Abstract
AIM: To evaluate the therapeutic effects of itopride vs other drugs (placebo, domperidone, mosapride) for functional dyspepsia (FD).
METHODS: Randomized controlled trials (RCTs) of itopride for FD were retrieved from databases. Relevant information was extracted and analyzed, using the relative risk (RR) and weighted mean deviation, as appropriate. A random or fixed effect model was used, based on the heterogeneity of the included articles, and visual inspection of funnel plots was used to evaluate publication bias.
RESULTS: Nine RCTs enrolling 2620 FD cases were included; 1372 cases received itopride treatment and 1248 cases received placebo or other drugs (control groups). Compared with control groups, itopride had superior RR values of 1.11 [95%CI: (1.03, 1.19), P = 0.006], 1.21 [95%CI: (1.03, 1.44), P = 0.02], and 1.24 [95%CI: (1.01, 1.53), P = 0.04] for global patient assessment, postprandial fullness, and early satiety, respectively. For the Leeds Dyspepsia Questionnaire score, the weighted mean deviation was -1.38 [95%CI: (-1.75, -1.01), P < 0.01]. The incidence of adverse effects was similar in the itopride and control groups. The funnel plots for all indicators showed no evidence of publication bias.
CONCLUSION: Itopride has good efficacy in terms of global patients assessment, postprandial fullness, and early satiety in the treatment of patients with FD and shows a low rate of adverse reactions. Itopride can greatly improve FD syndromes-score.
Keywords: Itopride, Functional dyspepsia, Meta-analysis, Randomized controlled trials, Prokinetic agents
INTRODUCTION
Functional dyspepsia (FD) is a common, functional gastrointestinal disorder[1]. In a multi-centre Asian study of 1115 patients with uninvestigated dyspepsia (UD) (Rome II criteria) from nine countries, 43% turned out to have FD after investigation[2]. FD places a heavy financial burden on society[3,4]. Globally the majority of patients suffering from dyspepsia, which account for approximately 5% of primary care, fall into the category of FD[5]. FD is a complex problem resulting from the interaction of gastric dysmotility[6,7], visceral hypersensitivity, and psychological factors, and causes delayed gastric emptying, abnormal gastric regulation, and aberrant myoelectricity. As many as 60% of FD patients have gastric dysmotility. Outcomes of drug therapy [including Chinese herbal medicines, antidepressant drugs, proton pump inhibitors (PPI), and Helicobacter pylori (H. pylori) eradication] for FD patients have not been satisfactory[8-10] compared with placebos. Although prokinetic agents have been proven to improve symptoms in FD patients by reducing gastro-esophageal reflux, promoting gastric emptying, and improving gastric regulation, metoclopramide is associated with a high incidence of central nervous system (CNS)-related adverse drug reactions (ADRs), domperidone can elevate serum prolactin levels and cause gynecomastia and galactorrhea, and cisapride has been withdrawn because of safety concerns including high risk of prolonging the QT interval and severe arrhythmias[11].
Itopride, a novel prokinetic agent, works by antagonizing dopamine D2-receptors and inhibiting acetylcholinesterase[12]. It does not cause any CNS-related ADRs because its high polarity does not allow it to cross the blood-brain barrier, it barely elevates prolactin levels and does not prolong the Q-T interval[5]. In a multicentre, randomised, double-blind, placebo-controlled trial, itopride significantly improved symptoms in patients with FD, and showed a greater response rate than placebo[13]. However, it was recently reported that itopride was no more effective in showing a difference in symptom response from placebo in FD[5]. Therefore, given the conflicting results for efficacy in some study reports and the possible serious adverse reactions (SARs) of itopride, a meta-analysis of randomised controlled trial (RCT) data published prior to December 2011 was conducted, with a view to evaluating more objectively the efficacy and safety of itopride in the treatment of FD.
