Abstract
A 60-year-old lady with type 2 diabetes mellitus and hypertension was referred for fever, bilateral loin pain, and renal failure. Investigations showed severe acute renal failure, bilateral renal papillary necrosis (RPN), urinary tract infection (E. coli), and infection with leptospirosis: Leptospira icterohemorrhagica; serovar hardjo. Renal biopsy showed tubulointerstitial nephritis with mesangial proliferation. The diagnosis was bilateral RPN in a diabetic lady with acute renal failure due to leptospirosis. The patient was successfully treated with hemodialysis, injection ceftriaxone, and benzyl penicillin.
Keywords: Diabetes mellitus, leptospirosis, renal papillary necrosis
Introduction
Leptospirosis is a common cause of acute kidney injury (AKI) in the tropics. The organism typically produces an acute tubulointerstitial nephritis, and has the propensity to damage the smaller blood vessels of the kidney. However, no association with papillary necrosis has so far been reported in the literature.
Case Report
A 60-year-old lady, a known case of type 2 diabetes mellitus for 15 years and hypertension for 3 years, presented to the clinic with fever, chills, and bilateral loin pain of 4 days duration. No other co-morbidities like chronic kidney disease or coronary artery disease were known in her in the past and she was apparently in normal state of health prior to the presenting illness.
She developed high-grade fever with chills and rigors. Fever was accompanied by dysuria and bilateral loin pain which was constant and aching in nature. Two days following the onset of fever and loin pain, she developed hematuria and subsequently her urine output started declining and she was anuric at the time of admission to the hospital. She also experienced severe generalized muscle ache. There was no arthralgia, skin rash or hemoptysis. Her medications included glipizide and multivitamin.
At the time of admission, she was conscious, irritable, febrile, and tachypneic. Her conjunctivae appeared suffused. There was no icterus, purpura, or lymphadenopathy. Muscle tenderness in limbs was noted. Her pulse was 104/min, BP–110/70 mm Hg, respiratory rate 23/min, temperature 101°F, oxygen saturation 95% at room air. Rales were appreciated in the basal regions of the chest. Examination of abdomen revealed no organomegaly, but both renal angles were tender on palpation.
Investigations revealed hemoglobin of 10.8 g/dL; leukocyte count of 28400 cells/μL; differential count revealed polymorphs 91%, lymphocytes 8%; platelet count 60000/μL. The blood film revealed normocytic normochromic RBCs, neutrophilic leucocytosis with toxic granulations. No hemoparasites were detectable. Urine analysis revealed 1+ protein, 40-50 WBCs/high power field, 20-25 RBCs/high power field, bile salts and pigments - negative, ketone-negative. Blood sugar on admission was 208 mg/dL; blood urea nitrogen 74 mg/ dL; serum creatinine 5.4 mg/dL; serum sodium 138 mmol/L; potassium 4.8 mmol/L; bicarbonate 7.0 mmol/L; chloride 104 mmol/L; plasma anion gap +27 mmol/L, pO290 mm Hg., pCO226 mm Hg. Serum total bilirubin measured 2.1 mg/dl; SGOT – 44 U/L; SGPT – 34 U/L; alkaline phosphatase – 242 U/L; serum total protein 6.5 g/L; and serum albumin measured 2.4 g/ dL. Urine culture revealed significant growth of E. Coli., (>105 colony forming units/ ml) sensitive to ciprofloxacin, amikacin, and ceftriaxone. However, blood culture grew no organism. Ultrasonogram of the abdomen revealed bilaterally enlarged kidneys with hydroureteronephrosis. No radioopaque lesion could be made out in the ultrasonogram or X-ray KUB region. An MR urogram performed to further evaluate the obstruction [Figure 1] revealed bilateral renal papillary necrosis (RPN) with bilateral midureteric obstruction.
Figure 1.
