Abstract
Background:
Application of different kinds of lasers in clinical and experimental studies causes photobiomodulation that works at localized cellular and humoral level on various biological systems. Increased numbers of fibroblasts, myofibroblast, and degranulation of mast cells have been the observed benefits post-irradiation.
Objective:
Was to find out the effect of irradiation with energy densities of 3.38 J/cm2, 8 J/cm2, and 18 J/cm2 on animal tissue (albino wistar rats) in an excisional wound model and to assess changes in biochemical (hydroxyproline) and histopathological levels in excisional wound model.
Materials and Methods:
The animals were divided into 4 groups, which were labeled as L1, diode laser (18 J/cm2), L2 Helium-neon (He-Ne, 8 J/cm2), L3 diode laser (3.38 J/cm2), and sham treatment for control was depicted by C, respectively. Histological and hydroxyproline analysis was performed on 7, 14, 21 days of post-wounding. One-way analysis of variance, ANOVA and Bonferroni's multiple comparison tests were done for tissue hydroxyproline levels.
Results:
There was no significant increase in the hydroxyproline content (P < 0.005) when observed in study group and compared to controls. Whereas significant epithelizations was seen in group treated with He-Ne laser of intensity of 8 J/cm2.
Conclusion:
The experimental observations suggest that low intensity helium-neon laser of 8 J/cm2 intensity facilitated photo stimulation by tissue repair, but failed to show significant tissue hydroxyproline levels in excisional wound model.
Keywords: Diode lasers, helium-neon laser, wound healing, acute and chronic inflammation
INTRODUCTION
Wound healing requires an integrated series of cellular and biochemical events that restore the structural and functional integrity and regain strength of an injured tissue.[1] The pattern of wound healing may be affected by cytokines, endocrines, or by manipulation of wound environment. The circumstance, in which the wound is sustained, clearly influences what bacteria's are carried or enters as foreign bodies.
Inflammation is a potential response intended to eliminate the initial cause of cell injury as well as necrotic cells and tissue resulting from original insult.
Inflammation can be acute or chronic. Acute inflammation is designed to deliver leukocytes and plasma protein to site of injury. Compromises of vascular changes i.e. alteration in vessel calibration resulting in an increase in blood flow (vasodilatation) and cellular events usually seen as migration of leucocytes from microcirculation and accumulation at the foci of injury.[2]
Tissue healing is one of the main claimed effect of laser and focus of varied body of research. Lasers are electromagnetic wave amplifiers, which can produce pencil-like beams of electromagnetic waves with special properties. Helium neon lasers and diode lasers are the commonly used laser in a clinical or experimental scenario. He-Ne lasers are produced in red visible region at 632.8 nm.[3] Diode lasers are commonly made of semi-conductive materials, and electrons can flow more readily in one direction. Sometimes, various laser diodes of assorted wavelength are mounted together and used as cluster probes.[3] Beneficial effects of laser are now understood, and merits of accelerated healing are cornerstone of laser therapy. However, still there is a dearth in literature regarding the role played by lasers in wound healing.
Hence, in this study, different dosages of laser were used to observe photobiomodulation by laser. The objectives of the present study were to irradiate the animal tissue (albino wistar rats) with different energy densities 3.38 J/cm2, 8 J/cm2, 18 J/cm2 and to assess the biochemical (hydroxyproline) and histopathological (capillarity proliferation/neovascularization, fibroblastic proliferation, granulation tissue vascularity and epithelialization) properties in open excisional wound using wistar rat model.