MATERIALS AND METHODS
Inclusion and exclusion criteria
Inclusion criteria for the studies used in the analysis required that they: (1) contained inclusion and exclusion criteria, and the study design was an RCT with a quality level above B; (2) were designed to study FD as the target population; (3) had a study group that was given itopride and a control group that was given placebo, domperidone, or mosapride, etc.; and (4) included one or more of the following indicators for comparison of efficacy between itopride and other therapy: Global patient assessment (GPA), postprandial fullness, early satiation, epigastric discomfort, adverse reaction, and the Leeds Dyspepsia Questionnaire (LDQ) score. Studies were excluded that had: (1) incomplete data; (2) been re-published (only those with credible data were chosen); (3) a control group that used itopride together with other drugs; (4) patients with obvious organic diseases such as gastritis, peptic ulcer, and cholecystitis, etc.; and (5) baseline data that were not similar.
Literature search and data collection
Databases searched included the Cochrane Library, PubMed, Elsevier, EMBASE, ISI, CNKI, VIP Chinese Scientific and Technological Periodical Database and Wanfang Data, prior to December 2011. Search terms and search strategy included: “itopride”, “functional dyspepsia”, “randomized or random or randomly or randomised”, “controlled trial”; “Yi Tuo Bi Li” (the Chinese character for “itopride”), “Gong Nen Xing Xiao Hua Bu Liang” (the Chinese character for “functional dyspepsia”), “sui ji dui zhao” (the Chinese character for “randomized control”), excluding studies involving children or pregnant women, as well as review papers. Meanwhile, articles published in core journals in China and abroad this year, such as Chinese Journal of Digestion, Chinese Journal of Internal Medicine, Chinese Journal of Gastroenterology, Gastroenterology, and Gut were searched manually. Conference papers published this year were also consulted, along with the references of the included articles, so as to include studies that may have been omitted. Extracted data included outcome measures, risk of bias and characteristics of trials, patients, and interventions. Authors of included trials were approached for additional information when necessary. The articles were screened by two reviewers independently, according to the steps for preliminary screening and full-text screening, and any differences were settled through discussions by the reviewers themselves or with assistance from a third party.
Quality evaluation
Study quality was evaluated according to the quality evaluation criteria recommended in the Cochrane Reviewers’ Handbook 4.2.2. Briefly, the quality of a study was rated A, B, or C based on its randomization method, allocation concealment, double-blind method, missing follow-up, and withdrawal from observation. Grade A completely conforms to the four quality standards and has the lowest possibility of bias. Grade B partially conforms to one or more quality standards and shows moderate possibility of bias. Grade C does not conform to any of the four quality standards and has a high possibility of bias.
Data analysis
Revman 5.0 (the Cochrane collaboration; http://www.cochrane.org/) was used for statistical analysis of the data. Relative risk (RR) was used to test the heterogeneity of such numerical data as GPA, epigastric fullness, early satiation, epigastric discomfort, and adverse reactions between the two groups of each study. Weighted mean deviation (WMD) was used for statistical analysis of the LDQ scores, and the effect variables were expressed by 95% confidence intervals. Statistical assessment was then performed using a χ2 test of homogeneity and evaluation of the inconsistency index (I2) statistic. The I2 statistic is defined as the percentage of variability caused by heterogeneity rather than chance with values > 50% representing the possibility for substantial heterogeneity. A fixed effect model was used to estimate the overall effect if RR was homogenous; if RR was non-homogenous, a random effect model was used.
Publication bias
Funnel plots were drawn using the RR values of each of GPA, epigastric fullness, early satiation, epigastric discomfort, and adverse reactions of the two groups included in the meta-analysis as the X coordinate and the standard error (SE) (log RR) as the Y coordinate, as well as using the mean deviation (MD) of LDQ scores as the X coordinate and the SE (MD) as the Y coordinate, after which the symmetry of the plots was observed to evaluate the impacts of publication bias. Subgroup analyses were performed to evaluate intervention effects in trials comparing itopride vs placebo or other prokinetic agents, trials with adequate bias control (assessed through randomization methods) and publication status.
