Abstract
Background:
Invasive breast carcinoma is one of the most common cancers of women. Parameters such as lymph node status, tumor grade, and the status of hormone receptors are among the main prognostic determinants of this cancer. Immunohistochemical detection of prostate-specific antigen (PSA) is widely used to identify metastatic prostatic adenocarcinoma. However, its immunoreactivity has been found in some non-prostatic tissues. This study was conducted to assess PSA expression in invasive breast carcinoma and its relationship with routine clinicopathologic parameters.
Materials and Methods:
100 formalin-fixed and paraffin-embedded invasive breast carcinoma tissue specimens from the pathology archive of Alzahra hospital (Isfahan, Iran) were studied for the expression of estrogen receptor (ER), progesterone receptor (PR), HER2/neu, and PSA by immunohistochemistry. Stained sections were classified according to the intensity of staining and the percentage of cells showing PSA staining. The relationship between PSA expression and other markers, age, lymph node status, tumor subtype, and tumor grade was then studied.
Results:
No association was found between PSA expression on one hand and PR, Her2/neu, age, lymph node status, tumor grade, and tumor subtype on the other. PSA score was reversely correlated with ER expression (P = 0.015).
Conclusion:
Despite the reverse relationship between PSA expression and the immunoreactivity of ER, PSA expression was not correlated with other prognostic factors. Therefore, the detection of PSA by immunohistochemistry does not seem to be a significant prognostic parameter in patients with invasive breast carcinoma.
Keywords: Estrogen receptor, immunohistochemistry, invasive breast carcinoma, prostate-specific antigen, progesterone receptor
INTRODUCTION
Breast carcinoma is one of the most common cancers in women. Parameters such as lymph node status, tumor grade, and the status of hormone receptors are important determinants of its prognosis.[1] Prostate-specific antigen (PSA), a 33 kDa kallikrein-like protease, was first extracted from prostatic tissue. Recently, it has also been found in a variety of other tissues including breast.[2,3] According to some studies, PSA does not seem to be a reliable prognostic parameter in breast cancer.[4]
In some previous studies, positive immunoreactivity for PSA has been unrelated to age, ER, and PR status and axillary lymph node involvement.[5] PSA has been found to be associated with better tumor differentiation in some studies[5,6] while others have reported no significant association between the levels of PSA with degree of differentiation.[7] PSA expression has also been found to be related to histologic type of tumor, seen more frequently in lobular and medullary carcinomas.[8]
Lack of the uniformity of the results regarding the relationship between PSA expression and classical prognostic parameters of breast cancer made us to conduct a study to investigate this issue. In the present study, we have assessed PSA expression by immunohistochemistry in invasive breast carcinoma to determine its relationship with routine clinicopathologic parameters including estrogen receptor (ER), progesterone receptor (PR), age, Her2/neu, lymph node status, tumor subtype, and tumor grade.
MATERIALS AND METHODS
In this cross-sectional study with a non-random sampling method, available formalin-fixed and paraffin-embedded tissue samples of invasive breast carcinoma from the pathology archive of Alzahra Hospital (Isfahan, Iran) during a 5-year-interval (2003-2007) have been evaluated.
Only the samples that had been assessed by immunohistochemistry for the expression of Her2/neu, ER, and PR at the time of diagnosis entered the study. The total number of patients with breast cancer during this time interval was 178. Of these, 100 samples fulfilled the entrance criteria.
The relationship of PSA expression with Her2/neu, ER, PR, tumor grade, tumor subtype, lymph node status, and age was studied. Age was classified as <50 vs. ? 50 years. Invasive ductal carcinomas were graded based on the Nottingham modification of Bloom and Richardson grading system.[2]
Immunohistochemical staining
4-Micron sections were prepared from formalin-fixed and paraffin-embedded tissue samples for PSA, ER, PR, and Her2/neu immunohistochemical staining. Tissue sections were deparaffinized in xylene, rehydrated in a series of graded alcohols, and placed in a Tris buffer bath (PH 7.6). Endogenous peroxidase activity was quenched using 3% hydrogen peroxide. Slides were rinsed with deionized water and placed in a Tris buffer bath. The antibodies used were as follow: ER (Mouse monoclonal anti-human ER- DAKO- Clone: 1D5), PR (Mouse monoclonal anti-human PR- DAKO), Her2/neu (Polyclonal rabbit anti-human c-erbB-2 oncoprotein- DAKO), and PSA (mouse anti-human antibody, Dakocytomation, Denmark).