MATERIALS AND METHODS
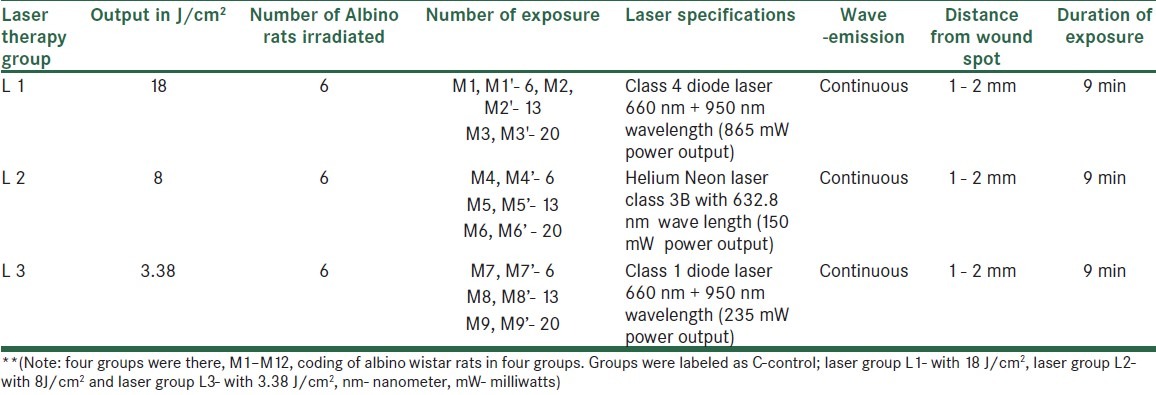
An ethical clearance for the study was obtained before the commencement of the experiment by manipal ethical committee. Materials used in the present study were helium-neon laser class 3B with 632.8 nm wave length (150 mW output), class 1 diode laser 660 nm + 950 nm wavelength (235 mW output), class 4 diode laser 660 nm + 950 nm wavelength (865 mW output), and continuous mode with hand held probes [Table 1].
Table 1.
Illustrating the groups and types of laser being used
Drugs and surgical materials (alloxan, gloves, solvent ether, ketamine, syringes, and saline from hospital pharmacy), reagents for biochemical investigations (Lobachem chemicals, Germany).
Animal selection and care
In-house bred albino wistar strain male rats were used in the study. The range of weight of animals is between 170 to 250 gm. All the animals were maintained at less than 12 hours day light environment. In each cage, only one animal was housed. For housing, the animals’ sterile propylene cages were used with husk bedding at 22 celsius and humidity 55 ± 5%. The animals were kept in hygienic environment, and the cages were changed every day. All the animals were provided with water and food, standard rat pellet by Mohur Lipton India Ltd. was provided. Breeding and maintenance of animals were done in accordance with the guidelines laid by government of India for use of laboratory animals.
Grouping
Animals were randomized into experimental and control group had n = 6 animals in each groups i.e. total of 24 animals in 4 groups (3 experimental and 1 control).
Excisional wound procedures
Animals were assigned into study and control group with 3 animals in each group, comparable in weight and age. The animals were anesthetized with intravenous ketamine of 2 mg/ kg weight. The dorsal furs of animals were shaved with blade. Animals were anesthetized prior to and during creation of the wounds. The rats were inflicted with excision wounds as described by Morton and Malone.[4] After the dorsal fur of the animals was shaved with a stainless steel blade, the anticipated area of the wound to be created was outlined on the back of the animals with methylene blue using a circular stainless steel stencil. A wound of circular area 5 cm by 5 cm was created with full thickness, 4 cm2 excisional wound was created using toothed forceps, no. 15 surgical blade, and pointed scissor. Then wound markings were marked on transparency sheets.
The entire wound was left open, and wistar rats were made to stabilize after the surgical procedure was over for at least 2 days.
Laser therapy protocol
All the equipments were calibrated prior to the study to make sure they deliver an accurate dose during the study protocol. The tissues were irradiated for varying length of time to have a desired influence on the animal's tissue. The dose was calculated according to the formula: D = P × T/A (D = dose in J/cm2; P = laser output in watts; T = irradiation time in sec; A = area of wound measured in cm2). The low energy He-Ne laser provides continuous mode of irradiation. Low-level laser therapy was provided through a fiber optic delivery system over the wound for 5 days a week until complete healing.
Study design
Experimental design: Male albino wistar rats were randomized into 4 groups; L1 (laser group), L2 (laser group), L3 (laser group) and control, each group comprised of 6 albino rats and (n = 24) twenty-four in total.
Tissue histology analysis: Group C- Control, group L1-3 optimum laser dose was given after creating an excisional wound model in wistar rats. Animals were sacrificed at 7th day, 14th day, and 21st day after creation of wound.
