Abstract
Background:
Bupivacaine, tramadol, and pethidine has local anesthetic effect. The aim of this study was to compare effect of subcutaneous (SC) infiltration of tramadol, pethidine, and bupivacaine on postoperative pain relief after cesarean delivery.
Materials and Methods:
120 patient, scheduled for elective cesarean section under spinal anesthesia, were randomly allocated to 1 of the 4 groups according to the drugs used for postoperative analgesia: Group P (Pethidine) 50 mg ,Group T (Tramadol) 40 mg, Group B (Bupivacaine 0.25%) 0.7 mg/kg, and Group C (control) 20CC normal saline injection in incision site of surgery. Pain intensity (VAS = visual analogous scale) at rest and on coughing and opioid consumption were assessed on arrival in the recovery room, and then 15, 30, 60 minutes and 2, 6, 12, 24 hours after that.
Results:
VAS scores were significantly lower in groups T and P compared with groups B and C except for 24 hours (VAS rest) and 6 hours (VAS on coughing) postoperatively (P < 0.05). The number of patients requiring morphine were significantly different between the groups (105 doses vs. 87, 56, 46, doses for group C, B, T and P, respectively, P < 0.05) in all the times, except for 2 and 6 hours postoperatively.
Conclusions:
The administration of subcutaneous pethidine or tramadol after cesarean section improves analgesia and has a significant morphine-sparing effect compared with bupivacaine and control groups.
Keywords: bupivacaine, pethidine, post-cesarean section pain, spinal anesthesia, tramadol
INTRODUCTION
Females undergoing cesarean section often wish to be awake postoperatively and to avoid excessive medications affecting interactions with their newborn infant and visitors.[1] Local anesthetics are widely used to provide postoperative pain relief, but analgesia is rarely maintained for more than 4-8 hours with largest acting local anesthetics (bupivacaine, ropivacaine, and levobbupivacaine) after administration incisional.[2] The systemic administration of high doses of opiates has been associated with side effects ranging from pruritus, nausea, and vomiting, to sedation and respiratory depression.[3–5] Subcutaneous administration of opiates is a method of postoperative pain control after cesarean section.[1] Opioids may produce analgesia through peripheral mechanisms.[6] Immune cells infiltrating the inflammation site may release endogenous opioid-like substances, which act on the opioid receptors located on the primary sensory neuron.[6] However, other studies do not support this conclusion.[7,8] Potential advantages of subcutaneous route include no first pass drug metabolism by the liver, improved patient compliance, convenience, and comfort; and consistent analgesia.[9] Local anesthetic effects of opioids have been demonstrated in several studies; tramadol is an analgesic with different spectrums of activity.[10] It cause the activation of both opioid and non-opioid (descending monoaminergic) systems, which are mainly involved in the inhibition of pain. Meperidine has been classified as an agonist of both μ- and K-receptors.[5] The postoperative analgesic effects of subcutaneous wound infiltration with tramadol have not been extensively studied and compared with the same routs of local anesthetics or opioids. To the best of our knowledge, there was no previous study to evaluate the analgesic effect locally infiltrated tramadol, bupivacaine, and pethidine after cesarean delivery. Therefore, we designed the present study to assess the effect of pethidine, tramadol, and bupivacaine wound infiltration before skin closure on postoperative pain relief in patients candidate for cesarean delivery.
MATERIALS AND METHODS
After institutional approval and obtaining an informed patient consent, 120 ASA physical status I-II females, scheduled for elective cesarean section under spinal anesthesia, were included in the study. We excluded patients with suspected or manifest bleeding disturbances, allergy to bupivacaine, tramadol or meperdine, atopia, diabetes mellitus, the presence of liver or kidney diseases, abuse of drugs patients with pregnancy-induced hypertension or pre-eclampsia, bradycardia, arrhythmia, A-V nodal block. Before the study began, a random-number table was used to generate a randomized schedule specifying the group to which each patient would be assigned upon entry into the trial. In case of exclusion, the next patient was randomized per schedule. In the operating room, standard monitoring was applied (the lead II EKG, Pulse oximetery and non-invasive blood pressure monitor.) During the 10 minutes preceding the spinal block, subjects were administered -10 cc/kg Ringer lactate solution via an 18- gauge IV cannula. Spinal anesthesia was performed in all patients at L2-L3 or L3-L4 or L4-L5 interspace with the patients in the sitting position using a 25- gauge whitacre needle. The block was done with 2.5 cc hyperbaric bupivacaine 0.5% in dextrose 8.25%. After injection, the parturient was turned to a supine position, and the operating table was tilted to the left. The sensory block to pinprick was repeatedly tested.
