Abstract
Background:
Due to the heterogeneity of traumatic brain injury (TBI), many of single treatments have not been successful in prevention and cure of these kinds of injuries. The neuroprotective effect of progesterone drug on severe brain injuries has been identified, and recently, the neuroprotective effect of vitamin D has also been studied as the combination of these two drugs has shown better effects on animal samples in some studies. This study was conducted to examine the effect of vitamin D and progesterone on brain injury treatment after brain trauma.
Materials and Methods:
This study was performed on patients with severe brain trauma (Glasgow Coma Scale (GCS) ≤ 8) from April to September, 2011. The patients were divided to 3 groups (placebo, progesterone, progesterone-vitamin D), each with 20 people. Upon the patients’ admission, their GCS and demographic information were recorded. After 3 months, they were reassessed, and their GCS and GOS (Glasgow outcome scale) were recorded. The collected data were analyzed using SPSS 18 software (SPSS Inc., Chicago IL, USA).
Results:
Before intervention, GCS mean of the placebo, progesterone, and progesterone-vitamin D groups were 6.3 ± 0.88, 6.31 ± 0.87, and 6 ± 0.88, respectively. They increased to 9.16 ± 1.11, 10.25 ± 1.34, and 11.27 ± 2.27, respectively 3 months after intervention. There was a significant difference among GCS means of the 3 groups (P-value = 0.001). GOS was classified to 2 main categories of favorable and unfavorable recovery, of which, favorable recovery in placebo, progesterone, and progesterone-vitamin D was 25%, 45%, and 60%, respectively which showed a statistical significant difference among the groups (P-value = 0.03).
Conclusion:
The results showed that recovery rate in patients with severe brain trauma in the group receiving progesterone and vitamin D together was significantly higher than that of progesterone group, which was in turn higher than that of placebo group.
Keywords: Progesterone, severe brain trauma, vitamin D
INTRODUCTION
Injuries arising from brain trauma are among the causes of death and severe disabilities. Management of brain traumatic injuries includes prevention of neurological complications, intracranial pressure monitoring, and surgical procedures. Finding drugs which are effectively neuroprotective is of special importance for prevention of secondary brain injury after trauma. Attempts have been made, for more than 30 years, to discover an effective and harmless compound which acts as a neuroprotective in brain injuries.[1] During last two decades, all the clinical trials phase II and III for the moderate and severe traumatic brain injuries failed.[1] So far, 130 drugs have been effective on brain injury in animal samples,[2] although they were not effective in clinical trials and do not have any neuroprotective effects.[2,3] One reason is the complexity and diversity of the mechanisms related to different types of TBI, which are not cured by a single drug and cover only one or few receptors.[1,4] Among the studied drugs, progesterone (PROG) has been identified as an effective and safe compound, which is a neuroactive steroidal hormone having neurosteroidal activity in central nervous system.[5–7] In traumatic brain injuries and strokes, this hormone causes blood-brain barrier protection, cerebral edema reduction, inflammatory response, necrosis and apoptosis and stimulation of myelin formation, free radicals reduction, and neuronal loss reduction.[8–11]
Similar to progesterone, vitamin D (VDH) is a neurosteroid acting like a PROG in neuroprotective process.[1] It causes the expression of more than 1000 genes that consequently results in activation of many pathways in CNS (central nervous system).[12] Therefore, VHD in combination with PROG may cure the patients. Recent studies have shown that vitamin D deficiency may intensify traumatic brain injury and reduce the effects of other therapies for TBI. This problem becomes important with respect to the elderly, since, of whom more than 50% suffer from vitamin D deficiency.[13] There are evidences that 30 to 50% of American people suffer from vitamin D deficiency, so that, all the patients with TBI of any age are at risk of unfavorable outcome.[13] Given that the studies examining the effect of the combined PROG and VDH on neural recovery after TBI were most conducted on animals, the present study assessed the effect of these two compounds on outcome improvement in patients with TBI.
MATERIALS AND METHODS
This clinical trial was performed in Alzahra Hospital, Isfahan, Iran, in 2010.
Inclusion criteria of the study
Patients with brain trauma and diffuse axonal injury
Patients with GCS < 8
Exclusion criteria of the study
Patient's legal representative does not consent to patient's participation in the study.
Sampling method
Simple random
Number of samples
20 patients were selected in each group through simple random sampling.
