Abstract
Despite the benefits of resistance, susceptibility to infectious disease is commonplace. Although specific susceptibility may be considered an inevitable consequence of the co-evolutionary arms race between parasite and host, a more general constraint may arise from the cost of an immune response. This “cost” hypothesis predicts a tradeoff between immune defense and other components of fitness. In particular, a tradeoff between immunity and sexually selected male behavior has been proposed. Here we provide experimental support for the direct phenotypic tradeoff between sexual activity and immunity by studying the antibacterial immune response in Drosophila melanogaster. Males exposed to more females showed a reduced ability to clear a bacterial infection, an effect that we experimentally link to changes in sexual activity. Our results suggest immunosuppression is an important cost of reproduction and that immune function and levels of disease susceptibility will be influenced by sexual selection.
Infectious diseases are ubiquitous (1) and can substantially reduce the fitness of their hosts (2). In response, hosts have evolved elaborate mechanisms of defense (3), and heritable variation for disease resistance exists in many populations (4–7). Yet, despite the benefits of resistance, natural selection does not eliminate susceptibility to disease (6, 8). This continuing susceptibility is generally considered an inevitable consequence of the co-evolutionary arms race between a host and its pathogens (9–11). Recently, however, it has been recognized that in addition to this process, disease susceptibility can be maintained by tradeoffs between costly immune defense and other components of fitness, such as reproduction (8, 12–16). This expectation is based on a model of resource allocation that underlies life history theory: finite resources are allocated among costly fitness-related traits to maximize fitness (17).
In Drosophila and other insects, the immune system includes cellular and humoral components offering defense against parasitoids, viruses, bacteria and fungi (18, 19). Cell-mediated immune responses are primarily involved in encapsulation reactions that defend insects against parasitoids (20). In contrast, the humoral immune response targets microbial pathogens and is characterized by the rapid production of a number of small antimicrobial peptides (18, 19).
Previous research in Drosophila melanogaster has investigated the induction of the cell-mediated encapsulation reaction in larvae. Encapsulation is associated with decreased adult size and female fecundity (21), there is genetic variation for encapsulation ability (5, 7, 22), and selection for immunologically mediated parasitoid resistance leads to a reduction in larval competitive ability (5, 7). Immune responses in larvae have delayed consequences on adult life history traits and on reproduction. In contrast, when facing adult infection, the optimum strategy must balance the costs of defense against current investment in reproduction. In fact, for highly virulent parasites, against which the effectiveness of defense is low, adults may forgo defense in favor of increased reproduction (23, 24), an option obviously not available for larvae. Tradeoffs involving humoral antimicrobial immune responses or, more generally, immune responses in the adult have not been explored in Drosophila.
Given an adult infection, the optimum allocation to immunological defense may differ between the sexes. The sexes differ in their reproductive strategies, with males expending more resources on current reproduction (specifically, obtaining mates) than do females (25). Consequently, males may invest fewer resources in immune-system mediated parasite defense (26, 27). This argument predicts that the prevalence and intensity of parasitic infection will be higher among males than among females. There is broad observational and experimental support for this prediction in vertebrates (26–30). The pattern is less clear among invertebrates. In a survey of parasitic infections in a broad range of arthropods, no consistent sex difference was observed (31). However, experimental studies have indicated that males are more susceptible to infection in crickets (32) and copepods (33), and that immune system function is suppressed in male scorpionflies (Panorpa vulgaris) (34). Such observations implicate sexual selection as a process influencing the evolution of resistance to disease: as males respond to increased sexual selection by increasing their mating effort, we expect a decrease in their ability to mount an immune response.
This prediction has been experimentally tested indirectly among a number of vertebrates (mostly birds) by using testosterone implants (35–42). Animals provided with testosterone implants show reduced immune system function in some (35, 36, 39, 40), but not all (37, 38, 41), studies. These experiments test for the simultaneous effect of testosterone on immunity and reproductive effort. They do not examine the possibility that changes in behavior directly affect immune system function. In a recent review, Møller et al. (43) cited only one published study that experimentally examined the relationship between a sexually selected trait and immune system function. In that study, divergent selection for an antibody response of chickens to sheep red blood cells resulted in a negatively correlated response in comb size, a character known to be important in mate choice, a result that is consistent with the tradeoff hypothesis (44).