A block level of T4 to T6 was required before surgery started, which took place 15 minutes after injection. The cesarean delivery was performed and at the time of skin closure, while still on the operating table, patients were randomly allocated to 1 to 4 groups. Each group consisted of 30 parturientes: Patients in group P received pethidine 50 mg, in group T treated with tramadol 40 mg, in group B treated with bupivacaine 0.25% 0.7 mg/kg, and in group C received 20 ml normal saline injection in incision site of surgery. All drugs were diluted with sterile normal saline to give 20 ml solutions, which were administered intraincisionally. Also, all drugs were labeled with the randomization number of the patient. Drug administration began at the time of skin closure. Patients and staff involved in data collections were unaware of the patient group assignment. In case of emergency, the anesthesiologist who was responsible for the patient had ready access to the nature of the drugs administered to the patient. On arrival in recovery room, pain intensity at rest and on coughing was assessed by visual analogous scale (VAS) ranging from 0 (no pain) to 10 (worst pain imaginable) and then 15, 30, 60 minutes and 2, 6, 12, 24 hours after arrival in the recovery. If analgesia was considered inadequate at any stage, the anesthesiologist could give additional bulous of morphine 0.08 mg/kg until VAS was <3. Recovery time (the time between arrival and discharge of parturient from the recovery room) was assessed based on Modified Aldrete's Score[9] for all the patients in 4 groups. The frequency of nausea and vomiting, mean arterial blood pressure, drug side effects, metoclopramide and opioid consumption, sedation score evaluated at the same time. Sedation was monitored using the following scale: 1 = alert; 2 = occasionally drowsy; 3 = frequently drowsy; 4 = sleepy, easy to arouse; 5 = somnolent, difficult to arouse. Nausea or vomiting was managed with metoclopramide 0.15 mg/kg as necessary. At the end of the 24 hours, patients were asked their overall opinion of the quality of pain relief they had received using the following- excellent; very good; good; poor. A sample size of 120 patients (4 groups of 30) was calculated to be required with standard errors 0.05, a power of 0.95 and d = 1.2 based on previous relevant clinical data. Statistical analysis was performed with SPSS version 10 software using Chi-Square, ANOVA, and Kruskal-wallis tests. Values for quantitative variables were reported as mean ±SD (standard deviation), and for qualitative variables as count and percent. A value of P < 0.05 was considered statistically significant.
