1 ± 5.1 | 50.9 ± 6.6 | 0.83 |
| Sex (male/female) | 17/13 | 16/14 | 0.80 |
| Mean BMI (kg/m2) | 21.63 | 21.79 | 0.54 |
| Previous surgeries, n (%) | |||
| Any prior abdominal surgery | 17 (57) | 16 (53) | 0.80 |
| Cholecystectomy | 9 (30) | 11 (37) | 0.58 |
| Hysterectomy | 3 (10) | 2 (7) | |
| Liver transplantation | 2 (7) | 1 (3) | |
| Other2 | 5 (17) | 2 (7) | 0.30 |
Endoscopic submucosal excavation treatment in the two groups
The patients’ diseased regions in the CO2 group consisted of esophagus (4/30), stomach (22/30), rectum (2/30) and sigmoid colon (2/30), while in the air group, the diseased regions included esophagus (6/30), stomach (21/30), rectum (2/30) and sigmoid colon (1/30). The mean diameters of tumors of the CO2 group and the air group were 1.6 ± 0.3 cm (range 0.5-5.0 cm) and 1.2 ± 0.5 cm (range 0.5-4.0 cm), respectively. The mean operating time was 35 ± 12 min in the CO2 group, and this was 41 ± 10 min in the air group. The success rate for complete resection of tumor was 100%. Both the CO2 group and the air group had light intraoperative bleeding; the mean bleeding volume was approximately 10 mL, without postoperative bleeding. Five cases were diagnosed with extraluminal type under endoscopic ultrasonography; it was recommended to perform full-thickness isolation and a perforation initiative for 3 cases of 5 belonging to the CO2 group, and for 2 cases of 5 belonging to the air group. There was no statistical difference in the ESE between the two groups (P > 0.05). The actual clinical data are shown in Table 2.
Table 2.
Endoscopic submucosal excavation treatment characteristics
| Group | Location (n) | Diameter (cm) | Operating time (min) | Success rate (%) | Full-thickness isolation (n) |
| CO2 | Esophagus (4) | 1.7 | 41 | 100 | 3 |
| Stomach (22) | 1.3 | 23 | |||
| Rectum (2) | 1.5 | 40 | |||
| Sigmoid colon (2) | 1.9 | 47 | |||
| Air | Esophagus (6) | 1.1 | 43 | 100 | 2 |
| Stomach (21) | 0.7 | 31 | |||
| Rectum (2) | 0.9 | 38 | |||
| Sigmoid colon (1) | 1.7 | 51 |
Comparison of PetCO2 between the two groups
The value of PetCO2 was compared at the following four time points: beginning of ESE, at removal of the tumors, at completed wound management, and 10 min after ESE. The value was expressed as mean ± SD; the clinical data are shown in Table 3. From the mean value at each time point above, we could conclude that the value of PetCO2 at each time point between the two groups had no statistical difference (P > 0.05, Figure 1A).
Table 3.
Comparison of PetCO2 between the two groups
| Time point | CO2 group (mmHg) | Air group (mmHg) | P value |
| Beginning of ESE | 34.01 ± 2.03 | 33.32 ± 2.21 | 0.78 |
| Removal of the tumors | 31.21 ± 2.35 | 30.59 ± 2.73 | 0.73 |
| Wound management | 32.75 ± 2.69 | 32.01 ± 2.22 | 0.92 |
| 10 min after ESE | 33.23 ± 2.56 | 32.61 ± 2.78 | 0.79 |
ESE: Endoscopic submucosal excavation.
Figure 1.