Among invertebrates the relationship between immune system function and adult sexual behavior and/or adult life history characters is even less well understood. In workers of the bumble bee (Bombus terrestris), experimental reductions of foraging activity resulted in an increased ability to mount an encapsulation response to a novel antigen (45), whereas activation of the humoral and/or cell-mediated immune response resulted in decreased survival under starvation conditions (46). In damselflies, there is indirect experimental evidence of a tradeoff between the cell-mediated encapsulation reaction and melanin-based wing spots on males, possibly due to the fact that both the immune response and the production of wing spots rely on the same phenol oxidase enzyme cascade (47).
To test the hypothesis that there is a tradeoff between immune system function and male reproductive behavior, we investigated the relationship between an inducible antibacterial immune response and courtship in male D. melanogaster. Specifically, we placed male flies under various conditions affecting their sexual activity and access to food resources and then measured the response of these males to injections of Escherichia coli bacteria. We first verified that the clearance of E. coli was immunological. This study addressed three questions concerning male immune function in D. melanogaster: (i) Does exposure to increasing numbers of females reduce immunological performance? (ii) Is the immunosuppression due to increased courtship, mating, or some other effect of increasing female number? (iii) Is the tradeoff influenced by adult food resource availability, and, in particular, does the tradeoff disappear under conditions of excess food?
Materials and Methods
Fly Stocks.
Wild-type flies (Riverside) were from a laboratory population established in 1995 from flies caught in the University of California, Riverside orange groves. The population had been on a 2-week generation time since 1998, 2 years before the experiments. relish mutants and their controls were kindly provided by Dan Hultmark (University of Umeå, Sweden). In all experiments, test flies were collected as virgins from bottles kept at low larval density. Low-density bottles were established by placing 20 males and 20 females in bottles provided with excess yeast and transferred every 24 h. All flies were kept on molasses–cornmeal–agar food at 25°C under constant light.
Assay of Humoral Immune System Function.
We measured humoral immune function as the rate of clearance of an injection of E. coli bacteria. The strain of bacteria used in all experiments was E. coli D21, which carries chromosomal resistance genes to both ampicillin and streptomycin. On the evening before injections, a bacterial culture was started and allowed to grow overnight. The resulting population was then centrifuged and the cells were resuspended in a Drosophila Ringer's solution to a concentration of ≈106 cells per ml, as determined from spectrophotometer readings. Injections were carried out using a nanoliter injector (Drummond Scientific, Broomall, CA) fitted with pulled glass capillary needles. Flies (under CO2 anesthesia) were injected in the thorax with 73.4 nl of the bacterial solution, which corresponds to ≈7.4 × 104 cells per fly.
To obtain estimates of the number of bacteria remaining in each fly, a single fly was placed in a 1.5-ml centrifuge tube and homogenized in 40 μl of Ringer's solution with a pipette tip. The appropriate dilution was then made and 100 μl of the solution was plated on LB agar plates containing 100 μg/ml streptomycin. These plates were then incubated for 15–18 h at 37°C. This incubation time ensured that the colonies were small, which minimized the amount of colony overlap. The plates were then scored for the number of E. coli D21 colonies by using a colony counter.
Determination of Immunological Clearance of Bacteria.
To establish the role of the humoral response in the clearance of E. coli D21 we used flies deficient in the relish gene. relish is involved in a cell-signaling pathway leading to the production of antibacterial peptides (48). Unlike wild-type flies, these relish-negative mutants (rel−) do not produce these peptides in response to injections of Gram-negative bacteria and show lowered responses to injections with fungi, although cell-mediated immunity is unaffected (49). In the first experiment we measured the rate of bacterial clearance in rel− and relish-positive (rel+) flies that shared the same genetic background along with wild-type flies from an outbred laboratory stock (Riverside). On day 0, 24 flies (3- to 4-days old) from each group were injected with ≈105 E. coli D21. Over the next 4 days, the number of remaining bacteria was assayed for 6 flies per population. Analyses were carried out using analysis of variance (ANOVA) and planned orthogonal contrasts comparing first the log-transformed means of the two control groups (rel+ and wild-type) and then the two controls with rel−.