RESULTS
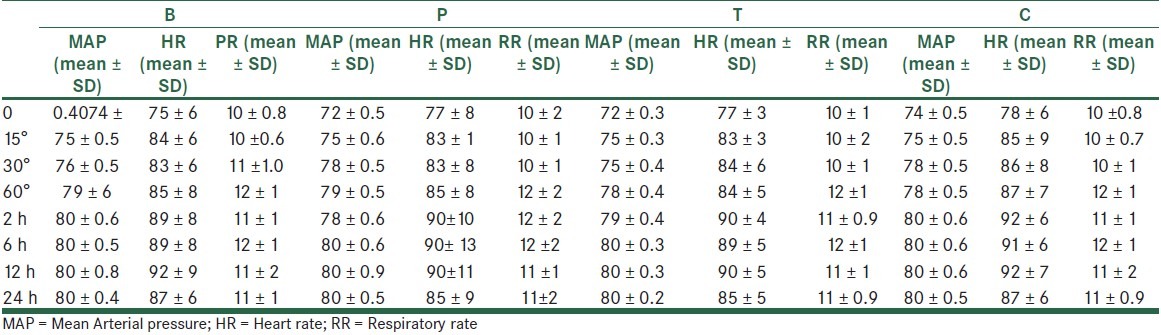
A total of 120 patients were studied. The 4 study groups comparable with respect to age, spinal needling site and basal MAP, HR and RR, as well as the obstetric history [Table 1]. Similarly, recovery times and level of block were unaffected by patient randomization [Table 2]. In all the times, VAS scores both at rest and on coughing were significantly different between the groups, except for 6 hours postoperatively. Also, VAS scores were significantly lower in groups tramadol (T) and meperidine (P) compared with groups bupivacaine (B) and control (C), except for 24 hours (VAS at rest) and 6 hours (VAS on coughing) postoperatively. [Table 3] VAS scores at rest were significantly lower in group P compared with group T at 0, 15, 30 minutes and 24 hours postoperatively. Also, VAS scores, on coughing, were significantly lower in group P compared with group T at 0, 15 minutes, 1, 2, and 24 hours postoperatively. The number of patients requiring morphine were significantly (P < 0.05) different between the groups (105 mg vs. 87, 56, 46 mg for groups control, bupivacaine, tramadol, and pethidine, respectively) in all the times, except for 2 and 6 hours postoperatively. None of the patients received morphine more than 1 dose (0.08 mg/kg) when VAS ≥ 3 postoperatively. The percentage of morphine administration at the different times is shown in Table 3. As shown in Table 4, the incidence of nausea and vomiting, and metoclopramide consumptions was similar for all groups. Patients’ satisfaction was significantly higher in groups P and T when compared with groups B and C [Table 3]. Sedation scores were similar for all groups (P > 0.05). None of the patients had sedation scores more than 3 during 24 hours postoperatively. 3 patients complained of shivering in group T. Also, only 1 patient had tremor and hypotension in group T. Mean arterial blood pressure (MAP), and respiratory rate (PR) were not different between the 4 groups during the study period [Table 5].
Table 1.
Demographics data of patients (Mean MAP, RR, PR) before intervention and spinal needling site in 4 groups
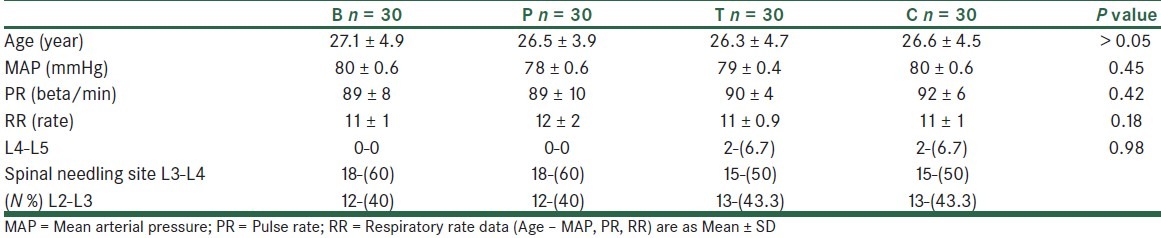
Table 2.
Recovery time, level of spinal block, Mean MAP, RR, PR after in 4 groups
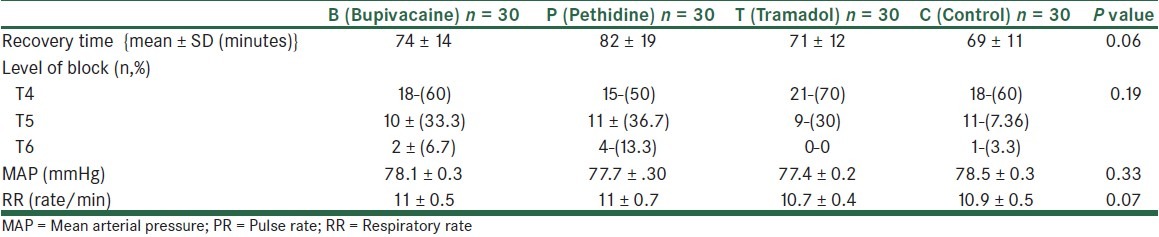
Table 3.
