Abstract
Background:
Alzheimer's disease (AD) is a prevalent disorder with severe learning and memory defects. Because it has been demonstrated that erythropoietin (EPO) has positive effects on the central nervous system, the aim of this study was to evaluate the effect of EPO on neuronal proliferation in dentate gyrus of hippocampal formation in a well-defined model for AD.
Materials and Methods:
A rat model of sporadic dementia of Alzheimer's type was established by a bilateral intracerebroventricular injection of streptozotocin (ICV-STZ). Impairment of learning and memory was confirmed 2 weeks after ICV-STZ injection by passive avoidance learning test and then rats were divided into fourgroups:Control, control-EPO, Alzheimer and Alzheimer-EPO. EPO was injected intraperitoneally every other day with a dose of 5000 IU/kg and, finally, the rats were anesthetized and decapitated for immunohistochemical study and neurogenesis investigation (by Ki67 method) in dentate gyrus of hippocampal formation.
Results:
The results driven from the histological study showed that EPO significantly increases neuronal proliferation in dentate gyrus of hippocampus in the Alzheimer-EPO group compared with the control, control-EPO and Alzheimer groups; however, there were no differences between the other groups.
Conclusion:
Our results show that even though EPO in intact animals doesnot change neurogenesis in dentate gyrus, it can nonetheless significantly increase neurogenesis if there is an underlying disorder like neurodegenerative diseases.
Keywords: Alzheimer's, erythropoietin, neuronal proliferation, rat, streptozotocin
INTRODUCTION
Alzheimer's disease (AD) is the most common reason of aging dementia, which has involved millions of people in the world. This disease is a progressive and irreversible brain disorder with unknown etiology,[1] which is accompanied with severe learning and memory impairment.[2] Its neuropathologic indices include massive accumulation of abnormal tau filaments in neurofibrillary tangles, deposition of amyloid-Beta (AB) plagues and extensive neuronal degeneration.[1,2] It has been determined that this disease is accompanied with neurons loss in several important areas for learning and memory, especially in the hippocampus.[1] AD is a medical and social problem; however, there is no effective therapeutic method and many studies are being conducted to find a suitable method for the prevention and cure for Alzheimer's.
Erythropoietin (EPO) is an effective factor in hematopoiesis, whose primary action is to rescue erythroid cells from apoptosis, increasing their survival.[3] This factor is used extensively for treatment of anemia. Human recombinant erythropoietin (rhEPO) is produced after human gene separation and expression in a cell line of Chinese hamster ovarian cells. The molecular weight of this protein is about 30.4KDa. Immunologically, it is similar to endogenous hormone and exhibits full biological activity and does not show species boundary.[4,5]
It has been demonstrated that EPO and its receptors are present in the central nervous system.[6,7] EPO receptor signaling is needed for natural evolution of the brain, and it has been shown that it has neuroprotective effects in neuronal disorders, such as Parkinson's disease.[8–10] EPO can induce neurogenesis in the hippocampus and improve spatial memory.[11,12] In addition, EPO protects neurons in medium against different stresses, such as stress oxidative, overstimulation toxicity resulting from glutamate and other factors that are involved in the pathogenesis of neurodegenerative diseases.[10,13,14] Although the exact mechanism of the neuroprotective effects of EPO has not been determined, it has been reported that EPO has a special role in messaging cascades for cell survival and increasing the expression of anti-apoptotic proteins in different laboratory models.[15]
One of the relevant animal models of AD can be induced by intracerebroventricular injection of streptozotocin (ICV-STZ).[16,17] STZ is a diabetogenic substance[18] that causes prolonged impairment of memory and brain metabolism as a sporadic dementia of the Alzheimer's type (SDAT) if injecting intracerebroventricularly in a sub-diabetogenic dose.[16,17]
Previous studies suggested that EPO is probably helpful in the treatment of Alzheimer's;[15,19] therefore, the aim of this study was to investigate the effects of EPO on neuronal proliferation in dentate gyrus of hippocampal formation in the rat ICV-STZ model of Alzheimer's.
MATERIALS AND METHODS
Male Wistar rats weighing 180-220g were housed four per cage and maintained on a 12-h light-dark cycle in an air-conditioned constant temperature (23±1°C) room, with food and water made available ad libitum, were used for the study. The Ethic Committee for Animal Experiments at Isfahan University approved the study and all experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised 1996.