Biochemical analysis: Group C- Control, group L1-3 optimum laser dose was given after creating an excisional wound model. Animals were sacrificed on 7th, 14th, and 21st day of post-wounding.
RESULTS
Tissue histology
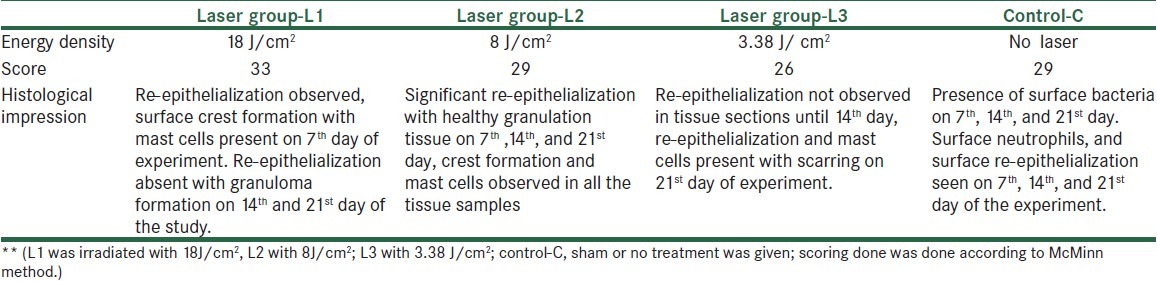
Histological samples were analyzed by a pathologist, and blinding was done for the samples to avoid bias for the results. For histological studies, the animals were euthanized and granulation tissues were collected on 7th, 14th, 21st day post-wounding. Wound specimen together with underlying muscle layers were excised and immediately fixed in bouin's fixative. Tissues were subjected to dehydration with different grades of alcohol, cleared in xylene, and embedded in paraffin wax. Thin sections of 5 μm each tissue were stained with haematoxylin-eosin and qualitatively assessed under the light microscope and observed for different parameters related to inflammation and repair. The list of assessing parameters is given in Table 2. Each parameter was graded further as described by McMinn.[5]
Table 2.
Scoring of histological section by McMinn method
Estimation of hydroxyproline
The tissues were collected for estimation on 7th, 14th, 21st day of post-wounding. The tissue was dried in an oven at 60°C, and the dry weight was noted. The acid hydrolysate of the dry tissue was used for the determination of hydroxyproline. To 0.1 mL acid hydrolysate added 0.4 mL of deionised water, 1.0 mL of 2.5 N NaOH, 1.0 mL 0.01 M CuSO4, and 1.0 mL 6% hydrogen peroxide. Tubes were covered with marbles and boiled for 15 min at 80°C. Then, 2.0 mL para- methylaminobenzaldehyde (Ehrlicks reagent) and 4.0 mL of 3 N H2SO4 was added. Incubated at 80°C for 15 min and the absorbance was read at 540 nm using spectrophotometer.[6]
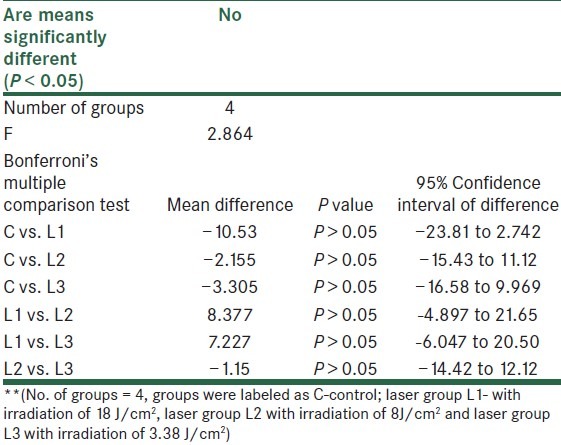
There were 4 groups with 24 wistar rats in total. Results were analyzed using one way ANOVA because there were 4 groups and between the groups, differences were to be known. It showed no difference within groups or on comparison with control [Tables 3 and 4].
Table 3.