RESULTS
Results of the literature search and information on included studies
328 articles were collected; 319 were excluded for not meeting the inclusion criteria, nine RCT articles[5,13-20] were finally included, as shown in Figure 1. Of the included RCT articles, seven were graded as grade B and two as grade A. Included studies contained a total of 2620 patients, 1372 of whom received itopride, and 1248 received placebo or other control drugs. Table 1 shows the basic characteristics of the studies included.
Figure 1.
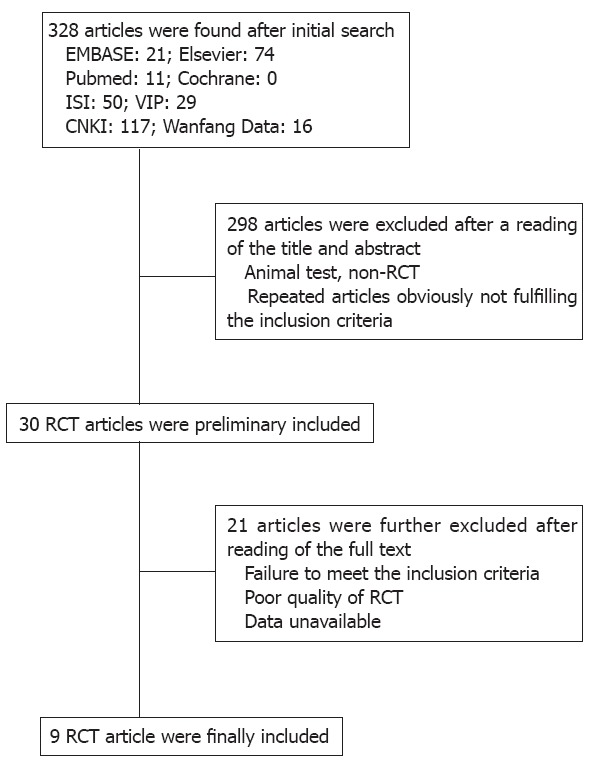
Flow chart of article inclusion and screening. RCT: Randomized controlled trial.
Table 1.
Clinical data of included articles
| Ref. | Year | Quality grade | Total cases | Duration of therapy (wk) |
Treatment group |
Control group |
||||
| Cases (male/female) | Average age (yr) | Itopride dosing regimen | Cases (male/female) | Average age (yr) | Dosing regimen | |||||
| Zhou et al[14] | 2000 | B | 208 | 2 | 105 | 43 | 50 mg tid | 103 | 46 | Domperidone 10 mg tid |
| Sun et al[15] | 2003 | B | 232 | 2 | 115 | - | 50 mg tid | 117 | - | Domperidone 10 mg tid |
| Mo et al[16] | 2003 | B | 80 | 2 | 40 | - | 50 mg tid | 40 | - | Domperidone 10 mg tid |
| Chen et al[17] | 2004 | B | 42 | 4 | 21 | 35 | 50 mg tid | 21 | 36 | Domperidone 10 mg tid |
| Amarapurkar et al[18] | 2004 | B | 60 | 2 | 30 (19/11) | 45 | 50 mg tid | 30 (11/19) | 40 | Mosapride 5 mg tid |
| Zhu et al[19] | 2005 | B | 236 | 4 | 119 | - | 50 mg tid | 117 | - | Domperidone 10 mg tid |
| Li et al[20] | 2005 | B | 200 | 4 | 100 (47/53) | 38 | 50 mg tid | 100 (47/53) | 38 | Domperidone 10 mg tid |
| Holtmann et al[13] | 2006 | A | 412 | 8 | 50 mg: 135 (48/87) | 47 | 50 mg tid 100 mg tid | 142 (53/89) | 49 | Placebo |
| 100 mg: 135 (57/78) | ||||||||||
| Talley et al[5], INT | 2008 | A | 524 | 8 | 264 (86/178) | 43 | 100 mg tid | 260 (99/161) | 43 | Placebo |