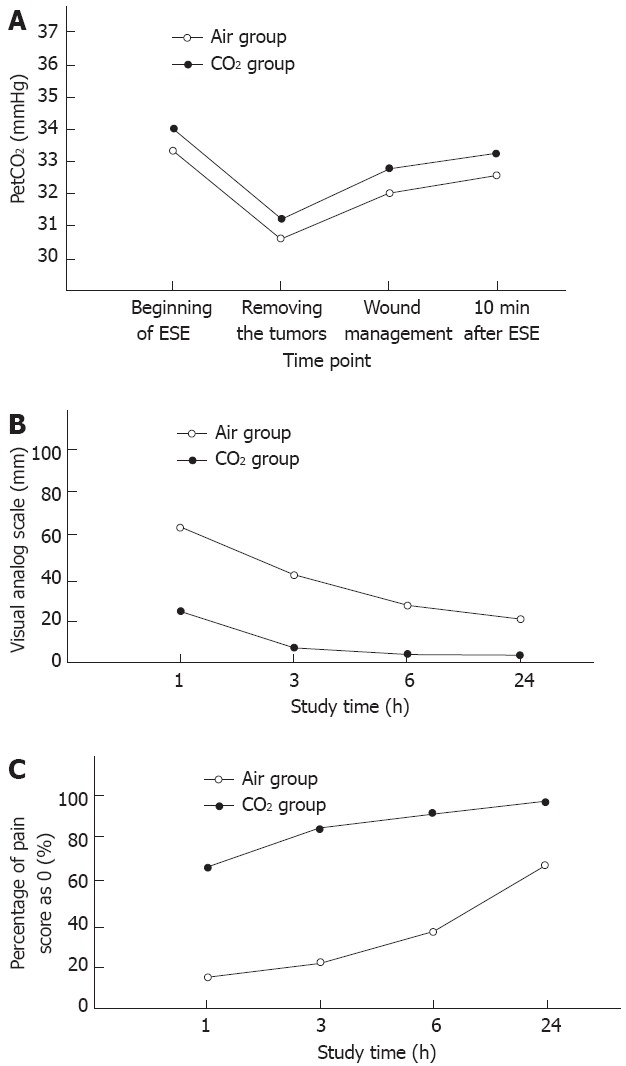
The comparison of PetCO2 (A), visual analog scale (B) and percentage of pain score of 0 (C) at each time point between the CO2 group and the air group. ESE: Endoscopic submucosal excavation.
Comparison of abdominal pain between the two groups after revival from anesthesia
The VAS was applied to evaluate the level of abdominal pain of the patients at the following four time points[8]: 1, 3, 6 and 24 h after anesthesia revival, respectively. The results showed that there was a significant difference of the value of VAS at 1, 3 and 6 h after revival from anesthesia between the two groups (P < 0.05, Figure 1B). Moreover, a comparison of the value of VAS at 24 h after revival from anesthesia between the two groups still had statistical difference. From Figure 1B, we can see that there was a slow decline in the air group, while the curved line declined more obviously in the CO2 group (1 h: 21.2 ± 3.4 vs 61.5 ± 1.7; 3 h: 8.5 ± 0.7 vs 42.9 ± 1.3; 6 h: 4.4 ± 1.6 vs 27.6 ± 1.2; 24 h: 2.3 ± 0.4 vs 21.4 ± 0.7, P < 0.05). Furthermore, the curved line returned to the baseline at 6 h after anesthesia revival in the CO2 group; however, in the air group the curved line never returned to baseline. The percentage of VAS scores of 0 at each time point was subjected to chi-square test, and the result demonstrated that the percentage of VAS scores of 0 in the CO2 group was significantly higher than that in the air group (1 h: 60.7 ± 1.4 vs 18.9 ± 1.5; 3 h: 81.5 ± 2.3 vs 20.6 ± 1.2; 6 h: 89.2 ± 0.7 vs 36.8 ± 0.9; 24 h: 91.3 ± 0.8 vs 63.8 ± 1.3, respectively, P < 0.05, Figure 1C). Consequently, in the ESE of gastrointestinal stromal tumors, the condition of abdominal pain at each time point after revival from anesthesia could be clearly aggravated by the application of CO2.