First, half of the rats received a bilateral ICV injection of STZ with a dose of 3 mg/kg STZ (in saline, 4 μL/injection site) to establish rat model of sporadic dementia of Alzheimer's type,[16,20] and the other rats received an equal amount of saline by the same method. To confirm the learning and memory impairments, rats were behaviorally studied 2 weeks after injection and those rats that had learning and memory impaired in them were selected for continuing in the study. From this time, rats were divided into four groups as follows: (1) control, (2) control-EPO, (3) Alzheimer and (4) Alzheimer-EPO; EPO-receiving groups received this drug with a dose of 5000 IU/kg intraperitoneally every other day for 2 weeks[21–24] and the control groups, similarly, received placebo;after cutting the head, animals underwent the histological study.
Surgical procedure
Animals were anesthetized with intraperitoneal injection of chloral hydrate with a dose of 400 mg/kg and a heating pad was used for maintaining the rat body temperature stable. After shaving the animal head, their heads were fixed into the brain surgery stereotaxic device and, by creating a longitudinal incision on the posterior part of the head, the skull was made evident. After determining the stereotaxic coordinates for brain lateral ventricles by using rat brain atlas guide[25] and also by using the obtained coordinates from the pilot study (AP=−0.8mm, L=±1.6mm, DV=−4.2mm), two holes were made in the skull by a drill as it did not hurt the brain tissue and the injection special cannula was entered into the ventricles slowly and STZ was injected into each ventricle by a Hamilton syringe in 3 min.After finishing the injection, the used needle remained in the ventricle for 5 min,after which the needle was brought out and all these stages were performed in the opposite ventricle. Sham groups were subjected to the same procedure, but these rats received an equal volume of saline instead of STZ. After surgery, rats were put in separated cages and had access to food and water without any limitation.
Passive avoidance learning
The learning apparatus consisted of two separate chambers connected through a guillotine door. One chamber was illuminated while the other was dark. The floor of both chambers consisted of steel grids used to deliver electric shocks. On the acquisition trial, each rat was placed in an illuminated chamber while its back was to the guillotine door. After 60 s of habituation, the guillotine door separating the illuminated and dark chambers was opened and the initial latency to enter the dark chamber was recorded. Rats with an initial latency time of more than 60 s were excluded from further experiments. The guillotine door was closed immediately after the rat entered the dark chamber and an electric foot shock (75 V, 0.2 mA, 50 Hz) was delivered to the floor grids for 3 s; then, the rat was removed from the dark chamber and returned to its home cage. Twenty-four hours later, retention latency time to enter the dark chamber was taken in the same way as in the acquisition trial, but foot shock was not delivered, and the latency time was recorded up to a maximum of 300 s.
Histological study
Rats were anesthetized and, after decapitation, their skulls were dissected and the brains were removed. After fixation in 10% buffered formalin and tissue passage, they were embedded in paraffin. Instantly after decapitation, trunk blood was collected for measuring hematocrit. After embedding, coronal cuts of the samples were prepared by cutting through the sample from the beginning to the end into 3-4μ cuts for immunohistochemical staining (Ki67 test). The slices were placed in an oven for 30-120 min at 56-58°C such that the paraffin of the tissue sections melted and the tissue stuck well on the slide, not being detached during staining. In the next stage, we put the slides in boiling citrate buffer for 10-15 min (based on kit instructions). Then, samples were washed two-times with washing solution, 5 min each time. Hydrogen peroxide solution was put on the slides for 15 min. Samples were washed again with washing solution two-times, 5 min each time. Then, slides were incubated in background+anti-ratKi67 at 37°C for 30 min, and again washed two-times with washing solution. Then, they were incubated in secondary AB+washing buffer for 20 minatroom temperature and were washed in washing solution and incubated in HRO/Streptavidin+wash buffer for 10 min at room temperature and washed again. Chromogen solution substrate (DAB+substrate) was the put on the samples for 15 min and washed in washing solution.Then, they were washed with running water and stained with hematoxylin. After washing with running water, the samples were put twice in ethanol 96° (30min in each time), once in ethanol 100° (30s) and twice in xylol (5min each time) and then the slides were covered with Canada balsam glue and pressed a little to be stuck without air bubbles. Then, the tissue slides were observed under an optic microscope for proliferated neurons.
Statistical analysis
Mann Whitney test was used for analyzing the results related to passive avoidance test,Kruskal-Wallis (non-parametric ANOVA) for was used for histological tests and Dunn's multiple comparison test was used for post-test. For analyzing the results related to hematocrit, one-way ANOVA was used and the Tukey test was used for the post-test.