Weight of the sample tissue and collagen content in Laser group and control group
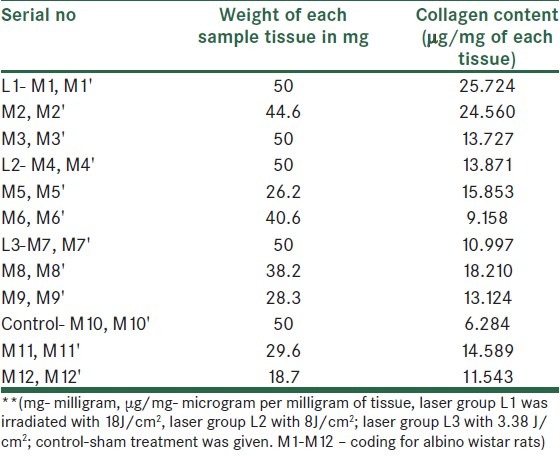
Table 4.
A comparison within group using one way ANOVA
DISCUSSION
Wound healing is a dynamic process involving series of phases
Inflammatory, proliferative, and maturation phase and is usually dependent on the extent of the tissue damage, host response to the wound, and health of the wound area. Irradiation of wound surface with laser depends on biological molecules, such as hemoglobin and melanin are particularly strong absorber of visible and infrared radiations.[7] Therefore, superficial absorption of laser remains unquestionable. What remains questionable is the effect of laser at depth produces any beneficial effects or not. This further depends on whether the biological process stimulated is sensitive enough to the spectrum of wavelength being used to finally produce any positive effect.
A study done by Jhangiri et al. on combination of 670 nm and 810 nm diode lasers for wound healing acceleration in diabetic rats showed no difference in wound parameters in laser or control groups.[8] Reddy et al. in an experimental study stated that the results from the biochemical analysis indicated that the Gallium-Arsenide (Ga-As) laser used in this study significantly increased wound tensile strain and toughness compared to the control wounds. Marginal increases in wound tensile strength (9%) and stress (7%) were observed in the Ga-As laser-treated wounds compared to the controls. Analysis of wound collagen revealed significant increases in total collagen (14%), salt soluble collagen (31%), acid soluble (14%), and insoluble collagen (50%) with simultaneous decrease in pepsin soluble collagen (19%) in the gallium-arsenide (Ga-As) laser-treated wounds compared to controls. Comparisons of these results with He-Ne lasers revealed it superiority over Ga-As lasers, at the parameters of treatment tested, in promoting the wound healing in diabetic rats.[9]
Table 2 shows results of histological section of tissue in experimental and control group, which reveals a distinction in observation and comparison of scores. In control, there was presence of bacterial infection with mild re-epitheliazation post 7th, 14th, and 21st day post-wounding. Whereas there was a clear re-epitheliazation seen in the entire laser-treated group.
Mendez et al., (2004) carried out a study with higher dosage of 50 J/cm2 in an experimentally-created wounds in rats that produced more advanced healing from day 3 to day 7.[10] Accelerated re-epitheliazation was seen in group L1 with dosage of 18J/cm2, but there were shortcomings with a higher dosage, which might have caused photo inhibition after a week of irradiation [Table 2].
Mester et al. (1985) suggested that any dosage from 1-4 J/cm2 is an appropriate dosage for wound healing, but a range of 1-48 J/cm2 is used clinically.[11,12] Hence, in L3 laser-treated group, a low dosage of 3.38 J/cm2 was used to know the effect on wound healing. Re-epitheliazation in this group was absent till 14th day post-wounding and was only seen on 21st day. However, the state of inflammation was lower in this group as seen by absence of edema and lower scores for leukocytes. This dosage of laser had a slower effect on re-epitheliazation, and scarring was seen on 21st day only. A study done by Vijyendra et al. in 2011 concluded that a dosage of 2 J/cm2 was an appropriate optimal dose for tissue histology and hydroxyproline levels.[13] Nevertheless, in our study, we didn’t have findings supporting the merits of low dosage.