Comparison of anal exsufflation between the two groups
In checking the anal exsufflation of patients in the two groups at 1, 2 and 4 h after treatment, only 21% of the patients in the CO2 group had anal exsufflation at 1 h after anesthesia revival, while 7% lasted for 2 h or more. However, 73.8% of the patients in the air group had anal exsufflation, and nearly 14.3% had a moderate or great amount of flatus, 28.6% lasted for 4 h or more. A comparison of the two groups at 1, 2 and 4 h after revival from anesthesia was carried out with a chi-square test, and a P value < 0.01 was found which was considered statistically significantly different. The amount of anal exsufflation had a negative correlation with the degree of abdominal pain and distension; furthermore, it also had a negative correlation with recovery time of abdominal distension in the air group.
DISCUSSION
GISTs should be considered as potentially malignant tumors owing to their unpredictable recurrence and metastasis; however, there are no definite clinical criteria for the diagnosis and treatment of GISTs[9]. EUS, especially an EUS-fine needle aspiration, plays an important part in the diagnosis of GISTs, can determine the nature of submucosal lesions of the digestive tract and is instructive in the choice of treatment methods. GISTs with a diameter of 3-5 cm shown in the endoscopic examination and by pathology are more likely to be malignant; therefore, such GISTs are supposed to be thoroughly surgically excised[10-14]. Although large GISTs are more inclined to be malignant, the small ones also have the possibility, so it is irrational to regard tumor size as the only standard for the malignancy of GISTs[11,15]. In this study, we defined the risk classification of GISTs according to the National Institutes of Health[16]. Consequently, the GISTs with definite diagnosis should be treated as much as possible.
Nowadays, a variety of surgical methods (as well as chemotherapy) for the treatment of GISTs are recognized in foreign and domestic studies. Surgical operation is still the traditional treatment; many patients with GISTs have been reported as being excised by undergoing laparoscopy[17,18], and it is significantly important to excise larger lesions by surgical treatment. Imatinib, a tyrosine kinase inhibitor, is currently being used to treat GISTs which have unique kinase mutations that serve as targets for medical therapy, but some disadvantages exist such as high cost of therapy, long-term treatment and indeterminate side-effects; meanwhile few studies are reported about the treatment for GISTs with unclear symptoms[19,20]. However, endoscopic therapy for these is much rarer. Choosing the treatment for GISTs that has lesser invasive injury and lower cost under endoscopy is rather clinically valuable.
Endoscopic mucosal resection (EMR) can be applied to the treatment of patients with distinguishable lesions of the digestive tract, such as early carcinoma and submucosal tumor. Moreover, EMR has not only the same therapeutic effect as surgical operation, but a short operating time, short hospitalization time, rapid recovery and low medical costs. However, it is hard to accomplish en bloc resection by the use of EMR for those lesions whose size is 2 cm or more. As a result, the remains are likely to recur and lead to many complications such as bleeding and perforation. Compared with EMR, ESE is able to excise a large majority of GISTs and provide intact data for pathological diagnosis. For preoperative evaluation of benign stromal tumors whose size is 5 cm or less, ESE is able to accomplish en bloc resection. ESE fully demonstrates the superiority of minimally invasive surgery as it has the advantage of rapid recovery, short hospitalization time and low medical costs. In our study, ESE was preferable for the GISTs originating from the muscularis propria, but not from the muscularis mucosae.
ESE is appropriate for GISTs originating from the muscularis propria; however, too much air insufflation because of a long operating time leads to pain for the patients in various degrees after revival from anesthesia. Pain caused by abdominal distension is the most common type, resulting from gastrointestinal gaseous tension. Therefore, it is recommended to select inhaling CO2 instead of air, as the CO2 is easily soluble in blood and other body liquids. It is not only rapidly absorbed by the gastrointestinal tract, but easily eliminated from the body by respiration. Patients never appear to have a metabolic disorder such as CO2 retention. Yamano et al[21] has reported that the usage of CO2 in enteroscopy could effectively alleviate the subjective pain of patients. In summary, our study investigated the comparison between the application of CO2 and air insufflation for the ESE operation; the postoperative subjective pain of patients was measured by VAS and results suggested that the absolute VAS was lower in the CO2 group than in the air group, and the number of patients with severe postoperative pain was also fewer in the CO2 group.