In a second experiment, we injected rel− and rel+ flies with either E. coli or Drosophila Ringer's solution (n = 72 for each treatment). Daily inspection of the number of surviving flies was carried out until all rel− flies had died. Data were analyzed using orthogonal χ2 tests of the proportion of individuals alive or dead on day 4, based on our a priori expectation that only rel− flies receiving injections of bacteria would experience lowered survival.
Female Number and Male Immune System Function.
On day 0, newly emerged wild-type virgin males were collected and placed immediately in vials with 0, 1, or 4 virgin females. Females were replaced daily under CO2 anesthesia (solitary males were similarly handled) until the end of the experiment, at which point males were homogenized. On day 4, the males were injected and placed in a new vial with their appropriate number of females. On day 8, the males were homogenized and 100 μl of a 1-ml dilution was plated on plates containing streptomycin. Colony counts were carried out on day 9. The experiment was divided into 2 blocks. Data were analyzed using analysis of covariance (ANCOVA) with the number of females as a continuously distributed covariate along with random factors of block and rearing vial (nested within block). Preliminary analysis found that the transformation (colony count)0.3 gave the best fit to the assumptions of ANCOVA. Analysis of untransformed and log-transformed data did not change the conclusions of the analysis. Final samples sizes were n = 42, n = 40, and n = 41 for males with 0, 1, and 4 females, respectively.
Social Proximity and Male Immune Function.
The timing of transfers, injections, homogenizations, and colony counts was exactly as in the previous experiment. Test males were kept in vials provisioned with excess yeast with either 4 other males (n = 36) or 4 virgin females (n = 38). Nontest males carried the eye mutation sparkling so that they could be distinguished from the test males. Results were analyzed by using ANOVA, with the transformation (colony count)0.3 as the dependent variable to meet the assumptions of ANOVA. The sex of interacting flies was entered as a fixed factor. Rearing bottle was included as a random factor in the analysis.
Female Mating Status, Resource Availability, and Male Immune System Function.
Again, the timing of transfers and injections was the same as before. Here, single males were housed with either 4 nonvirgin or 4 virgin females (females were replaced daily). Flies were maintained in vials representing three levels of food availability: 50% regular media, 100% regular media, and vials supplemented with excess yeast. Vials with 50% regular media were made by remelting regular food and making a 50% dilution with a 2% agar solution. To create the yeast-supplemented vials, yeast paste was spread on the surface of regular food. Preliminary experiments showed that these dietary treatments resulted in large differences in female fecundity, indicating that resources are limiting in vials with 100% regular media, and even more so in vials with 50% regular media (K.A.M, unpublished data). Results were analyzed by using ANOVA, with the transformation (colony count)0.3 as the dependent variable to meet the assumptions of ANOVA. Female mating status, diet, and their interaction were entered as fixed factors. The random factors day (corresponding to each temporal block) and rearing bottle (nested within day) were also included in the model.
In addition to vials with females, we also included males housed alone in yeast-supplemented vials. Males housed in yeast-supplemented vials (with or without females) were analyzed separately by using ANOVA and planned orthogonal comparisons, first comparing the means of males housed with virgin versus nonvirgin females and then the means of males with females (virgin and nonvirgin) versus males housed alone.
Analysis of Male Sexual Behavior.
In a first experiment, virgin males were collected and housed singly in vials for 3 days, after which they were either left alone or combined, without anesthesia, with either 1 (n = 42) or 4 (n = 40) nonvirgin females. These vials were then observed for a few seconds every hour for 12 h and scored for whether the male was seen courting and/or mating. Courtship included wing vibrations, chasing, licking, and attempted copulations. The measure “proportion of males ever observed mating” was the proportion of males that was seen mating at least once during the 12 observation periods. The analysis of courtship was based on an ANOVA of the arcsine–square root transformation of the proportion of times each male was seen courting out of the 12 observation periods. Males alone were never observed courting, so the analysis compared only males with 1 female and males with 4 females. Data on the number of males seen mating were analyzed using a G-test with William's correction (50).