| Talley et al[5], NOR | 2008 | A | 626 | 8 | 308 (109/199) | 43 | 100 mg tid | 318 (96/222) | 43 | Placebo |
Analysis results of efficacy indicators
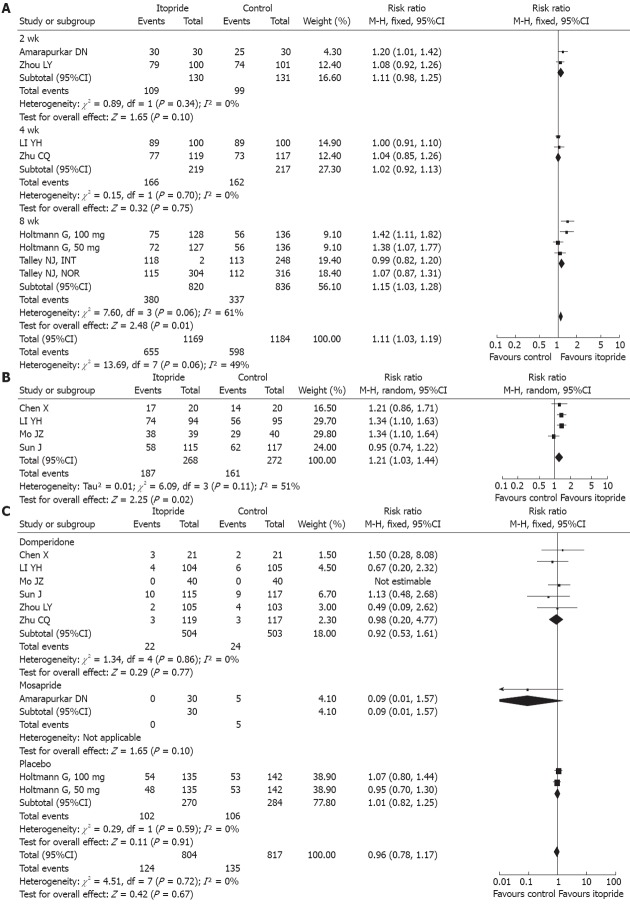
GPA: Six RCT articles[5,13,14,18-20]reported the GPA of itopride in FD patients, of which three were domperidone-controlled, one was mosapride-controlled, and the other two were placebo-controlled. The chi-square value of the test for heterogeneity was 13.69, with an I2 value of 49%, indicating that there was homogeneity of effects among the trials. Therefore, a fixed effect model was used, and the calculated RR value was 1.11 [95%CI: (1.03, 1.19), P = 0.006], as shown in Figure 2A. Itopride improved the GPA of FD patients more significantly than control groups.
Figure 2.
Forest plot for global patient assessment (A), postprandial fullness (B) and adverse reactions (C) with itopride treatment for functional dyspepsia.
Postprandial fullness: Four RCT articles[15-17,20] reported the efficacy of itopride with respect to postprandial fullness of FD patients, all of which were domperidone-controlled. The chi-square value of the test for heterogeneity was 6.09, with an I2 = 51%, indicating that there was heterogeneity of effects among the trials. Therefore, a random effect model was used, and the calculated RR value was 1.21 [95%CI: (1.03, 1.44), P = 0.02], as shown in Figure 2B. Itopride improved the postprandial fullness of FD patients more significantly than domperidone.
Early satiation: Four RCT articles[15-17,20] reported the efficacy of itopride with respect to early satiation of FD patients, all of which were domperidone-controlled; the chi-square value of the test for heterogeneity was 9.18, with a I2 = 67%, indicating that there was heterogeneity of effects among the trials. Therefore, a random effect model was used, and the calculated RR value was 1.24 [95%CI: (1.01, 1.53), P = 0.04]. Compared with domperidone, itopride improved the early satiation of FD patients more significantly.