We compared the value of PetCO2 at the following four time points: beginning of ESE, at total removal of the tumors, at completed wound management, and 10 min after ESE. From the above data, we could draw conclusions that there were no significant differences of PetCO2 at each time point between the two groups, suggesting that CO2 is not able to cause postoperative retention as well not influencing the safety during the operation.
Comparing postoperative anal exsufflation between the two groups, the results revealed that the time of anal exsufflation in the CO2 group is shorter than that in the air group, and that the flatus of patients in the CO2 group is also less, which demonstrates that CO2 is much easier to be absorbed. Both the difficulty of operation and the ratio of various related complications will increase in the case of the existence of a large amount of remaining gas.
The GISTs partly derived from muscularis propria are diagnosed as extraluminal type or clinging to the serosa by EUS. Those tumors clinging to the serosa layer cannot be excised completely by ESE; it is suggested to perform full-thickness excision and bring out a perforation initiative. In our study, there were five patients with full-thickness excision of GISTs who had little gas entry into the abdominal cavity so that there was less obvious abdominal pain, and no postoperative abnormal conditions happened compared with other patients by ESE. However, the patients with full-thickness excision among the air group had severe abdominal pain as well as long-term gastrointestinal decompression.
In summary, CO2 insufflation could effectively alleviate the pain of patients when the GISTs were excised by ESE, without the risk of CO2 retention. The safety of CO2 insufflation is comparable to that of air insufflation, and less pain exists after operation. Therefore, it is hopeful that CO2 insufflation will become the standard method for ESE with full-thickness excision and it is apparent that this method will be widely applied in the future.
COMMENTS
Background
Gastrointestinal stromal tumors are the most common tumors of mesenchymal tissue in the digestive system. In recent years, endoscopic submucosal excavation has been used to treat gastrointestinal stromal tumors (GISTs) instead of surgical excision. The application of CO2 in endoscopic submucosal excavation (ESE) could reduce the complications of the procedure effectively.
Research frontiers
Foreign experts and scholars have begun investigating the application of CO2 in endoscopic submucosal dissection, endoscopic retrograde cholangiopancreatography, as well as in double-balloon enteroscopy, etc. So far, there has not been a report about the effect of endoscopic submucosal excavation with the insufflation of CO2. In this study, the authors evaluate the safety and efficacy of CO2 insufflation in ESE compared with the insufflation of air as control.
Innovations and breakthroughs
In this study, the authors have detailed the superiority of CO2 insufflation in ESE. Compared with air insufflation, the pain scores after ESE at different time points in the CO2 group decreased significantly. Meanwhile, the percentage of visual analog scale (VAS) scores of 0 in the CO2 group after 1, 3, 6 and 24 h was significantly higher than that in the air group. Moreover, the condition of the CO2 group was better than that of the air group in respect of anal exsufflation.
Applications
CO2 insufflation could effectively alleviate the pain of patients when GISTs are excised by ESE without the risk of CO2 retention. Therefore, it is hopeful that CO2 insufflation will become the standard method for ESE with full-thickness excision and it will certainly be widely applied in the future.
Peer review
The authors examined the application of CO2 insufflation in endoscopic submucosal excavation. The results suggested that the postoperative pain of patients measured by VAS seems to be lower in the CO2 group than that in the air group, and the time of anal exsufflation in the CO2 group is also shorter than that in the air group. So, CO2 insufflation may be the standard method for the ESE with full-thickness excision in the future.