The patients were randomly divided into 3 groups, each with 20 people. Within 8 hours after traumatic injury, the patients in the first group were injected 1 mg/kg of progesterone intramuscularly every 12 hours for 5 days, and the patients in the second group were injected 1 mg/kg of progesterone intramuscularly every 12 hours for 5 days and also 5 μg/kg vitamin D once-a-day for 5 days. The third group as the control group received placebo (Both placebos were injected intravenously). Patients’ level of consciousness was periodically controlled based on Glasgow Coma Scale (GCS) during hospitalization and 1 month after treatment, and Glasgow Outcome Scale (GOS) was controlled after 3 months. GOS criteria were defined as follows:
5 = Good recovery: Normal or near normal recovery
4 = Moderate disability: Disable but independent
3 = Severe disability: Dependent with physical / psychological disabilities
2 = Persistent vegetative state
1 = Dead
Finally, these criteria were divided into 2 categories of “favorable” (good recovery and moderate disability) and “unfavorable” (the other 3 criteria).[5] Analysis of the data was done using SPSS 18 software. Qualitative and quantitative data of the groups were compared using Chi square and One-way ANOVA tests, respectively. Comparison of GCS and GOS variations, before and after intervention and among the 3 groups, was done using repeated measurement ANOVA.
RESULTS
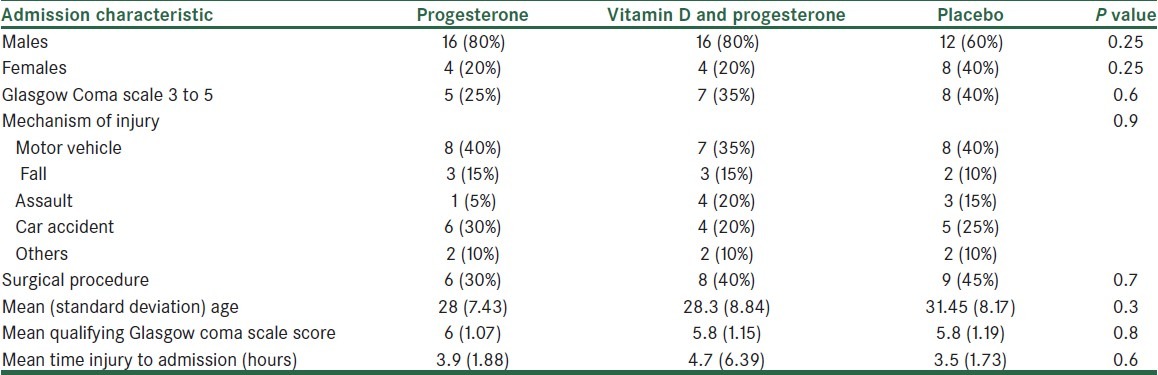
This study was carried out in Trauma Center of Alzahra Hospital, Isfahan, Iran, from April to September, 2011. 3 groups of 20 patients were studied and followed up over 3 months.[5] Demographic specifications showed no significant difference among the 3 groups, i.e., the patients were matched in this regard [Table 1].
Table 1.
Clinical and demographic characteristics between 3 groups
Before intervention, GCS mean of the placebo, progesterone, and progesterone-vitamin D groups comprised 6.3 ± 0.88, 6.31 ± 0.87, and 6 ± 0.88, respectively, which 3 months after intervention, increased to 9.16 ± 1.11, 10.25 ± 1.34, and 11.27 ± 2.27, respectively. There was a significant difference among GCS means of the 3 groups (P-value = 0.001) [Figure 1].
Figure 1.
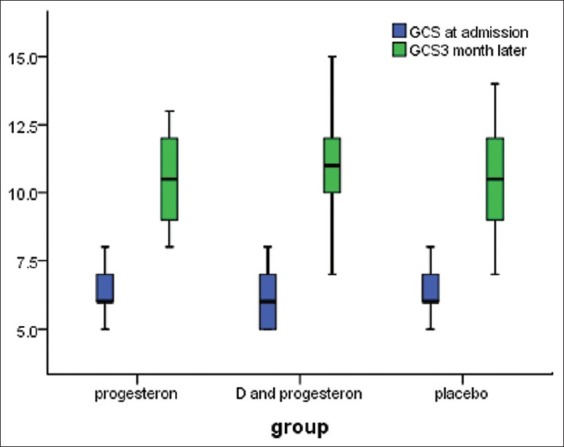
Comparison of GCS at administration and 3 month later between 3 groups
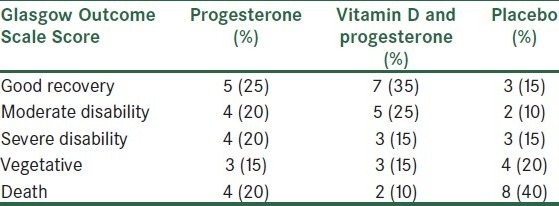
GOS values of the patients, 3 months after trauma, are shown in Table 2. Recovery rate in the group receiving progesterone and vitamin D together was higher than that of other groups; consequently, mortality rate in this group was less than that of others (P-value = 0.03).