Epigastric discomfort: Three RCT articles[15,16,20] reported the efficacy of itopride with respect to epigastric discomfort of FD patients, all of which were domperidone-controlled; the chi-square value of the test for heterogeneity was 2.67, with a I2 = 25%, indicating that there was homogeneity of effects among the trials. Therefore, a fixed effect model was used, and the calculated RR value was 1.00 [95%CI: (0.88, 1.14), P = 0.98]. Itopride and domperidone had similar efficacy on epigastric discomfort of FD patients.
LDQ: Two RCT articles[5,13] reported that itopride improved the LDQ scores of FD patients, both of which were placebo-controlled; the chi-square value of the test for heterogeneity was 18.53, and I2 = 84%, indicating that there was heterogeneity of effects between the trials. Therefore, a random effect model was used, and the calculated WMD value was -1.38 [95%CI: (-1.75, -1.01), P < 0.01]. Thus, itopride improved the LDQ scores of FD patients more significantly than placebo.
Incidence of ADRs: Eight RCT articles[13-20] reported the ADRs of itopride in the treatment of FD patients, of which six were domperidone-controlled, one was mosapride-controlled, and the other one was placebo-controlled; the chi-square value of the test for heterogeneity was 4.51, with a I2 = 0%, indicating that there was homogeneity of effects among the trials. Therefore, a fixed effect model was used, and the calculated RR value was 0.96 [95%CI: (0.78, 1.17), P = 0.67], as shown in Figure 2C. Analysis of the sub-groups showed that itopride did not have a higher incidence of ADRs than domperidone, mosapride, or placebo.
Analysis of publication bias
As compared with the control groups, itopride’s funnel plots of GPA, postprandial fullness, early satiation, epigastric discomfort, and ADR all showed a symmetrical shape that was narrow at the top and wide at the bottom, indicating that there was no publication bias.
DISCUSSION
The pathogenesis of FD is far from fully understood, but gastrointestinal motility and visceral sensitivity are proven to play very important roles[21,22] in the occurrence of FD symptoms. Clinically, prokinetic agents, such as domperidone, cisapride, and mosapride, are often used to treat these patients. Recently a meta-analysis by Hiyama[23] showed a significant treatment benefit in favour of prokinetic agents in patients with FD. However, in that study, itopride is rarely involved. Given the concern for safety and efficacy of the existing prokinetic agents, a novel agent that is safer and more effective is urgently needed. Itopride is a prokinetic agent that has a completely different mechanism of action from existing ones; it works by both antagonizing dopamine receptors and inhibiting the activity of acetylcholinesterase. It not only stimulates release of acetylcholine, but also inhibits its degradation, thus promoting gastrointestinal motility. There are a few well-designed RCTs on the efficacy of itopride in the treatment of FD, and the reported efficacy was controversial. Therefore, a meta-analysis of previously published high quality RCTs was conducted.
In the present study, when compared with the control groups, the RRs of itopride for GPA, postprandial fullness, and early satiation of FD patients indicate that this drug could significantly improve the GPA scores, postprandial fullness, and early satiation in FD patients. However, it did not improve epigastric discomfort more significantly than the comparator, which could be a result of itopride’s action of increasing postprandial gastric receptive relaxation[24] and gastrointestinal motility[20]. To further evaluate the efficacy of itopride in improving the symptoms of FD patients, the LDQ was used to evaluate FD patients’ symptoms at baseline and after treatment, and the calculated WMD was -1.38 [95%CI: (-1.75, -1.01), P < 0.01], suggesting that the drug could significantly reduce the LDQ scores of FD patients, which made the results more convincing. As for safety, it showed that the incidence of ADRs was no higher for itopride than for domperidone, mosapride, or placebo. The ADRs attributed to itopride were mainly abdominal pain and diarrhoea, which were all mild to moderate, without clinically related changes in the electrocardiogram, particularly prolongation of QT intervals. This appears to be different from other prokinetic agents, possibly because the polarity of itopride largely prevents it from entering the brain or the CNS[25]. In addition, as compared with other dopamine receptor antagonists, itopride caused a much lower incidence of CNS-related ADRs and hyperprolactinaemia while keeping dopamine active. Meanwhile, there were fewer drug interactions of itopride compared with other prokinetic agents[26], probably because itopride is metabolized by a monooxygenase, while mosapride and other prokinetics are metabolized by cytochrome P450, as reported by Mushiroda[26].