Immunohistochemical staining was visualized, using the LSAB method (Link + strepavidin-HRP from DAKO, Denmark) according to the manufacturer's instructions.
Assessment of immunostaining
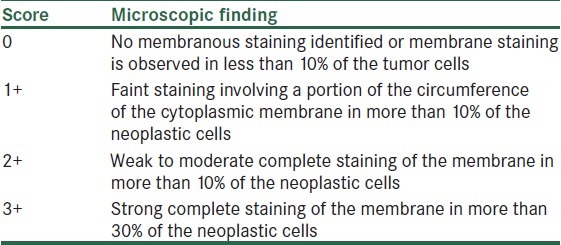
Expression of ER and PR was determined as positive when more than 1% of nuclei showed immunoreactivity. Expression of Her2/neu was scored as 0 to 3 according to Table 1. PSA expression in epithelial cells of normal prostate tissue was used as positive control. A granular staining at the apical aspect of cytoplasm, predominantly adjacent to the nucleus where the Golgi complex is located was observed. To score the expression of PSA, both the extent and intensity of immunoreactivity were considered. The intensity of expression was scored from 0 to 3 as follow: 0: Non-stained, 1: Weak, 2: Moderate, and 3: Strong (the same as positive control). The percentage of stained cells for each staining intensity was estimated. The final composite score was determined by multiplying the intensity of staining and the percentage of stained cells. According to the scoring system for PSA immunoreactivity, the lowest and highest expected final composite scores are 0 and 3, respectively.
Table 1.
Scoring of Her2/neu expression[2]
The data have been presented as mean ± standard error (SE).
Statistical analysis
Quantitative variables have been presented as mean ± SD. Independent sample t-test and one-way ANOVA were used to compare normally-distributed measurements. P value less than 0.05 was considered as statistically significant. Data analysis was performed using SPSS software, version 16 (SPSS Corp, Chicago, USA).
RESULTS
100 tissue samples from patients with invasive breast carcinoma were included in the study. The mean age of these patients was 50.96 ± 13.52 years (Range: 23-83 years). 28% of the cases were PSA positive. The mean, minimum, and maximum scores of PSA expression were 0.226, 0.00, and 2.40, respectively [Figures 1 and 2].
Figure 1.
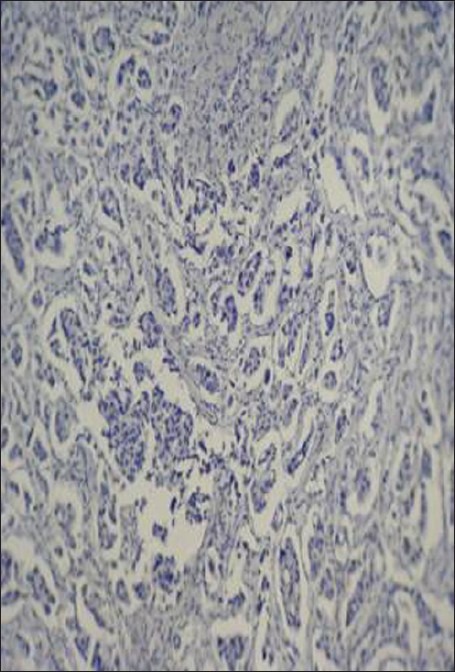
Staining intensity of prostate specific antigen (PSA): No staining of the cytoplasm with PSA in invasive breast carcinoma (Immunohistochemical staining (×400)
Figure 2.