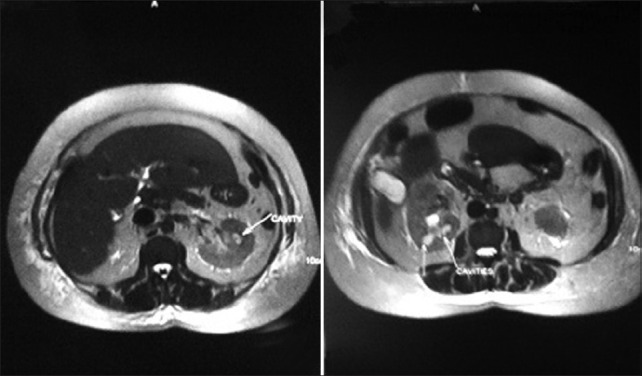
MR urogram demonstrating multiple cavities in the region of renal papillae
Thrombocytopenia and severe myalgia prompted a search for leptospirosis, a common cause of AKI in this part of the country. The organism could not be isolated from the urine or blood. However, the MAT (Microscopic Agglutination Test) done in the second week of the illness detected Leptospira icterohemorrhagica; serovar hardjo. Serologic investigations for dengue were negative.
She was subjected to a USG-guided renal biopsy which revealed acute tubular necrosis, interstitial inflammation, and mild mesangial proliferation [Figure 2a–d].
Figure 2.
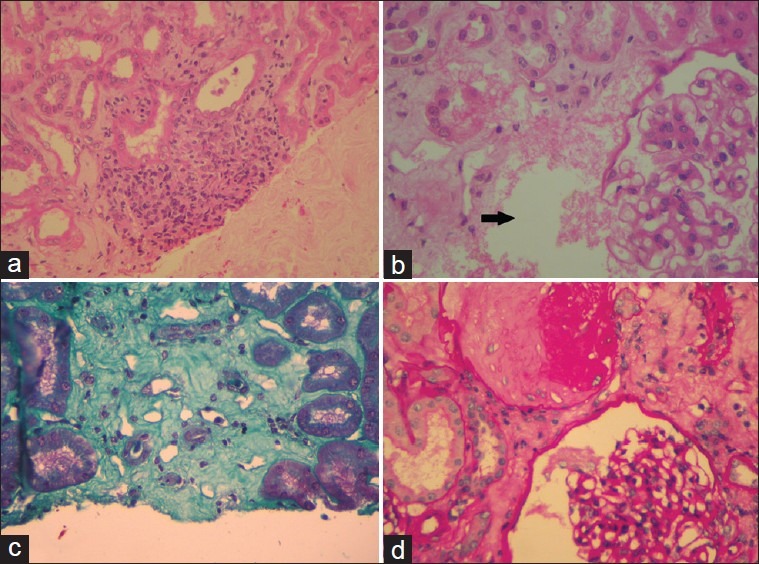
(a) Infiltration of the interstitium by mononuclear cells. (b) Arrow points to an area of tubular necrosis. (c) Interstitial fibrosis appreciated with Massons Trichrome. (d) Glomerulus demonstrating mesangial proliferation
The patient was treated with hemodialysis and her antibiotics included benzyl penicillin and ceftriaxone. Gradually, the fever settled, her general condition stabilized, and renal function started to improve. By the 20th day of the illness, serum creatinine was 1.7 mg/ dL and platelet count was 190,000/μL and patient was discharged. Follow-up at 3 months revealed a serum creatinine of 1.4 mg/dL.
Discussion
Infectious diseases contribute to a major burden of AKI in the tropics. Acute diarrheal disease, leptospirosis, dengue fever, and falciparum malaria are leading causes of community acquired AKI in this part of the country.[1]
This patient presented with fever, AKI, and thrombocytopenia. AKI was multifactorial in origin – E. coli urinary tract infection (UTI) and sepsis, leptospiral infection, and bilateral ureteric obstruction were apparent contributory factors.