RESULTS
Measuring of hematocrit has shown that there were no significant differences between the groups.
The mean initial latency in the acquisition trial showed no change among the groups. Results from the retention phase of passive avoidance learning (PAL), as measured by mean retention latency time, showed that the mean retention latencies in the ICV-STZ group (171.79±31.4s) were significantly less than that in the sham group (290.6±9.4s; P<0.001) [Figure 1], confirming induction of learning and memory impairments.
Figure 1.
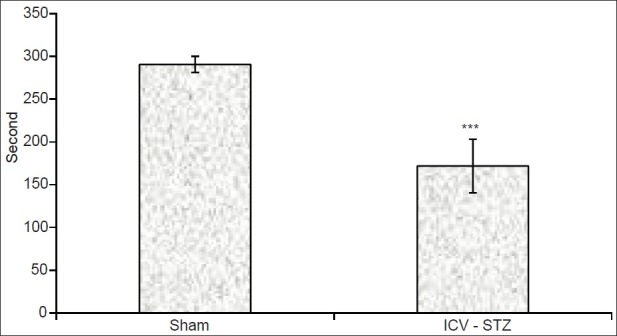
Effects of intracerebroventricular injection of streptozotocin (ICV-STZ) on step-through latency in the rats 24 h after the acquisition phase of passive avoidance learning. Data are expressed as mean±SEM (***P<0.001 with respect to the sham group; n=15)
The results obtained from the histological study showed that STZ injection into brain ventricles decreased the number of newly made neurons in the granular layer of dentate gyrus, but this decrease was not significant. Nevertheless, although EPO did not effect the amount of neurogenesis in healthy rats, it significantly increased the neurogenesis in the granular layer of dentate gyrus, and there was a significant difference between EPO-receiving Alzheimer's group and control group (P<0.001) and Alzheimer (P<0.001) [Figures 2 and 3].
Figure 2.
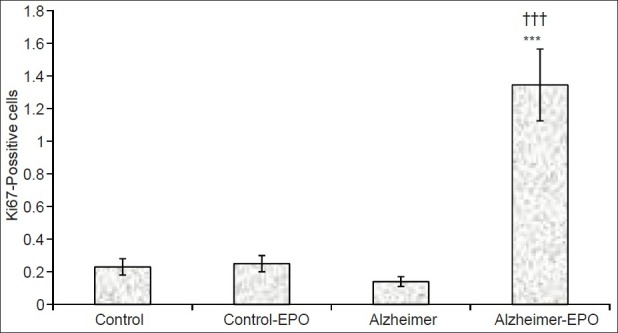
Amount of neuronal proliferation in the granular layer of the hippocampal dentate gyrus of rats with intracerebroventricular injection of streptozotocin after receiving erythropoietin for 2 weeks (n=5, ***P<0.001 in comparison with the control group; †††P<0.001 in comparison with the Alzheimer-erythropoietin[EPO] group)
Figure 3.
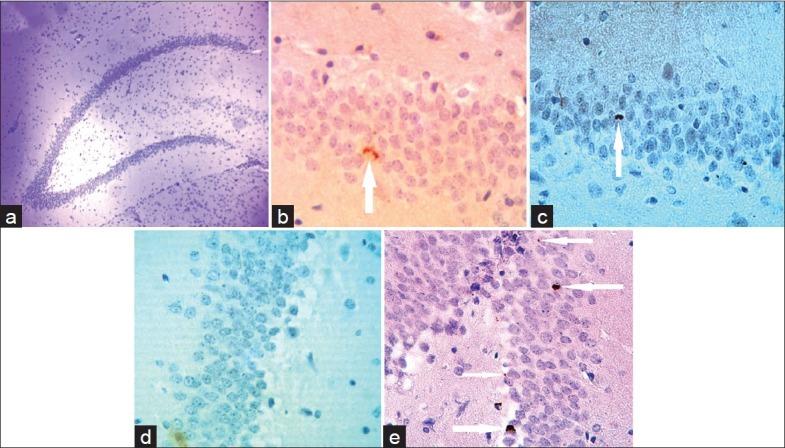
Photomicrograph of optical microscope from the granular layer of the hippocampal dentate gyrus of rats (a) (M=×4). Ki67-positive cells in the granular layer of the studied groups; control (b), control-erythropoietin (EPO) (c), Alzheimer (d) and Alzheimer-EPO (e) are visible on the arrow tip (M=×40)
DISCUSSION
These results are consistent with previous studies that showed that ICV injection of STZ causes severe learning and memory disorder.[20] Studies have shown that ICV-STZ impairs brain glucose metabolism and reduces ATP/ADP ratio, probably through creation of an imbalance between energy production and consumption.[17]
ICV-STZ induces impairments of cognitive performances by decreasing energy metabolism and stress oxidative by inhibiting ATP synthesis and acetyl co-enzyme A and therefore acetylcholine synthesis. Also, it has been determined that acetylcholine transferase activity decreases in the hippocampus of STZ-induced Alzheimer rats. It has been shown that acetylcholine is necessary for formation and improvement of memory; its synthesis needs glucose metabolism and insulin in order to control choline acetyltransferase activity.[16,17]
In addition, like in AD, ICV-STZ through prolonged impairment of brain energy metabolism and oxidative damage increases the inflammatory cytokines, such as interleukin-8 (IL-8) and interleukin-1 (IL-1). These inflammatory cytokines and severe oxidative stress lead to mitochondrial dysfunction and increase the risk of cell apoptosis in the brain, particularly in the hippocampus.[26–28]