In a second experiment, we examined the sexual behavior of males housed with either 4 virgin or 4 nonvirgin female flies. It was expected that differences in behavior would be concentrated in the few hours after the introduction of females, during which time males would be mating with virgin females. We therefore observed behavior every half an hour for the first 4 h, every hour for the next 5 h, and then every 4 h for the next 16 h. For the analysis, the proportion of times that a male was seen (i) courting (but not mating) and (ii) mating were estimated for each of seven 4-h periods, and then averaged for each male over the entire 25-h period. The arcsine–square root transformed average proportion of times that a male was seen courting, when he was not mating, was analyzed by using ANOVA. The average proportion of males observed mating or not mating with either virgin or nonvirgin females was analyzed by using a G test with the William's correction (50).
Results and Discussion
This study addressed three questions concerning male immune function in D. melanogaster: (i) Does exposure to increasing numbers of females reduce immunological performance? (ii) Is the immunosuppression due to increased courtship, mating, or some other effect of increasing female number? (iii) Is the tradeoff influenced by adult food resource availability?
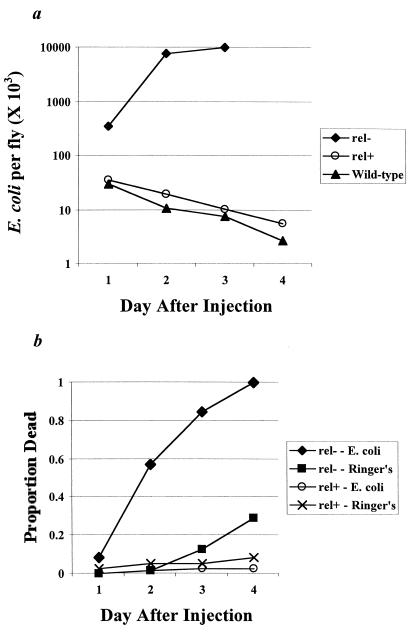
To assay humoral immune function, we measured the clearance of experimental injections of E. coli D21 bacteria. We first established that the decline of bacterial numbers was due to immunological activity, and not simply that flies were an inhospitable host. We compared the rates of clearance in flies with (rel+) and without (rel−) the gene relish, which is involved in a signaling pathway leading to the production of antibacterial peptides while leaving cell-mediated immunity unimpaired (49). Bacteria in rel− flies grew exponentially to a concentration of about 107 bacteria per fly, whereas in rel+ and wild-type (Riverside) controls, bacterial numbers declined in a log-linear manner (Fig. 1a). Because all rel− flies had died by day 3, the rel− counts on day 3 were based on counts from flies that had died on day 2. Analyses for days 1 and 2 after injection were carried out by using ANOVA and planned orthogonal contrasts comparing first the log-transformed means of the two control groups (rel+ and wild-type) and then the two controls with rel−. On both days, rel− flies had significantly greater bacteria counts than their controls (contrast rel− versus rel+ and wild-type: day 1: F = 5.67, df = 1, 15, P = 0.031; day 2: F = 135.75, df = 1,15, P < 0.0001). The two controls were not significantly different for days 1 or 2. However, analysis for all 4 days revealed that outbred wild-type flies (Riverside) cleared more bacteria than did rel+ flies, suggesting inbreeding depression (Strain, rel+ versus wild-type, F = 11.63, df = 1, 40, P = 0.0015; day, F = 15.57, df = 3, 40, P < 0.0001; Day × Strain, not significant).
Figure 1.
The level of E. coli infection and its effect on the survival of flies with (rel+) or without (rel−) the wild-type humoral immune response. (a) The number of E. coli D21 recovered from relish mutants (rel−) or controls (rel+ or wild-type) in the first 4 days after injection. (b) Survival curves for rel− mutants or their controls (rel+) injected with either E. coli D21 or Drosophila Ringer's solution.
Mortality rates were significantly increased in rel− flies injected with E. coli D21 (Fig. 1b); in fact, all injected rel− flies had died by day 4. There was no significant difference in survival of rel+ flies receiving injections of either Ringer's solution or E. coli D21 (χ2 = 2.12, P > 0.05). However, rel− flies receiving an injection of Ringer's solution experienced significantly lower survival than the combined Ringer's solution- or E. coli D21-injected rel+ controls (χ2 = 23.01, df = 1, P < 0.001). Previous research has demonstrated that even one bacterial cell is potentially pathogenic in rel− flies (49), which likely explains the rise in the death rate of rel− Ringer's solution-injected controls. Even so, mortality in the bacteria-injected rel− was much greater than that seen in their Ringer's solution-injected controls. This comparison was highly significant, even taking into account multiple comparisons (χ2 = 78.97, df = 1, P < 0.001). Therefore, experimental injections of E. coli are potentially pathogenic, but only in severely immune-compromised hosts.