Researchers have postulated in past that at higher dosages of laser, calcium ion imbalance is an important indicator of cell damage and dysfunction. Therefore, the effect of higher spectrum of wavelength in laser with greater power output still needs to be demonstrated. A Cochrane review on effect of low level lasers on venous leg ulcers in human by Flemming and Cullum was inconclusive about the evidence and benefits of low level laser therapy (2001).[14] Another review done by Lucas et al. in 2002 on wound healing in animals showed that out of 49 outcomes of the studies included, 30 were positive for granulation tissue. However, after excluding methodologically poor studies, 13 out of 21 were negative for evidence of healing.[15] Further evidence supporting laser for wound healing is complicated by difference in skin between humans and animals.
In laser group irradiated with 8 J/cm2 showed a lesser inflammation, re-epitheliazation post 7 day of irradiation i.e. lower scores for edema and leucocytes, re-epitheliazation was marked by 14th, and closure of wound site was seen by 21st day [Figures 1 and 2]. It has also been claimed that there is a window for an effective photobiostimulation, above threshold, beneficial effect are produced, but if the intensity is too high, the beneficial effects do not occur. The windows are hypothesized to be narrow. Though this concept had been challenged in past by simple statistics, still arndt-schultz law is used to explain the hypothetical narrow ‘windows’, the effect would be appreciable and positive above some dosage but negative at increasingly higher dosages.[16]
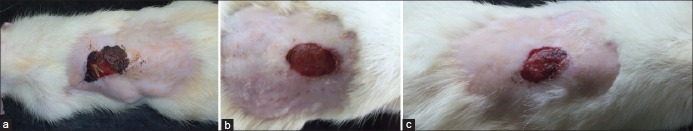
Figure 1.
Irradiation with energy density 8 J/ cm2. (a) Figure depicts wound healing on 7th day of irradiation by He-Ne laser with an energy density of 8 J/cm2, (b) Figure depicts wound healing on 14th day of irradiation by He-Ne laser with an energy density of 8 J/cm2, (c) Figure depicts 21st day of irradiation by He-Ne laser with an energy density of 8 J/cm2
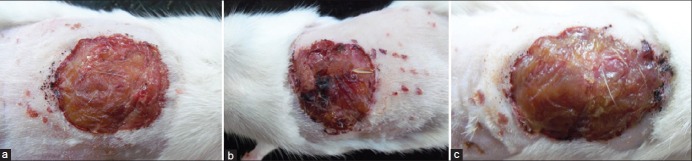
Figure 2.
Control group with no laser irradiation (a) Figure depicts wound healing of control group on 7th day of study, (b) Figure depicts wound healing of control group on 14th day of the study, (c): Figure depicts wound healing of control group on 21st day of study
In laser group treated with 18 J/cm2, re-epitheliazation was significant after one week of irradiation but with subsequent sessions of irradiation, the re-epitheliazation became negative by 21st day. Another shortcoming in this group was development of granuloma on 14th day with this dosage. Granuloma is defined as circumscribed, tiny lesions may be due to localized inflammatory mass.[2] Usually, formation of granuloma is hypothesized to be a protective defense reaction by host, but eventually causes tissue destruction because of persistent poorly-digested antigen. Another study done with too low dosages resulted in insignificant changes in horses following treatment with 2 J/cm2 (Peterson et al. 1999).[17]
Nayak et al. in 2007 reported proliferative action of He-Ne laser at the dosage of 2.1 J/cm2, which demonstrated an increase in hydroxyproline levels that is a reflection of collagen content in the tissue.[4] Similar finding were reported by Vijyendra et al. in 2011; there samples were taken on 5th, 10th, and 15th day of post-wounding with single exposure of He-Ne laser with same dosage.[13] Findings of the previous studies did not correlate with the results in our study, we didn’t find significant changes in the tissue hydroxyproline levels in either laser treated, or in sham-treated group.
CONCLUSION
In present study, we investigated the dose response of He-Ne and diode lasers on animal tissue to observe the resultant cell response. The experimental observation suggests that low intensity He-Ne laser of 8 J/cm2 intensity facilitated photo stimulation by tissue repair, but failed to show any tissue hydroxyproline changes in an excisional wound model. The results indicate that He-Ne laser resulted in more stimulatory effect than diode lasers. From the histopathological examination, we can conclude that 8 J/cm2 of He-Ne lasers appear to be the threshold dosage for an enhanced regeneration of tissue and reduced inflammation.