Leptospirosis, a spirochetal zoonosis, is a common cause of AKI in this part of the country.[1] The clinical manifestations of leptospirosis vary from that of subclinical presentation to potentially fatal Weil's disease characterized by icterus, renal failure, and coagulopathy. The other known manifestations of the infection include pulmonary hemorrhage, meningitis, and febrile illness without icterus or renal involvement. The mechanism of renal failure in leptospirosis is tubulointerstitial damage that is mediated by the migration of the organism and the elaboration of virulent toxins including the products of lysis of the organism. The outer membrane protein (OMP) of the organism contributes to the tubular damage. Even though tubular damage and interstitial inflammation are well known in leptospirosis, no association with RPN has so far been reported in the literature to our knowledge. The association maybe postulated as being due to sepsis and urinary tract infection. The patient was probably dehydrated from the fever which was present prior to admission predisposing the diabetic lady to papillary necrosis on the background of the leptospira infection.
For convenience, the renal pathologic changes in leptospirosis are described here based on location. Interstitial nephritis is the basic renal lesion of leptospirosis. There is mononuclear cell infiltration in a diffuse or focal manner around blood vessels or glomeruli. Tubular necrosis, interstitial edema, and interstitial hemorrhage are other renal manifestations.[2–4] Glomeruli are usually unremarkable. Mesangio proliferative glomerulonephritis is sometimes observed.[3] Vasculitis with focal hemorrhage may be observed in the acute phase of the disease, but might not be seen in the biopsied material that is taken in the later course of the disease. Platelet aggregation is noted in the corticomedullary capillaries in experimental animals, and there is segmental necrosis of endothelium causing interstitial hemorrhage. C3 deposition in the afferent arterioles might be observed.[5]
Renal biopsy in this patient revealed interstitial mononuclear infiltration, tubular necrosis, and mesangial proliferation, findings consistent with leptospirosis.
RPN is characterized by coagulative necrosis of the renal medullary pyramids and papillae brought on by several associated conditions and toxins that exhibit synergism toward the development of ischemia. Often two or more risk factors coexist and contribute to renal medullary necrosis. The common denominator underlying renal papillary necrosis is ischemia to the medullary pyramids and histologically papillary necrosis can be recognized by an area of coagulative necrosis. Some important etiological factors for papillary necrosis include pyelonephritis due to bacterial uropathogens like E. coli, Klebsiella sp., fungi, etc., urinary tract obstruction; Sickle cell hemoglobinopathies including sickle cell trait; tuberculosis; cirrhosis of liver, chronic alcoholism; analgesic abuse; diabetes mellitus; renal transplant rejection; radiation; systemic vasculitis.
This patient had well-known risk factors for papillary necrosis viz diabetes mellitus and urinary tract infection with E. coli. She denied ingestion of analgesics or non-steroidal anti-inflammatory drugs in the recent past. The propensity of leptospira to affect the tubulointerstitum and renal vasculature raises the question if this could be another contributory factor to the bilateral papillary necrosis seen in this patient.
With institution of appropriate antibiotics and other supportive measures this patient recovered from renal failure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jayakumar M, Prabahar MR, Fernando EM, Manorajan R, Venkatraman R, Balaraman V. Epidemiologic trend changes in acute renal failure - a tertiary center experience from South India. Ren Fail. 2006;28:405–10. doi: 10.1080/08860220600689034. [DOI] [PubMed] [Google Scholar]
- 2.Arean VM. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil's disease) Am J Pathol. 1962;40:393–423. [PMC free article] [PubMed] [Google Scholar]
- 3.Sitprija V, Evans H. The kidney in human leptospirosis. Am J Med. 1970;49:780–8. doi: 10.1016/s0002-9343(70)80059-6. [DOI] [PubMed] [Google Scholar]
- 4.Lai KN, Aarons I, Woodroffe AJ, Clarkson AR. Renal lesion in leptospirosis. Aust N Z J Med. 1982;12:276–9. doi: 10.1111/j.1445-5994.1982.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 5.De Brito T, Bhom GM, Yasuda PH. Vascular damage in acute experimental leptospirosis of the guinea-pig. J Pathol. 1979;128:177–82. doi: 10.1002/path.1711280403. [DOI] [PubMed] [Google Scholar]