Considering the discrepancy in contradictory trial results[12,20], study design issues are important. There were several probable reasons, including heterogeneity of the conditions and differences in patient selection. In the Tally’s trial, the requirement that all patients had to be H. pylori negative, exclusion of heartburn and that the LDQ score needed to be > 9 at baseline meant high intensity scores for the typical symptoms of pain and fullness were needed for LDQ, all of which might contribute to the high placebo response rate[12]. On the other hand, the majority of dyspeptic subjects overlap with heartburn symptoms as well as H. pylori infection, and heartburn also is a predictor of response, so the exclusion criteria were much stricter in the Tally’s study, as Veldhuyzen mentioned[27].
This meta-analysis covered a wide range of high-quality articles, and all studies included were randomized controlled trials RCTs. In addition, the diagnostic criteria for inclusion of articles were uniform. Considering publication bias, that is, the disproportionate publication of research articles with a positive result than of those with a negative result, an effort was made to collect as full a range of related literature as possible through many different approaches (including computer search, manual search, and literature tracing), and repeated publications were excluded. All nine studies included in this analysis had definitive inclusion criteria and baseline descriptions of sex, age, disease severity, and concomitant medications of the population included, and the ratios of the population in the study groups and the control groups were reasonable.
However, the present study did have some limitations. Firstly, the ethnic groups of the populations in the articles were varied. Race and/or western lifestyle are important risk factors[28,29]. Secondly, because of differences in trial design, comparators used for the control group, and follow-up, there was a large degree of heterogeneity among the studies included, as well as in the GPA, early satiation, and LDQ scores. For this reason, a random effect model was used for the meta-analysis, which probably affected the results of the evaluation. Thirdly, Helicobacter pylori may play a role in pathogenesis of functional dyspepsia[1]. However, seven of the FD trials included in this meta-analysis were from Asia, which has a higher prevalence of Hp, and this probably affected the results.
In summary, the results of this meta-analysis suggest that itopride has therapeutic benefits with respect to GPA, postprandial fullness, early satiation, and the LDQ of FD patients, with a lower incidence of ADRs. However, because of the existence of heterogeneity, further studies of more high-quality RCTs with consistent indicators are probably warranted to validate the safety and efficacy of itopride.
ACKNOWLEDGMENTS
We would like to acknowledge and thank Min Zhao and Bing-Bing Chen for their help with searching for trials and their comments.
COMMENTS
Background
Functional dyspepsia (FD) is a complex problem resulting from the interaction of gastric dysmotility, visceral hypersensitivity, and psychological factors, which causes delayed gastric emptying, abnormal gastric regulation, and aberrant myoelectricity. As many as 60% of FD patients have gastric dysmotility. Itopride works by antagonizing dopamine D2-receptors and inhibiting acetylcholinesterase. In a multicentre, randomised, double-blind, placebo-controlled trial, itopride significantly improved symptoms in patients with FD, and showed a greater rate of response than placebo. However, it was recently reported that itopride was not more effective in showing a difference in symptom response from placebo in FD. Therefore, it is necessary to perform a comprehensive meta-analysis to evaluate more objectively the efficacy and safety of itopride in the treatment of FD.
Research frontiers
Although prokinetic agents are proven to improve symptoms in FD patients, metoclopramide is associated with a high incidence of central nervous system-related adverse drug reactions, domperidone can elevate serum prolactin levels and cause gynecomastia and galactorrhea, and cisapride has been withdrawn due to safety concerns including high risk of prolonging the QT interval and severe arrhythmias. It is essential to search for more effective and safe drugs.
Innovations and breakthroughs
The study comprehensively searched for all randomised controlled trials involving itopride in the treatment of FD, and used meta-analysis to analyze the effects and safety of itopride.