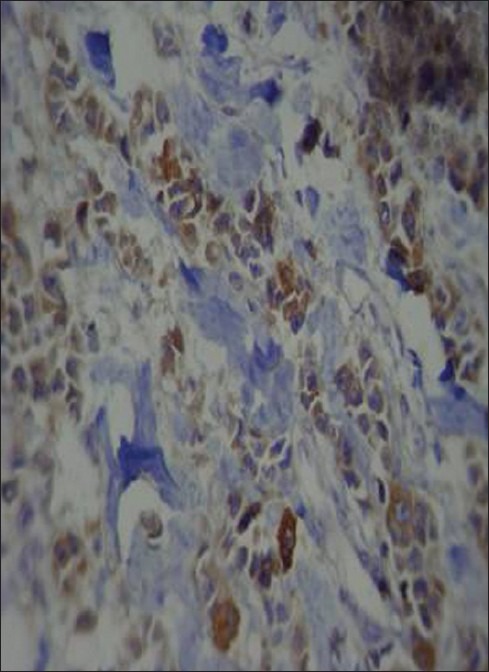
Staining intensity of prostate specific antigen (PSA): Strong diffuse staining of the cytoplasm with PSA in invasive breast carcinoma (Immunohistochemical staining (×400)
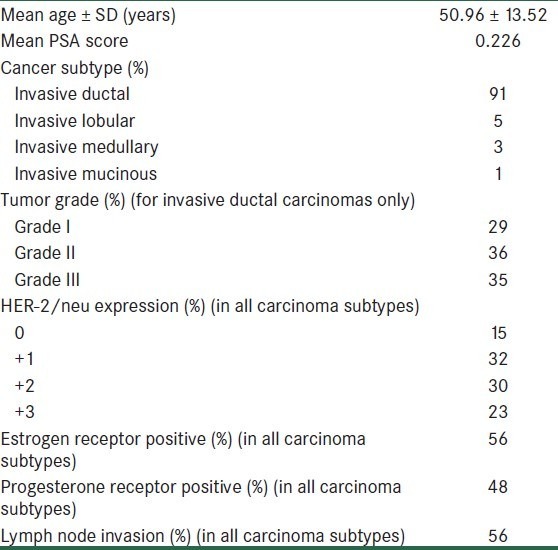
Other clinicopathologic characteristics of specimens have been summarized in Table 2.
Table 2.
Clinicopathologic characteristics of patients with invasive breast carcinoma
Mean score of PSA in specimens with positive ER was 0.172 ± 0.364. In estrogen receptor negative specimens, it was 0.294 ± 0.578. There was a significant difference between the mean PSA score in these 2 groups, and PSA score was found to be reversely correlated with ER expression (P = 0.015).
Mean PSA score in specimens with positive PR was higher than the specimens with negative PR (0.253 ± 0.409 vs. 0.201 ± 0.525). However, the difference between the 2 groups was not statistically significant (P = 0.8).
Mean PSA score was 0.117, 0.258, 0.137, and 0.370 in 0, +1, +2, and +3 Her2/neu scores, respectively. ANOVA method did not show any significant relationship between PSA expression and Her2/neu expression (P = 0.246).
No relationship was found between PSA score and either clinicopathologic parameters of age (≥50 and <50 yrs), subtype of invasive breast carcinoma, grade of tumor, and axillary lymph node status (P > 0.05).
DISCUSSION
Variable clinical outcome of patients with breast cancer has stimulated the search for powerful prognostic markers, which can predict tumor behavior. Although tumor size, axillary lymph node status, and tumor grade are still among the most important prognostic factors, a long list of potential prognostic biomarkers has now been identified. The aim of this study was to determine the expression of prostate-specific antigen in invasive breast carcinoma and its relationship with routine clinicopathologic parameters. A reverse relationship was found between PSA immunoreactivity and expression of ER. However, no relationship was found between PSA expression on one hand and PR and Her2/neu status on the other.