VAS at rest and on coughing and frequency of morphine consumption at recovery o, 15’, 30’, 60’, 2, 6, 12, 24 hours postoperative in the 4 groups
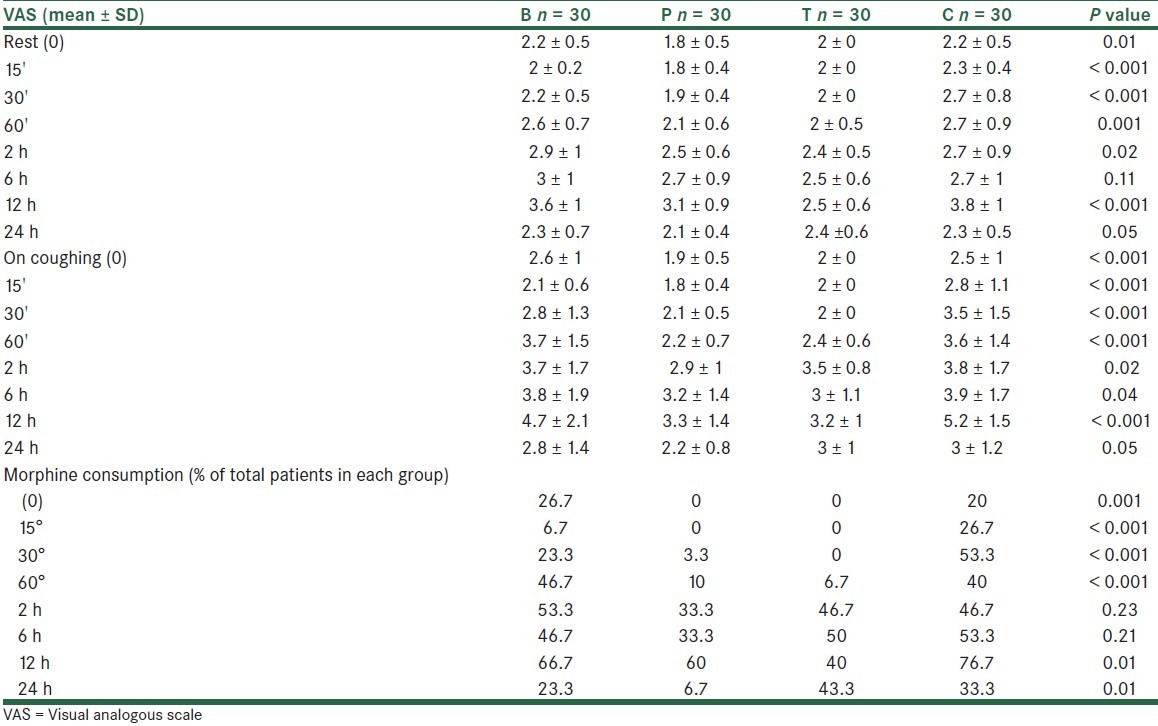
Table 4.
Frequency of side effects, metoclopramide consumption, and satisfaction scores in the 4 groups
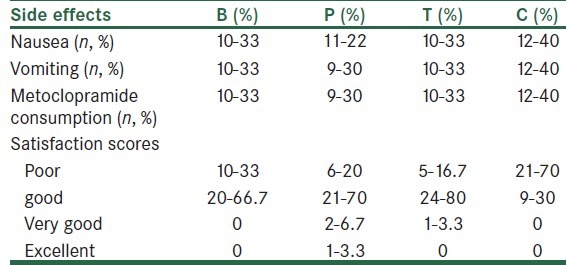
Table 5.