Applications
The results indicate that itopride has therapeutic benefits with respect to Global Patient Assessment, postprandial fullness, early satiation, and the Leeds Dyspepsia Questionnaire of FD patients, with a lower incidence of adverse drug reactions.
Peer review
The author investigated the efficacy of itopride for functional dyspepsia in a meta-analysis. The article is overall easy to understand, and the method of meta-analysis is correct. The results are interesting and suggest that itopride shows good efficacy for the treatment of global patients assessment, postprandial fullness, and early satiety in patients with FD.
Footnotes
Supported by The Natural Science Foundation of Zhejiang Province of China, No. LY12H29002; and Traditional Chinese Medicine Science Foundation of Zhejiang Province of China, No. 2011ZB032
Peer reviewer: Zhao-Shen Li, MD, PhD, Professor, Department of Gastroenterology, Changhai Hospital, Second Military Medical University, 168 Changhai Road, Shanghai 200433, China; Fan-Rong Liang, Chengdu University of Traditional Chinese Medicine, Chengdu 610075, Sichuan Province, China
S- Editor Huang XZ L- Editor A E- Editor Zhang DN
References
- 1.Miwa H, Ghoshal UC, Gonlachanvit S, Gwee KA, Ang TL, Chang FY, Fock KM, Hongo M, Hou X, Kachintorn U, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–168. doi: 10.5056/jnm.2012.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan AC, Bao T, Chakkaphak S, Chang FY, Ke M, Law NM, Leelakusolvong S, Luo JY, Manan C, Park HJ, et al. Validation of Rome II criteria for functional gastrointestinal disorders by factor analysis of symptoms in Asian patient sample. J Gastroenterol Hepatol. 2003;18:796–802. doi: 10.1046/j.1440-1746.2003.03081.x. [DOI] [PubMed] [Google Scholar]
- 3.Mahadeva S, Yadav H, Everett SM, Goh KL. Economic impact of dyspepsia in rural and urban malaysia: a population-based study. J Neurogastroenterol Motil. 2012;18:43–57. doi: 10.5056/jnm.2012.18.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahadeva S, Goh KL. Burden of Dyspepsia in Rural and Urban Asia: Author’s Reply. J Neurogastroenterol Motil. 2012;18:230. doi: 10.5056/jnm.2012.18.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 6.Hou XH, Li Q, Zhu L, Xie X, Chen JD. Correlation of gastric liquid emptying with various thresholds of sensation in healthy controls and patients with functional dyspepsia. Dig Dis Sci. 2004;49:188–195. doi: 10.1023/b:ddas.0000017437.20932.8b. [DOI] [PubMed] [Google Scholar]
- 7.Quartero AO, de Wit NJ, Lodder AC, Numans ME, Smout AJ, Hoes AW. Disturbed solid-phase gastric emptying in functional dyspepsia: a meta-analysis. Dig Dis Sci. 1998;43:2028–2033. doi: 10.1023/a:1018803129779. [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–1337. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–1780. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698–1703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga Y, Kimura T, Miyashita N, Morikawa K, Nagata O, Itoh Z, Kondo Y. Characterization of acetylcholinesterase-inhibition by itopride. Jpn J Pharmacol. 1994;66:317–322. doi: 10.1254/jjp.66.317. [DOI] [PubMed] [Google Scholar]
- 13.Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354:832–840. doi: 10.1056/NEJMoa052639. [DOI] [PubMed] [Google Scholar]
- 14.Zhou LY, Li BC, Lin SL, Li AY, Dong XY, Li ZS, Yuan YZ, Yu ZL, Liu XG, Wang HJ, et al. A Multi-centre Clinical Trial on Itopride Hydrochloride for Treatment of Functional Dyspepsia. Zhongguo Linchuang Yaolixue Zazhi. 2000;16:403–407. [Google Scholar]