As a secondary observation, our results demonstrated that although EPO didnot have any significant effect on neurogenesis in the control rats, it increased the neurogenesis in our Alzheimer's rats. Therefore, similar to previous studies, EPO has positive effects on neural functions when there is a disorder.[29]
It has been shown that there is programmed cell death or apoptosis in the central nervous system of adult mammalians.[30] This programmed cell death often presents in the area where neurogenesis is seen even in adulthood; like dentate gyrus of hippocampal formation, which has an effective role in learning and memory.[31] Becausea very high number of new neurons are made during adult neurogenesis and only some of them survive and others encounter apoptosis,[32] it has been suggested that apoptosis is actually dependent on neurogenesis.[33] Apoptosis is a complicated process that can be inducedand regulated by many factors or conditions; therefore, the factors that induce neurogenesis or prevent apoptosis in the dentate gyrus can improve learning and memory.[34–36] EPOcan induce neurogenesis in the hippocampus and therefore improve spatial memory.[37,38] Studies have shown that in cerebral stroke and head injury, besides increasing neurogenesis, EPO has neuroprotective effects due to the enhancement of glucose transporters, glycolytic enzymes and growth factors,[12,39] rather than hematopoietic effects,[23] as revealed in our study. For the hematopoiesis effect, EPO needs about 1 month and, in our study, neurogenesis was evaluated only after 2 weeks. Our results showed that hematocrit was indifferent between the groups.
According to previous studies, EPO can increase gene expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), insulin growth factor 1 (IGF-1) and certain proteins in the hippocampus. BDNF is a factor for maintenance and survival of neurons, and can reduce phosphorylation of tyrosine kinase B and lead to neuronal differentiation.[38]
Because EPO is a potent antioxidant, it has protective effects against oxidative stress. It was able to protect neurons in medium against diverse stressors, such as oxidative stress and pathogen stress; therefore, it can probably reverse oxidative effects in ICV-STZ rats.[16,29] Also,EPO reduced neuroinflammation in many of experimental models, and this reduction is associated with a marked decrease in proinflammatory cytokines within the brain; hence, it can be helpful in AD.[22,40]
In conclusion, our results showed that EPO increases neurogenesis or prevents apoptosis of new born cells in the dentate gyrus in the Alzheimer's model. Thus, EPO can be considered as an option for treating AD. Also, on the margin of this study, it was found that the ICV-STZ rats had a high mortality and symptoms before their death, including bleeding from the nose, eyes, ears, paralysis of hands and feet and, eventually, death. However, it was observed that those ICV-STZ animals that were treated with EPO,had dramatically reduced complications and mortality rates. Therefore, because this drug is used as a chemotherapeutic drug in cancer patients, perhaps the use of EPO could reduce the adverse effects of this drug.