Male Immune Function and Sexual Activity.
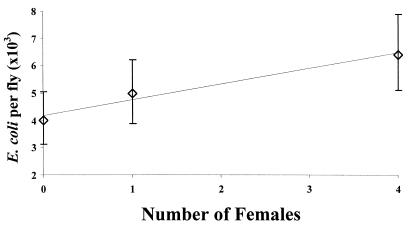
We first tested whether exposure to increasing numbers of females reduced male immunological performance. We found, consistent with expectations, that when a male was housed with increasing numbers of females there was a highly significant linear decrease in his ability to immunologically clear an experimental injection of E. coli bacteria (Fig. 2; ANCOVA, Number of females, F = 10.85, df = 1, 118, P = 0.0013).
Figure 2.
The number of E. coli (±95% confidence interval) recovered from males kept in vials alone, or with either 1 or 4 virgin females that were replaced daily.
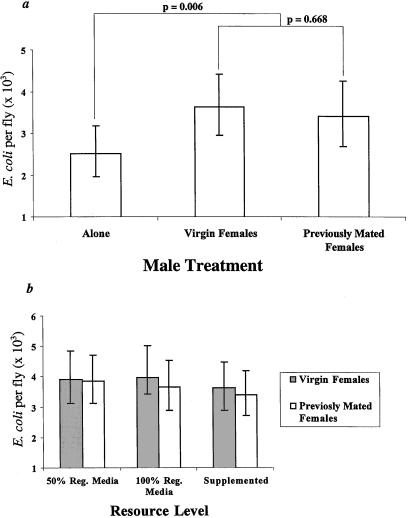
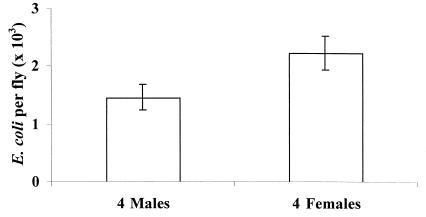
We suspected that changes in the level of male courtship or mating were causing this relationship; however, it was necessary to eliminate the potential role of a simple increase in the density of flies per vial. Increasing density could cause immunosuppression in two ways, indirectly by reductions in the amount of food per fly, or directly by an increase in the opportunity for interactions among flies regardless of sex. We tested for the potential influence of food limitation by determining if the tradeoff disappeared under conditions of excess food. We found that this was not the case. Males kept alone with excess food still cleared injections of E. coli significantly faster than similarly provisioned males housed with 4 females (Fig. 3a; a priori orthogonal contrast of males alone versus with females (either virgin or nonvirgin), F = 7.62, df = 1, 100, P = 0.006). Because excess food did not mitigate the tradeoff, we tested for the direct effect of increased interactions under these same conditions by using males kept with 4 males and males kept with 4 virgin females. Males with females cleared bacteria significantly slower than males kept with other males (Fig. 4; F = 18.08, df = 1, 69, P < 0.0001). Thus, neither the indirect effect of density on food availability nor its direct effect on proximity can explain the immunosuppression observed in Fig. 2.
Figure 3.
The influence of food availability and female mating receptivity on the humoral immune response of male flies. (a) The number of E. coli (±95% confidence interval) recovered from males in vials supplemented with excess yeast either alone, or with 4 virgin or nonvirgin females. Shown are P values for planned orthogonal contrasts. (b) The number of E. coli (±95% confidence interval) recovered from males kept with either 4 virgin or 4 nonvirgin females (replaced daily) under three levels of increasing resource availability.
Figure 4.
The number of E. coli (±95% confidence interval) recovered from males kept in vials with either 4 males or 4 females that were replaced daily.
The persistence of the tradeoff under conditions of excess food suggests that sexual behavior and mounting an immune response are in some sense mutually exclusive. Otherwise, we would expect the tradeoff to be approximately proportional to the available resource, and that it would disappear when the resource intake was sufficiently high.