- 15.Sun J, Zhang CL, Chu Y, Yuan YZ, Li ZS, Liu XG, Luo HS. A multi-center, double-blind, randomized and controlled trial of itopride hydrochloride in treatment of functional dyspepsia. Shanghai Yixue. 2003;26:227–229. [Google Scholar]
- 16.Mo JZ, Li DG, Jiang JH, Jiang YB, Wang XP, Gong ZH, Cao ZJ. A multi-center clinical trial of itopride hydrochloride in the treatment of functional dyspepsia. Zhongguo Xinyao Zazhi. 2003;12:467–469. [Google Scholar]
- 17.Chen X, Hu NZ, Xie HJ, Li BK, Xu JM. Effect of itopride hydrochloride on functional dyspepsia. Zhongguo Linchuang Yaolixue Zazhi. 2004;20:25–29. [Google Scholar]
- 18.Amarapurkar DN, Rane P. Randomised, double-blind, comparative study to evaluate the efficacy and safety of ganaton (itopride hydrochloride) and mosapride citrate in the management of functional dyspepsia. J Indian Med Assoc. 2004;102:735–77, 760. [PubMed] [Google Scholar]
- 19.Zhu CQ, Mao YM, Zeng MD, Dong SX, Xu GM, Wang GS, Li YM, Cai JT. A clinical study of hydrochloride itopride in the treatment of functional dyspepsia. Zhongguo Yaoke Daxue Xuebao. 2005;6:580–583. [Google Scholar]
- 20.Li YH, Gong PL, Hou XH, Chen J, Liu NZ, Tian DA, Tang FA, Feng CW, Yang YX, Li HB. Itopride in treatment of 104 patients with functional dyspepsia: a randomized, double-blind controlled clinical trial. Zhongguo Xinyao Yu Linchuang Zazhi. 2005;7:524–528. [Google Scholar]
- 21.Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37–II42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689–696. doi: 10.1111/j.1572-0241.2001.03521.x. [DOI] [PubMed] [Google Scholar]
- 23.Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Chayama K, Haruma K. Treatment of functional dyspepsia with serotonin agonists: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2007;22:1566–1570. doi: 10.1111/j.1440-1746.2006.04723.x. [DOI] [PubMed] [Google Scholar]
- 24.Choung RS, Talley NJ, Peterson J, Camilleri M, Burton D, Harmsen WS, Zinsmeister AR. A double-blind, randomized, placebo-controlled trial of itopride (100 and 200 mg three times daily) on gastric motor and sensory function in healthy volunteers. Neurogastroenterol Motil. 2007;19:180–187. doi: 10.1111/j.1365-2982.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Nishizawa T, Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. J Gastroenterol. 2006;41:513–523. doi: 10.1007/s00535-006-1847-5. [DOI] [PubMed] [Google Scholar]
- 26.Mushiroda T, Douya R, Takahara E, Nagata O. The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: comparison with cisapride and mosapride citrate. Drug Metab Dispos. 2000;28:1231–1237. [PubMed] [Google Scholar]
- 27.Veldhuyzen Van Zanten SJ. Pitfalls in designing trials of functional dyspepsia: the ascent and demise of itopride. Gut. 2008;57:723–724. doi: 10.1136/gut.2007.139923. [DOI] [PubMed] [Google Scholar]
- 28.Mahadeva S, Raman MC, Ford AC, Follows M, Axon AT, Goh KL, Moayyedi P. Gastro-oesophageal reflux is more prevalent in Western dyspeptics: a prospective comparison of British and South-East Asian patients with dyspepsia. Aliment Pharmacol Ther. 2005;21:1483–1490. doi: 10.1111/j.1365-2036.2005.02455.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahadeva S, Yadav H, Rampal S, Everett SM, Goh KL. Ethnic variation, epidemiological factors and quality of life impairment associated with dyspepsia in urban Malaysia. Aliment Pharmacol Ther. 2010;31:1141–1151. doi: 10.1111/j.1365-2036.2010.04270.x. [DOI] [PubMed] [Google Scholar]