Future recommendation
However, 8 J/cm2 appears to have a better response to granulation tissue post-wounding for histological section. A 3-day irradiation frequency with 18 J/cm2 may be incorporated for histopathological and biochemical analysis in a diabetic excisional model for wound healing, which might be useful to augment rate of healing in acute and chronic wounds.
ACKNOWLEDGMENTS
The present study is being carried out under the project, no. entitled, “5/3/8/41/2007 RHN”. This study is a part of ongoing project funded by Indian council of medical research (ICMR), under government of India. The authors are grateful to Mr. Sridhar, Animal house facility, KMC, Manipal University for his help during the study. The authors also thank Dr. Padmanabha Udupa, Professor, Department of Biochemistry for his support and encouragement during the study.
Footnotes
Source of Support: Indian Council of Medical Research
Conflict of Interest: None declared
REFERENCES
- 1.Henry M, Thompson J. Clinical Surgery. 2nd edn. London: Elsevier Saunders Limited; 2002. Wound healing and management; pp. 105–13. [Google Scholar]
- 2.Kumar V, Abul AK, Nelson F, Richard M. Robbins Basic Pathology. 8th edition. New Delhi: Reed Elsevier India private limited; 2007. Acute and chronic inflammation; pp. 31–58. [Google Scholar]
- 3.Robertson V, Ward A, Low J, Reed A. Electrotherapy explained principles and practice. 4th ed. Noida: Reed Elsevier India Private limited; 2009. Infrared and visible radiations; pp. 459–98. [Google Scholar]
- 4.Morton JJ, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196:117–26. [PubMed] [Google Scholar]
- 5.McMinn RH, Pritchard JJ. Tissue repair. New York and London: Academic Press; 1969. pp. 1–40. [Google Scholar]
- 6.Nayak BS, Maiya A, Kumar P. Influence of helium-neon laser photostimulation on excision wound healing in wistar rats. Online J Biol Sci. 2007;7:89–92. [Google Scholar]
- 7.Sullivan S, Schmitz T. Physical rehabilitation. 5th ed. Philadelphia: FA Davies Company; 2001. Vascular, lymphatic and integumentary disorders; p. 643. [Google Scholar]
- 8.Jahangiri Noudeh Y, Shabani M, Vatankhah N, Hashemian SJ, Akbari K. A combination of 670 nm and 810 nm diode lasers for wound healing acceleration in diabetic rats. Photomed Laser Surg. 2010;28:621–7. doi: 10.1089/pho.2009.2634. [DOI] [PubMed] [Google Scholar]
- 9.Reddy GK. Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg Med. 2003;33:344–51. doi: 10.1002/lsm.10227. [DOI] [PubMed] [Google Scholar]
- 10.Mendez TM, Pinheiro AL, Pacheco MT, Nascimento PM, Ramalho LM. Dose and wavelength of laser light have influence on the repair of cutaneous wounds. J Clin Laser Med Surg. 2004;22:19–25. doi: 10.1089/104454704773660930. [DOI] [PubMed] [Google Scholar]
- 11.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–9. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 12.Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–5. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- 13.Vijendra P, Rao SBS, Kumar P, Rao L, Mahato KK. Photobiomodulatory effects of He-Ne laser on excision wounds. Proc of SPIE. 2011;7887 [Google Scholar]
- 14.Flemming K, Cullum NA. Laser therapy for venous leg ulcers. Cochrane Database Syst Rev. 1999 doi: 10.1002/14651858.CD001182. [DOI] [PubMed] [Google Scholar]
- 15.Lucas C, Poublon C, Cockrell C, Haan RJ. Wound healing in cell studies and animal experimental mode by Low level Laser therapy were clinically justified? A systematic review. Lasers Med Sci. 2002;17:100–34. doi: 10.1007/s101030200018. [DOI] [PubMed] [Google Scholar]
- 16.Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 17.Petersen SL, Botes C, Olivier A, Guthrie AJ. The effect of low level laser therapy (LLLT) on wound Healing in Horses. Equine Vet J. 1999;31:228–31. doi: 10.1111/j.2042-3306.1999.tb03177.x. [DOI] [PubMed] [Google Scholar]