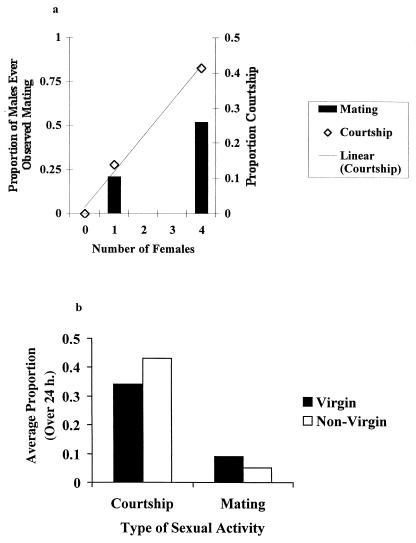
To further establish a causal link between the level of male sexual behavior and immunosuppression, we quantified the relationship between the number of females per vial and levels of courtship and mating. As expected, males with 4 females courted significantly more and were more likely to be seen mating than males with just 1 female (Fig. 5a; Courtship: ANOVA, F = 220.75, df = 1, 78, P < 0.0001; Proportion mating, G = 8.532, df = 1, P < 0.01). These highly linear relationships support the hypothesis of a direct tradeoff between male sexual activity and the humoral immune response.
Figure 5.
Male sexual behavior. (a) The effect of female number on reproductive behavior. The proportion of males ever observed mating and the average proportion of times males were observed courting over a period of 12 h for males kept in vials either alone or with 1 or 4 nonvirgin females. (b) Female mating status (virgin or nonvirgin) and male reproductive behavior. Shown is the average proportion of males courting or mating over a 24-h period.
To exclude mating as the sole cause of the immunosuppression, we investigated the immunological and behavioral differences among males housed with virgin or nonvirgin females. We found that males housed with 4 virgin females mate more and court less than males with 4 previously mated females (Fig. 5b; Courtship, F = 4.78, df = 1, 66, P = 0.032; Mating, Gadj = 6.803, df = 1, P < 0.01). As expected, the most drastic difference was in the level of mating, in which males with virgin females were found to mate 1.98 times as often as males with nonvirgin females. Males with nonvirgin females did court significantly more, but this difference was much less dramatic (a 1.24-fold increase). These data predict that, if mating is primarily responsible for reduced immunological performance, then males housed with virgin females should have slower clearance rates than similarly provisioned males housed with nonvirgin females. This possibility was tested by measuring the clearance of bacteria in males housed with either 4 virgin or 4 nonvirgin females (changed daily). In addition, we also wished to investigate further the possible role of food availability by testing whether very low food levels exaggerated the tradeoff. Thus, the roles of courtship, mating, and food availability were simultaneously investigated by adding three levels of food availability ranging from a minimal maintenance level to excess.
We found that, regardless of food availability (at least across the nonstarvation levels that we studied), males with virgin or nonvirgin females had similar rates of clearance, demonstrating that the effectiveness of the immune response was not significantly affected by the increased mating rate of males with virgin females (Fig. 3b). In addition, clearance rates were not affected by food level or by any interaction of food level with mating regime (ANOVA: Female, F = 0.21, df = 1, 204, P = 0.646; diet, F = 1.02, df = 2,204, P = 0.362; Female × Diet, F = 0.04, df = 2, 204, P = 0.958). From this experiment we can conclude that mating alone cannot explain the pattern observed in Fig. 2. Immunosuppression results either from increased courtship or (perhaps more likely) as a consequence of an increase in some weighted sum of mating and courtship.
The direct phenotypic tradeoff between male sexual activity and the humoral immune response demonstrated here supports the view that immune system function and levels of disease susceptibility are traits shaped by tradeoffs with other costly fitness components (8, 12, 26, 27). Assuming that immunosuppression increases the probability of a fitness-reducing infection, any such tradeoff in immune function will contribute to the cost of reproduction, defined as a decline in future reproduction as a consequence of current reproductive effort (17, 51).
Here we exposed the immunological consequences of increasing mating effort by means of experimental manipulations. Similar experimental work has revealed courtship costs expressed as reduced survival in male D. melanogaster (52, 53). Courtship rates are likely higher in laboratory populations of D. melanogaster than natural populations (54). However, in this study, courtship rates of males kept with one female were similar to rates reported in field studies (55). In addition, the laboratory environment represents a condition to which our flies were likely well adapted. Thus, even a subtle change in the mating system in an environment to which this population was well adapted resulted in decreased immune system function.