Mean arterial blood pressure (mmHg), heart rate (beta/min) and respiratory rate (rate/min) at the recovery (0), 15, 30, 60’, 2, 6, 12, 24 hours postoperative in the 4 groups
DISCUSSION
We performed a double-blind, prospective, randomized study to compare the effect of subcutaneous bupivacaine, meperidine, and tramadol on postoperative pain relief, morphine requirements, patients satisfaction and side effects after an elective cesarean section. Our data showed that VAS scores both at rest and on coughing were significantly lower in groups P and T when compared with groups B and C. Previous works demonstrated that, subcutaneously tramadol provided local anesthesia equal to lidocaine in patients undergoing minor surgery (lipoma excision and scar revision) under local anesthesia.[11] Moreover, tramadol extended the pain-free period after operation and significantly decreased the need for postoperative analgesia.[11] Also, it was shown that tramadol had a local anesthetic effect similar to that of prilocaine after intradermal injection.[10] Initially, it was thought that tramadol produced its anti-nociceptive and analgesic effects through spinal and supraspinal sites rather than via a local anesthetic action.[12] However, several clinical studies have shown that it might have peripheral local anesthetic type properties.[13–16] By direct tramadol application to the sciatic nerve in rats, it was proven that tramadol exerts a local anesthetic type effect.[15] In the present study, tramadol had a local anesthetic action similar to that of bupivacaine and because of its anti-nociceptive effect, it could extend the postoperative pain-free period. When extracellular sodium concentration decreases, the nerve fiber becomes sensitive to local anesthetics.[17] . Jou et al. suggested that tramadol affects sensory and motor nerve conduction by a similar mechanism to that of lidocaine, which acts on the voltage-dependent sodium channel, leading to an axonal blockage.[18] However, Mert et al. proposed that tramadol might have a mechanism, different from that of lidocaine for producing conduction blocks, the presence of a large Ca2+ concentration in the external medium increases tramadol's activity whereas decreasing lidocaine activity.[19] Tramadol is structurally-related codeine, which is, in fact, a methyl-morphine.[16,20] Tramadol exerts its action on central monoaminergic systems, and this mechanism may contribute to its analgesic effect.[20] After IM injection, tramadol was rapidly and almost completely absorbed, and peak serum concentrations were reached in 45 minutes on average;[21] subcutaneous pethidine infusion analgesia has advantage over conventional intramuscular bolus injections. It was judged acceptable to both patients and ward staff.[22] The findings of the other studies in this regards is in accordance with the results of our study. In our study, the total amount of consumed analgesic in the postoperative period was considerably less in groups P and T compared with groups B and C, respectively.
Bupivacaine wound instillation induced relatively poor post-cesarean analgesia.[23] It should be remembered that tissue response to surgery-induced injury initiates in nociception, inflammation, and hyperalgesia.[24,25] Thus, it is not surprising that agents with different mechanisms of action modulate this cascade. Local anesthetic agents modulate peripheral pain transduction by inhibiting the transmission of noxious impulses from the site of injury.[23] Furthermore, despite the fundamental differences in mechanism of action, basic science investigations suggest that both local anesthetic agents and opioids decrease peripheral and central sensitization via direct central nervous system effect.[9] As our study showed, one study demonstrated that subcutaneous wound infiltration with bupivacaine 0.5% did not decrease morphine requirements on the first postoperative day after lower segment cesarean section.[26]
The frequency of nausea, sedation, and metoclopramide consumptions was low and not significantly different among the 4 groups. Nausea and vomiting have been major side effects of opioid used for postoperative analgesia.[9] Tramadol appeared to cause substantially more postoperative nausea and vomiting than morphine.[27]
The mutagenic effect of tramadol is well-described and recognized as one of its more troublesome side effects.[28–30] The rate of titration of the opioids dose, rather than target dose, is the major determinant of a patient's tolerability.[31,32] In our study, patient satisfaction was significantly higher in groups P and T when compared with groups B and C [Table 4]. However, it should be remembered that the analgesic efficacy is likely dependent upon multiple variables. First, it is possible that by altering the volume and concentration of the drug administered, an improved analgesia may be achieved. Second, the relative efficacy of the analgesic regimens investigated is study design-dependent.[23] Therefore, we suggest that by altering the delivery set-up, different results may be achieved. However, this hypothesis requires further investigations. Again, no difference was found between groups in mean arterial blood pressure, and respiratory rate. This finding is comparable to other studies.[11,22,23,26] Our study challenges some of the claimed clinical differences between pethidine and tramadol. It demonstrates that the effectiveness of pethidine is similar to tramadol. Therefore; we suggest altering the study design and further investigations.
ACKNOWLEDGMENT
The authors wish to sincerely thank the support of all the colleagues in Beheshti Hospital Medical Center affiliated to Isfahan University of Medical Sciences in Isfahan, Iran. Furthermore, our special thanks go to the patients who wholeheartedly and actively assisted us to carry out this research. No conflict of interest existed. We thank Mrs. Tahery and the nurses at the Post-anesthesia Care Unit for their contribution to the study.