Although some previous studies have indicated an association between PSA expression and positive ER and PR status,[4,9,10] there are some other studies that have not found any relationship between PSA expression and ER immunoreactivity.[5,11–13] Still others have found a reverse relationship between PSA expression and ER status.[7,8] The latter finding is the same as what we found about the relationship of PSA and ER status. In one of the most recent studies in this field conducted by Kraus et al., the number of positive PSA cases was too small to make any significant conclusion about the relationship between PSA immunoreactivity and hormone receptor status[14].
The absence of any relationship between PSA and PR status in our study is the same as the observation of some previous studies.[5,11] However, there are studies that have reported a significant relationship between PSA expression and positive PR.[4,9,15] A reverse relationship has been observed between PSA expression and PR immunoreactivity in some other studies.[7]
Although we couldn’t find any relationship between PSA expression and Her2- neu status, Narita et al. found an inverse correlation between Her2- neu and PSA.[8] This discrepancy may result from the different scoring systems used for the interpretation of HER2- neu status in our study and the study of Narita et al. Since Narita et al. have published their study in 2006, they have considered those cases with strong complete staining of the membrane in more than 10% of the neoplastic cells as 3+ while we have used the criterion of strong complete staining of the membrane in more than 30% of the neoplastic cells as 3+ HER2- neu immunoreactivity according to the newest version of HER2- neu scoring system, recommended by American Society of Clinical Oncology/College of American Pathologists published in 2007.[16]
In our study, there wasn’t any significant relationship between PSA expression and age of the patients. This has been the finding of some previous studies[4,5,12] while others have found a significant relationship between PSA expression and younger age of the patients.[7,17]
Axillary lymph node metastasis is one of the most important prognostic parameters in breast cancer. There is a sharp difference in survival rates between patients with positive and negative nodes. In our study, no association was found between PSA expression and lymph node status. The same finding has been reported in previous studies that have investigated the relationship between PSA expression and lymph node status.[4,5,7,8,11] In the study of Alanen et al., PSA positivity among postmenopausal patients was not associated with axillary lymph node status.[18]
In this study, we didn’t find any correlation between PSA expression and histologic subtype of the tumor. Narita et al. have reported a statistically significant higher expression of PSA in medullary and invasive lobular carcinomas.[8]
In our study, there wasn’t any significant relationship between PSA expression and tumor grade. This has been the same as the finding of some previous studies.[4,7,8,11] Others have found a reverse relationship between PSA expression and tumor grade.[5,6]
As is obviously seen, the results of the current study are different in many aspects from those of some previous similar studies in this field. Some but not all of this discrepancy may be explained by different PSA antibodies and different staining methods used in the studies based on immunohistochemistry technique. Heterogeneity of the studied populations may be another factor, which results in some differences in the results.
CONCLUSION
According to the results of the current study, PSA immunoreactivity does not seem to be a significant prognostic determinant in invasive breast carcinoma. Since data from patients’ outcome were not available, we could not study the relationship between PSA expression and patients’ survival. Of course, the relatively small population of the patients in this study is one of its limitations. Moreover, discrepancies between the results from different studies in this field still remain in our study. This makes further investigations necessary to finally clarify the issue of significance of PSA expression in breast cancer. Further studies with larger sample volume and accurate survival data using more accurate techniques such as PCR for detection of PSA in tissue samples are needed to validate our knowledge about the significance of PSA expression in invasive breast carcinoma.
ACKNOWLEDGMENTS
This study as a research project conducted in Isfahan University of Medical Sciences has been approved and financially supplied by the university vice chancellery for research (project number: 388372). The authors wish to acknowledge the chancellery for their contribution to this work.