In D. melanogaster there is no paternal care, and male reproductive effort is composed entirely of investment in successfully mating. In nature, an evolutionary increase in mating effort will be favored by increased sexual selection (56). Hence, provided that the phenotypic correlation between courtship and immune function is unchanged by selection for increased courtship, strong sexual selection can be expected to exaggerate the negative immunological consequences of sexual activity. This selection could have far-reaching consequences on the life-history evolution of a species by amplifying the cost of reproduction.
Acknowledgments
We thank A. Agulnick, N. Schiller, T. Wong, and R. Hatch for technical assistance and advice, D. Hultmark and M. Hendegren for providing fly stocks, and T. Panhuis, D. Reznick, Judy Stamps, M. Zuk, and four anonymous reviewers for comments on the manuscript. This work was supported by a National Science Foundation Dissertation Improvement Grant to M.Z., K.M, and L.N., as well as a Department of Education Graduate Assistance in Areas of National Need fellowship to K.M.
Abbreviation
- ANCOVA
analysis of covariance
References
- 1.Grenfell B T, Dobson A P. Ecology of Infectious Diseases in Natural Populations. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 2.Ewald P W. Evolution of Infectious Disease. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 3.Warr G W, Cohen N. Phylogenesis of Immune Functions. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 4.Ebert D, Zschokke-Rohringer C D, Carius H J. Proc R Soc London Ser B. 1998;265:2127–2134. [Google Scholar]
- 5.Fellowes M D E, Kraaijeveld A R, Godfray H C J. Proc R Soc London Ser B. 1998;265:1553–1558. doi: 10.1098/rspb.1998.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henter H J, Via S. Evolution. 1995;49:427–438. doi: 10.1111/j.1558-5646.1995.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 7.Kraaijeveld A R, Godfray H C J. Nature (London) 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- 8.Kraaijeveld A R, Godfray H C J. Am Nat. 1999;153:S61–S74. doi: 10.1086/303212. [DOI] [PubMed] [Google Scholar]
- 9.May R M, Anderson R M. Parasitology. 1990;100:S89–S101. doi: 10.1017/s0031182000073042. [DOI] [PubMed] [Google Scholar]
- 10.Frank S A. Evol Ecol. 1993;7:45–75. [Google Scholar]
- 11.Frank S A. Heredity. 1991;67:73–84. doi: 10.1038/hdy.1991.66. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon B C, Verhulst S. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 13.Read A F, Keymer A E. The Evolutionary Biology of Parasitism. Cambridge: Cambridge Univ. Press; 1990. [DOI] [PubMed] [Google Scholar]
- 14.van Baalen M. Proc R Soc London Ser B. 1998;265:317–325. doi: 10.1098/rspb.1998.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simms E L, Triplett J. Evolution. 1994;48:1973–1985. doi: 10.1111/j.1558-5646.1994.tb02227.x. [DOI] [PubMed] [Google Scholar]
- 16.Frank S A. Evolution. 1993;47:325–327. doi: 10.1111/j.1558-5646.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 17.Roff D A. The Evolution of Life Histories. New York: Chapman & Hall; 1992. [Google Scholar]
- 18.Brey P T, Hultmark D. Molecular Mechanisms of Immune Responses in Insects. London: Chapman & Hall; 1998. [Google Scholar]
- 19.Khush R S, Lemaitre B. Trends Genet. 2000;16:442–449. doi: 10.1016/s0168-9525(00)02095-3. [DOI] [PubMed] [Google Scholar]
- 20.Carton Y, Nappi A J. Parasitol Today. 1997;13:218–227. doi: 10.1016/s0169-4758(97)01058-2. [DOI] [PubMed] [Google Scholar]
- 21.Fellowes M D E, Kraaijeveld A R, Godfray H C J. J Evol Biol. 1999;12:123–128. [Google Scholar]