Footnotes
Source of Support: Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared
REFERENCES
- 1.McIntosh DG, Rayburn W. Patient-controlled analgesia in obstetrics and gynecology. Obstet Gynecol. 1991;78:1129–35. [PubMed] [Google Scholar]
- 2.Greene NM. Distribution of local anesthetic solutions within the subarachnoid space. Anesth Analg. 1985;64:715–30. [PubMed] [Google Scholar]
- 3.Eisenach J, Grice S, Dewan D. Patient-controlled analgesiafollowing cesarean section: A comparison with epidural and intramuscular narcotics. Anesthesiology. 1988;68:444–8. doi: 10.1097/00000542-198803000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Sinatra R, Morgese L, Chung J. Epidural narcotic and patient-controlled analgesia for cesarean section pain relief. Anesthesiology. 1988;68:454–7. doi: 10.1097/00000542-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Sidawi E, Ramin S, Lucas M, Leveno K, Cunningham G. Cesarean delivery- A randomized trial of epidural versus patient-controlled meperidine analgesia during labour. Anesthesiology. 1997;87:487–97. doi: 10.1097/00000542-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–90. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 7.Heard SO, Edwards WT, Ferrari D, Hanna D, Wong PD, Liland A, et al. Analgesic effect of intraarticular bupivacaine or morphine after arthroscopic knee surgery: A randomized, prospective, double-blind study. Anesth Analg. 1992;74:822–6. doi: 10.1213/00000539-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Picard PR, Tramer MR, McQuar HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): A qualitative systematic review of randomized controlled trialls. Pain. 1997;72:309–18. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda K. Intravenous opioid anesthetics. In: Miller RD, editor. Miller's Anesthesia. 6th ed. Philadelphia, Pennsylvania: Elsevirer Churchill Livingstons; 2005. p. 415. [Google Scholar]
- 10.Atunkaya H, Ozer Y, Kargi E, Babuccu O. Comparison of local anaesthetic effects of tramadol with prilocaine for minor surgical procedures. Br J Anaesth. 2003;90:320–2. doi: 10.1093/bja/aeg079. [DOI] [PubMed] [Google Scholar]
- 11.Altunkaya H, Ozer Y, Kargi E, Ozkocak I, Hosnuter M, Demirel CB, et al. The postoperative Analgesic effet of tramadol when used as subcutaneous local Anesthetic. Anesth Analg. 2004;99:1461–4. doi: 10.1213/01.ANE.0000135640.21229.A0. [DOI] [PubMed] [Google Scholar]
- 12.Langlois G, Estèbe JP, Gentili ME, Kerdilès L, Mouilleron P, Ecoffey C, et al. The addition of tramadol to lidocaine does not reduce tourniquet and postoperative pain during IV regional anesthesia. Can J Anaesth. 2002;49:165–8. doi: 10.1007/BF03020489. [DOI] [PubMed] [Google Scholar]
- 13.Pang WW, Huang PY, chang DP, Huang MH. The peripheral analgesic effect of tramadol in reducing propofol injection pain: A comparison with lidocaine. Reg Anesth Pain Med. 1999;24:246–9. doi: 10.1016/s1098-7339(99)90136-0. [DOI] [PubMed] [Google Scholar]
- 14.Acalovschi I, Cristea T, Margarit S, Gavrus R. Tramadol added to lidocaine for intravenous regional anesthesia. Anesth Analg. 2001;92:209–14. doi: 10.1097/00000539-200101000-00040. [DOI] [PubMed] [Google Scholar]
- 15.Kapral S, Gollmann G, Waltl B, Likar R, Sladen RN, Weinstabl C, et al. Tramadol added to mepivacaine prolongs the duration of an axillary brachial plexus blockade. Anesth Analg. 1999;88:853–6. doi: 10.1097/00000539-199904000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Wagner LE, II, Eaton M, Sabnis SS, Gingrich KJ. Meperidine and lidocaine block of recombinant voltage-dependent Na+channels: Evidence that meperidine is a local anesthetic. Anesthesiology. 1999;91:1481–90. doi: 10.1097/00000542-199911000-00042. [DOI] [PubMed] [Google Scholar]