Footnotes
Source of Support: University vice chancellery for research (project number: 388372)
Conflict of Interest: None declared
REFERENCES
- 1.Fabian CJ, Kimler BF, Elledge RM, Grizzle WE, Beenken SW, Ward JH. Models for early chemoprevention trials in breast cancer. Hematol Oncol Clin North Am. 1998;12:993–1017. doi: 10.1016/s0889-8588(05)70038-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosai J. Rosai and Ackerman's surgical pathology. 10th ed. Edinburgh: Mosby; 2011. pp. 1681–722. [Google Scholar]
- 3.Diamandis EP, Yousef GM, Luo LY, Magklara A, Obiezu CV. The new human kallikrein gene family: Implications in carcinogenesis. Trends Endocrinol Metab. 2000;11:54–60. doi: 10.1016/s1043-2760(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 4.Ilvan S, Celik V, Cetinaslan I, Calay Z, Ferahman M. Immunohistochemical analysis of prostate-specific antigen in female breast cancer. J BUON. 2004;9:183–6. [PubMed] [Google Scholar]
- 5.Hall RE, Clements JA, Birrell SN, Tilley WD. Prostate-specific antigen and gross cystic disease fluid protein-15 are co-expressed in androgen receptor-positive breast tumours. Br J Cancer. 1998;78:360–5. doi: 10.1038/bjc.1998.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narita D, Raica M, Anghel A, Suciu C, Cimpean A. Immunohistochemical localization of prostate-specific antigen in benign and malignant breast conditions. Rom J Morphol Embryol. 2005;46:41–5. [PubMed] [Google Scholar]
- 7.Foekens JA, Diamandis EP, Yu H, Look MP, Meijer-van Gelder ME, van Putten WL, et al. Expression of prostate-specific antigen (PSA) correlates with poor response to tamoxifen therapy in recurrent breast cancer. Br J Cancer. 1999;79:888–94. doi: 10.1038/sj.bjc.6690142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narita D, Raica M, Suciu C, Cimpean A, Anghel A. Immunohistochemical expression of androgen receptor and prostate-specific antigen in breast cancer. Folia Histochem Cytobiol. 2006;44:165–72. [PubMed] [Google Scholar]
- 9.Zarghami N, Grass L, Diamandis EP. Steroid hormone regulation of prostate-specific antigen gene expression in breast cancer. Br J Cancer. 1997;75:579–88. doi: 10.1038/bjc.1997.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Giai M, Diamandis EP, Katsaros D, Sutherland DJ, Levesque MA, et al. Prostate-specific antigen is a new favorable prognostic indicator for women with breast cancer. Cancer Res. 1995;55:2104–10. [PubMed] [Google Scholar]
- 11.Narita D, Cimpean AM, Anghel A, Raica M. Prostate-specific antigen value as a marker in breast cancer. Neoplasma. 2006;53:161–7. [PubMed] [Google Scholar]
- 12.Heyl W, Wolff JM, Biesterfeld S, Schroder W, Zitzelsberger D, Jakse G, et al. Immunohistochemical analysis of prostate-specific antigen does not correlate to other prognostic factors in breast cancer. Anticancer Res. 1999;19:2263–5. [PubMed] [Google Scholar]
- 13.Poh BH, Jayaram G, Sthaneshwar P, Yip CH. Prostate-specific antigen in breast disease. Malays J Pathol. 2008;30:43–51. [PubMed] [Google Scholar]
- 14.Kraus TS, Cohen C, Siddiqui MT. Prostate-specific antigen and hormone receptor expression in male and female breast carcinoma. Diagn Pathol. 2010;5:63. doi: 10.1186/1746-1596-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Diamandis EP, Sutherland DJ. Immunoreactive prostate-specific antigen levels in female and male breast tumors and its association with steroid hormone receptors and patient age. Clin Biochem. 1994;27:75–9. doi: 10.1016/0009-9120(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. Epub 2006 Dec 11. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Levesque MA, Clark GM, Diamandis EP. Prognostic value of prostate-specific antigen for women with breast cancer: a large United States cohort study. Clin Cancer Res. 1998;4:1489–97. [PubMed] [Google Scholar]
- 18.Alanen KA, Kuopio T, Collan Yu, Kronqvist P, Juntti L, Nevalainen TJ. Immunohistochemical labeling for prostate-specific antigen in breast carcinomas. Breast Cancer Res Treat. 1999;56:169–76. doi: 10.1023/a:1006210627219. [DOI] [PubMed] [Google Scholar]