- 22.Carton Y, Frey F, Nappi A. Heredity. 1992;69:393–399. doi: 10.1038/hdy.1992.141. [DOI] [PubMed] [Google Scholar]
- 23.Forbes M R L. Oikos. 1993;67:444–450. [Google Scholar]
- 24.Jokela J, Schmid-Hempel P, Rigby M C. Oikos. 2000;89:267–274. [Google Scholar]
- 25.Trivers R L. In: Sexual Selection and the Descent of Man 1871–1971. Campbell B G, editor. Chicago: Aldine; 1972. pp. 163–179. [Google Scholar]
- 26.Zuk M. Parasitol Today. 1990;6:231–233. doi: 10.1016/0169-4758(90)90202-f. [DOI] [PubMed] [Google Scholar]
- 27.Zuk M, McKean K A. Int J Parasitol. 1996;26:1009–1023. [PubMed] [Google Scholar]
- 28.Alexander J, Stimson W H. Parasitol Today. 1988;4:189–193. [Google Scholar]
- 29.Bundy D A P. Parasitol Today. 1988;4:186–189. doi: 10.1016/0169-4758(88)90076-2. [DOI] [PubMed] [Google Scholar]
- 30.Poulin R. Am Nat. 1996;147:287–295. [Google Scholar]
- 31.Sheridan L A D, Poulin R, Ward D F, Zuk M. Oikos. 2000;88:327–334. [Google Scholar]
- 32.Gray D A. J Invertebr Pathol. 1998;71:288–289. doi: 10.1006/jipa.1997.4742. [DOI] [PubMed] [Google Scholar]
- 33.Wedekind C, Jakobsen P J. Oikos. 1998;81:458–462. [Google Scholar]
- 34.Kurtz J, Wiesner A, Goetz P, Sauer K P. Dev Comp Immunol. 2000;24:1–12. doi: 10.1016/s0145-305x(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 35.Evans M R, Goldsmith A R, Norris S R A. Behav Ecol Sociobiol. 2000;47:156–163. [Google Scholar]
- 36.Duffy D L, Bentley G E, Drazen D L, Ball G F. Behav Ecol. 2000;11:654–662. [Google Scholar]
- 37.Hasselquist D, Marsh J A, Sherman P W, Wingfield J C. Behav Ecol Sociobiol. 1999;45:167–175. [Google Scholar]
- 38.Lindstrom K M, Krakower D, Lundstrom J O, Silverin B. Proc R Soc London Ser B. 2001;268:207–211. doi: 10.1098/rspb.2000.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters A. Proc R Soc London Ser B. 2000;267:883–889. [Google Scholar]
- 40.Saino N, Møller A P, Bolzern A M. Behav Ecol. 1995;6:397–404. [Google Scholar]
- 41.Ros A F H, Groothuis T G G, Apanius V. Am Nat. 1997;150:201–219. doi: 10.1086/286063. [DOI] [PubMed] [Google Scholar]
- 42.Veiga J P, Salvador A, Merino S, Puerta M. Oikos. 1998;82:313–318. [Google Scholar]
- 43.Møller A P, Christe P, Lux E. Q Rev Biol. 1999;74:3–20. doi: 10.1086/392949. [DOI] [PubMed] [Google Scholar]
- 44.Verhulst S, Dieleman S J, Parmentier H K. Proc Natl Acad Sci USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.König C, Schmid-Hempel P. Proc R Soc London Ser B. 1995;260:225–227. [Google Scholar]
- 46.Moret Y, Schmid-Hempel P. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 47.Siva-Jothy M T. Proc R Soc London Ser B. 2000;267:2523–2527. doi: 10.1098/rspb.2000.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engstrom Y. Dev Comp Immunol. 1999;23:345–358. doi: 10.1016/s0145-305x(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 49.Hedengren M, Asling B, Dushay M S, Ando I, Ekengren S, Wihlborg M, Hultmark D. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 50.Sokal R R, Rohlf F J. Biometry: The Principles and Practice of Statistics in Biological Research. New York: Freeman; 1995. [Google Scholar]
- 51.Williams G C. Am Nat. 1966;100:687–690. [Google Scholar]
- 52.Cordts R, Partridge L. Anim Behav. 1996;52:269–278. [Google Scholar]
- 53.Partridge L, Farquhar M. Nature (London) 1981;294:580–582. [Google Scholar]
- 54.Gromko M H, Markow T A. Anim Behav. 1993;45:253–262. [Google Scholar]
- 55.Partridge L, Hoffmann A, Jones J S. Anim Behav. 1987;35:468–476. [Google Scholar]
- 56.Andersson M B. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]