- 17.Jou IM, Chu KS, Chen HH, Chang PJ, Tsai YC. The effects of intrathecal tramadol on spinal somatosensory evoked potentials and motor evoked responses in rats. Anesth Analg. 2003;96:783–8. doi: 10.1213/01.ANE.0000049683.58980.30. [DOI] [PubMed] [Google Scholar]
- 18.Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439:77–81. doi: 10.1016/s0014-2999(02)01368-7. [DOI] [PubMed] [Google Scholar]
- 19.Shipton EA. Tramadol: Present and future. Anaesth Intensive Care. 2000;28:363–74. doi: 10.1177/0310057X0002800403. [DOI] [PubMed] [Google Scholar]
- 20.Radbruch L, Grond S, Lehmann KA. A risk-benefit assessment of tramadol in the management of pain. Drug Saf. 1996;15:8–29. doi: 10.2165/00002018-199615010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Lintz W, Beier H, Gerloff J. Bioavailability of tramadol after i.m. injection in comparison to i.v. infusion. Int J Clin Pharmacol Ther. 1999;37:175–83. [PubMed] [Google Scholar]
- 22.Gleeson RP, Rodwell S, Shaw R, Seligman SA. Post cesarean analgesia using a subcutaneous pethidine infusion. Int J Gynaecol Obstet. 1990;33:13–7. doi: 10.1016/0020-7292(90)90648-5. [DOI] [PubMed] [Google Scholar]
- 23.Zohar E, Shapiro A, Eidinov A, et al. Postcesarean analgesia: the efficacy of bupivacaine wound instillation with and without supplemental diclofenac. J Clin Anesth. 2006;18:415–21. doi: 10.1016/j.jclinane.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Woolf CJ. Recent advances in the pathophysiology of acute pain. Br J Anaesth. 1989;63:139–46. doi: 10.1093/bja/63.2.139. [DOI] [PubMed] [Google Scholar]
- 25.Woolf CJ, Chong MS. Preemptive analgesia: Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 26.Trotter TN, Hayes-Gregson P, Robinson S, Cole L, Coley S, Fell D. Wound in filtration of local anesthetic after lower segment caesarean section. Anaesthesia. 1991;6:404–7. doi: 10.1111/j.1365-2044.1991.tb09558.x. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins D, Shipton EA, Potgieter D, Van derMerwe CA, Boon J, De Wet C, et al. Comparison of tramadol and morphine via subcutaneous PCA following major orthopedic surgery. Can J Anesthesia. 1998;45:436–42. doi: 10.1007/BF03012579. [DOI] [PubMed] [Google Scholar]
- 28.Demiraran Y, IIce Z, Kocaman B, Bozkurt P. Does tramadol wound infiltration offer an advantage over bupivacaine for postoperative analgesia in children following herniotomy? Paediatr Anaesth. 2006;16:1047–50. doi: 10.1111/j.1460-9592.2006.01910.x. [DOI] [PubMed] [Google Scholar]
- 29.Matkap E, Bedirli N, Akkaya T, Gümüş H. Preincisional local infiltration of tramadol at the trocar site versus intravenous tramadol for pain control after laparoscopic cholecystectomy. J Clin Anesth. 2011;23:197–203. doi: 10.1016/j.jclinane.2010.08.010. Epub 2011 Apr 16. [DOI] [PubMed] [Google Scholar]
- 30.Khajavi MR, Aghili SB, Moharari RS, Najafi A, Mohtaram R, Khashayar P, et al. Subcutaneous tramadol infiltration at the wound site versus intravenous administration after pyelolithotomy. Ann Pharmacother. 2009;43:430–5. doi: 10.1345/aph.1L494. [DOI] [PubMed] [Google Scholar]
- 31.Ayatollahi V, Behdad S, Hatami M, Moshtaghiun H, Baghianimoghadam B. Comparison of peritonsillar infiltration effects of ketamine and tramadol on post tonsillectomy pain: A double-blinded randomized placebo-controlled clinical trial. Croat Med J. 2012;53:155–61. doi: 10.3325/cmj.2012.53.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasapoglu F, Kaya FN, Tuzemen G, Ozmen OA, Kaya A, Onart S. Comparison of peritonsillar levobupivacaine and bupivacaine infiltration for post-tonsillectomy pain relief in children: Placebo-controlled clinical study. Int J Pediatr Otorhinolaryngol. 2011;75:322–6. doi: 10.1016/j.ijporl.2010.11.015. Epub 2010 Dec 18. [DOI] [PubMed] [Google Scholar]