Table 2.
Comparison of Glasgow outcome Scale scores between the progesterone, progesterone–Vitamin D, and placebo groups at 3 months later
Favorable responses in placebo, progesterone, and progesterone-vitamin D comprised 25%, 45%, and 60%, respectively which showed a statistical significant difference among the groups (P-value = 0.03) [Figure 2].
Figure 2.
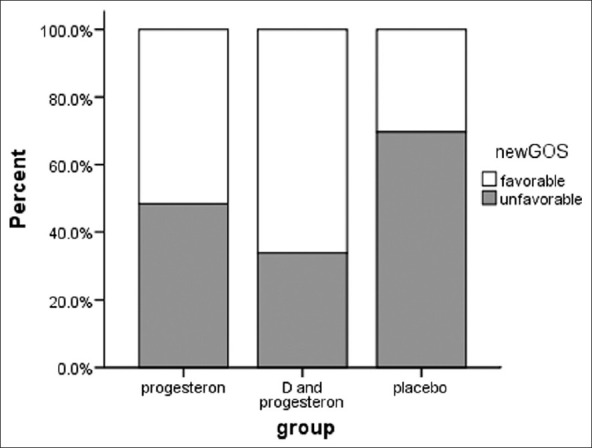
Comparison of dichotomized Glasgow Outcome scale score for patients receiving placebo or progesterone or progesterone and vitamin D after 3 month
In this study, mean mortality rate was 23.3% (14 patients) as the mortality rate in the placebo, progesterone-vitamin D, and progesterone groups was 40% (8 patients), 10% (2 patients), and 20% (4 patients), respectively. These rates showed a significant difference among groups (P-value = 0.000).
DISCUSSION
Numerous studies have been done on TBI treatment. However, most of them were conducted under in vitro and in vivo conditions. There have been also few studies on TBI treatment with combined progesterone and vitamin D, especially on human samples. In this study, progesterone and progesterone-vitamin D were found to improve the patients’ outcome as the recovery rate in the group with combination therapy was significantly more than that in other groups. Moreover, GCS variations and mortality rate in the group with combination therapy were significantly less than those in other groups. Favorable outcome of the group with combination therapy was significantly more than those in other groups. A similar study performed under in vitro condition[1] showed that low dose PROG (0.01, 0.1, and 1.5 μmol/l) did not reduce glutamate-induced cell death, whereas with higher doses (10, 24, 40, and 80 μmol/l), PROG significantly reduced LDH and MTT as the optimum dose of PROG was reported as 20 μmol/l. Furthermore, therapeutic responses to vitamin D were found to have a U-shaped pattern as the most favorable response was found in low doses (0.001 - 0.5 μmol/l), and the least neuroprotective effect was found in higher doses (1 - 10 μmol/l).[1] In the above study, the combination of vitamin D (0.1 μmol/l) and PROG (20 μmol/l) showed no significant preventive effect than the other group, and this was contrary to the U-shaped pattern of response to vitamin D; however, the combined PROG (20 μmol/l) and VDH (20 μmol/l) significantly reduced cell death and was more effective than any of the drugs alone.
Another study on the effect of progesterone alone showed more favorable outcome and much less mortality rate for the patients in progesterone group.[5]
The effect of progesterone on σ1 receptor acts as a competitive inhibitor and may reduce N-methyl-D-aspartate (NMDA) glutamate signaling.[14,15] Moreover, PROG affects nicotinic acetylcholine receptor (nAChR)[16] and stimulates gamma-aminobutyric acid (GABA) as the most important inhibition transmitter in brain. All the above 3 mechanisms are responsible for the positive neuroprotective effects of PROG as they inhibit the excitotoxic responses in injuries.[13]
Vitamin D belongs to the secosteroid class, which directly causes the expression of more than 100 genes.[17] This neuroactive steroid can be dispersed in CNS since it is the final activator of enzymes and has an intracellular receptor. The primary effects of vitamin D are the inhibition of cell proliferation and stimulation of cellular differentiation, especially in immune system.[18,19] It has been found that vitamin D deviates all the dimensions of immune performance, which is near the immune response type 2 and generally is anti-inflammatory and regulatory.[13] Similar to progesterone, vitamin D reduces the pro-inflammatory level of TH1 cytokines such as IL6, IL12, IL1B, and TNFα.[20] Furthermore, vitamin D maintains the intracellular surface of calcium through following ways: 1. Maintenance of PTH at an appropriate level, 2. Adjustment of L-type voltage-sensitive calcium channels, and 3. Control of intracellular Ca2+ buffering.