Footnotes
Supported by Grants from Project of Science and Technology Commission of Shanghai Municipality, No. 10441901702; Nano-specific Project of Science and Technology Commission of Shanghai Municipality, No. 11nm0503700; and Shanghai Key Laboratory of Pediatric Digestion and Nutrition, No. 11DZ2260500
Peer reviewers: Braden B, Reprint Author, Univ Hosp, Dept Med 2, Theodor Stern Kai 7, D-60590 Frankfurt, Germany; Kiriyama S, Reprint Author, Natl Canc Ctr, Dept Endoscopy, 1-1 Tsukiji 5 Chome, Tokyo, Japan; Mekky MA, Reprint Author, Aichi Canc Ctr Hosp, Dept Gastroenterol, Chikusa Ku, 1-1 Kanokoden, Nagoya, Aichi 4648681, Japan
S- Editor Jiang L L- Editor Logan S E- Editor Li JY
References
- 1.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 2.Zong CH, Xu LM, Chen HF. The diagnostic value of endoscopic ultrasonography in gastric stromal tumors. Zhongguo Neijing Zazhi. 2006;12:917–921. [Google Scholar]
- 3.Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537–542. doi: 10.1016/j.gie.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:58–64. doi: 10.1055/s-2006-945036. [DOI] [PubMed] [Google Scholar]
- 5.Kerns SR, Hawkins IF. Carbon dioxide digital subtraction angiography: expanding applications and technical evolution. AJR Am J Roentgenol. 1995;164:735–741. doi: 10.2214/ajr.164.3.7863904. [DOI] [PubMed] [Google Scholar]
- 6.Goldman JM. A simple, easy, and inexpensive method for monitoring ETCO2 through nasal cannulae. Anesthesiology. 1987;67:606. doi: 10.1097/00000542-198710000-00038. [DOI] [PubMed] [Google Scholar]
- 7.Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50:604–607. doi: 10.1136/gut.50.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilbert DM, Heinz A, Jocham D, Eisenberger F, Chaussy C. Complications with portable ESWL--a multicenter study. Urologe A. 1997;36:217–221. doi: 10.1007/s001200050092. [DOI] [PubMed] [Google Scholar]
- 9.Nowain A, Bhakta H, Pais S, Kanel G, Verma S. Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol. 2005;20:818–824. doi: 10.1111/j.1440-1746.2005.03720.x. [DOI] [PubMed] [Google Scholar]
- 10.Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468–473. doi: 10.1016/s0016-5107(97)70175-5. [DOI] [PubMed] [Google Scholar]
- 11.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995;103:41–47. doi: 10.1093/ajcp/103.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Raut CP, Morgan JA, Ashley SW. Current issues in gastrointestinal stromal tumors: incidence, molecular biology, and contemporary treatment of localized and advanced disease. Curr Opin Gastroenterol. 2007;23:149–158. doi: 10.1097/MOG.0b013e32802086d0. [DOI] [PubMed] [Google Scholar]
- 16.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Ke ZW, Cai JL, Chen DL. Laparoscopic resection of submucosal tumors in gastric fundus. Zhonghua Waike Zazhi. 2008;46:1780–1783. [PubMed] [Google Scholar]
- 18.Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Noh SH. Laparoscopic resection of a huge intraluminal gastric submucosal tumor located in the anterior wall: eversion method. J Surg Oncol. 2005;89:95–98. doi: 10.1002/jso.20195. [DOI] [PubMed] [Google Scholar]
- 19.American Gastroenterological Association Institute. American Gastroenterological Association Institute medical position statement on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2215–2216. doi: 10.1053/j.gastro.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Davila RE, Faigel DO. GI stromal tumors. Gastrointest Endosc. 2003;58:80–88. doi: 10.1067/mge.2003.317. [DOI] [PubMed] [Google Scholar]
- 21.Yamano HO, Yoshikawa K, Kimura T, Yamamoto E, Harada E, Kudou T, Katou R, Hayashi Y, Satou K. Carbon dioxide insufflation for colonoscopy: evaluation of gas volume, abdominal pain, examination time and transcutaneous partial CO2 pressure. J Gastroenterol. 2010;45:1235–1240. doi: 10.1007/s00535-010-0286-5. [DOI] [PubMed] [Google Scholar]