The progesterone activity through GABAergic system to inhibit extracellular activity and the performance of vitamin D to increase intracellular Ca2+-binding proteins together affect the calcium metabolism through sub-pathways.
Other reasons for the use of combined PROG and vitamin D in TBI treatment are as follows:
Reduction of the effects of glutamate release and calcium inflex
Protection against toxic effects of heme breakdown products
Enhancement of free radical scavenging
Modulation of the renin-angiotensin system
Protection of the axonal and cytoskeleton infrastructure
CONCLUSION
As mentioned before, vitamin D and progesterone are pleiotropic hormones with a lot of common pathways, which consequently reduce the CNS injury and increase recovery rate of nervous system after TBI. Many studies performed on mice and humans have reported significant favorable outcome after TBI.[21–23] Recently, progesterone has been found to reduce inflammatory response and oxidative stress.[24,25] Moreover, progesterone activates the protective pathways and increases the expression of genes and proteins related to neuroprotection after TBI.[13] There are also reports about the neuroprotective effect of vitamin D under in vitro and in vivo conditions and cortical infarcts.[26] Furthermore, studies have shown that vitamin D deficiency disrupts the processes associated with CNS health such as mitosis, mitogenesis, neurite outgrowth, possibly adult neurogenesis in hippocampal cells, and mitochondria function.[27]
Regarding the foregoing, the use of combined PROG and vitamin D is reasonable in that vitamin D in combination with PROG improves repair mechanisms of CNS considering their common pathways, and also compensates other mechanisms, which are not performed by PROG. This reduces the heterogeneity of TBI and the probable failure of a single treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with Vitamin D affords better neuroprotectionagainst excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;15:328–36. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margulies S, Hicks R. Rockville, MD: 2008. Combination Therapies for Traumatic Brain Injury Workshop. Participants. [Google Scholar]
- 3.Roberts I, Schierhout G, Alderson P. Absence of evidence for the effectiveness of five interventions routinely used in the intensive care management of severe head injury: A systematic review. Neurol Neurosurg Psychiatry. 1998;65:729–33. doi: 10.1136/jnnp.65.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzelaar JP, Mararon A, Young HF. Improving the outcome of severe head injury with oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: A phase trial. J Neurosury. 1993;78:375–82. doi: 10.3171/jns.1993.78.3.0375. [DOI] [PubMed] [Google Scholar]
- 5.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–18. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 7.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–88. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 8.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–97. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 10.Roof RL, Duvdevani R, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosci. 1992;4:425–7. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Tani M. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 1998;21:154–9. doi: 10.1016/s0166-2236(97)01198-3. [DOI] [PubMed] [Google Scholar]
- 12.Eelen G, Verlinden L, van Camp M, van Hummelen P, Marchal K, de Moor B, et al. The effects of 1alpha, 25- dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–46. doi: 10.1359/JBMR.0301204. [DOI] [PubMed] [Google Scholar]
- 13.Milos C, Iqbal S, Donald G. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Neuroendocrinology. 2009;30:158–72. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergeron R, de Montigny C, Debonnel G. Pregnancy reduces brain sigma receptor function. Br J Pharmacol. 1999;127:1769–76. doi: 10.1038/sj.bjp.0702724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–7. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–53. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eelen G, Verlinden L, van Camp M, van Hummelen P, Marchal K, de Moor B, et al. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–46. doi: 10.1359/JBMR.0301204. [DOI] [PubMed] [Google Scholar]
- 18.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 19.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25- dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–62. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 21.Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–28. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- 22.Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: What animal models teach us. Front Biosci. 2008;13:1083–9. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–97. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–86. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- 25.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- 26.Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, et al. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39:873–80. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 27.Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics. 2007;7:769–80. doi: 10.1002/pmic.200600392. [DOI] [PubMed] [Google Scholar]