ACKNOWLEDGMENTS
The present study was financially supported by the Vice Chancellors of both KashanUniversity of Medical Sciences, Kashan, Iran and Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Vice Chancellors of both KashanUniversity of Medical Sciences, Kashan, Iran and Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared
REFERENCES
- 1.Herring A, Ambrée O, Tomm M, Habermann H, Sachser N, Paulus W, et al. Environmental enrichment enhances cellular plasticity in transgenic mice with Alzheimer-like pathology. Exp Neurol. 2009;216:184–92. doi: 10.1016/j.expneurol.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Bothwell M, Giniger E. Alzheimer's disease: Neurodevelopment converges with neurodegeneration. Cell. 2000;102:271–3. doi: 10.1016/s0092-8674(00)00032-5. [DOI] [PubMed] [Google Scholar]
- 3.Lasne F, de Ceaurriz J. Recombinant erythropoietin in urine-An artificial hormone taken to boost athletic performance can now be detected. Nature. 2000;405:635. doi: 10.1038/35015164. [DOI] [PubMed] [Google Scholar]
- 4.Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res. 2004;153:77–86. doi: 10.1016/j.bbr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Squadrito F, Altavilla D, Squadrito G, Campo GM, Arlotta M, Quartarone C, et al. Recombinant human erythropoietin inhibits iNOS activity and reverts vascular dysfunction in splanchnic artery occlusion shock. Br J Pharmacol. 1999;127:482–8. doi: 10.1038/sj.bjp.0702521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F, Chong ZZ, Maiese K. Erythropoietin on a tightrope: balancing neuronal and vascular protection between intrinsic and extrinsic pathways. Neurosignals. 2004;13:265–89. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- 7.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher JW. Erythropoietin: Physiology and pharmacology update. Exp Biol Med(Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 9.Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Shang Y, Sun SG, Liu RG, Yang WQ. Protective effect of erythropoietin against 1-methyl-4-phenylpyridinium-induced neurodegenaration in PC12 cells. Neurosci Bull. 2007;23:156–64. doi: 10.1007/s12264-007-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–7. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Wang HD, Yu XG, Jin W, Qiao L, Lu TJ, et al. Erythropoietin prevents zinc accumulation and neuronal death after traumatic brain injury in rat hippocampus: in vitro and in vivo studies. Brain Res. 2009;1289:96–105. doi: 10.1016/j.brainres.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276:39469–75. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 14.Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, et al. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J Biol Chem. 2004;279:55578–86. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- 15.Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, et al. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027–34. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, et al. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 18.Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455:79–83. doi: 10.1016/j.neulet.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Ma R, Huang C, Tang Q, Fu Q, Liu H, et al. Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Lett. 2008;442:143–7. doi: 10.1016/j.neulet.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Ishrat T, Hoda MN, Khan MB, Yousuf S, Ahmad M, Khan MM, et al. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer's type (SDAT) Eur Neuropsychopharmacol. 2009;19:636–47. doi: 10.1016/j.euroneuro.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, et al. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008;6:37. doi: 10.1186/1741-7007-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu K, Jung KH, Lee ST, Kim JH, Kang KM, Kim HK, et al. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia. 2008;49:1723–32. doi: 10.1111/j.1528-1167.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- 23.El-Kordi A, Radyushkin K, Ehrenreich H. Erythropoietin improves operant conditioning and stability of cognitive performance in mice. BMC Biol. 2009;7:37. doi: 10.1186/1741-7007-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van BF. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego: Academic Press; 2005. [Google Scholar]
- 26.Shin EJ, Jeong JH, Bing G, Park ES, Chae JS, Yen TP, et al. Kainate-induced mitochondrial oxidative stress contributes to hippocampal degeneration in senescence-accelerated mice. Cell Signal. 2008;20:645–58. doi: 10.1016/j.cellsig.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Vendramini AA, de Lábio RW, Rasmussen LT, Dos Reis NM, Minett T, Bertolucci PH, et al. Interleukin-8-251T> A, Interleukin-1 alpha-889C> T and Apolipoprotein E polymorphisms in Alzheimer's disease. Genet Mol Biol. 2011;34:1–5. doi: 10.1590/S1415-47572010005000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Qu ZQ, Zeng YS, Lin YK, Li Y, Chung P, et al. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp Toxicol Pathol. 2011 doi: 10.1016/j.etp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Wiese L, Hempel C, Penkowa M, Kirkby N, Kurtzhals JA. Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J. 2008;7:3. doi: 10.1186/1475-2875-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White LD, Barone S., Jr Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death Differ. 2001;8:345–56. doi: 10.1038/sj.cdd.4400816. [DOI] [PubMed] [Google Scholar]
- 31.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 32.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–7. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–75. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- 34.Ferri P, Cecchini T, Ciaroni S, Ambrogini P, Cuppini R, Santi S, et al. Vitamin E affects cell death in adult rat dentate gyrus. J Neurocytol. 2003;32:1155–64. doi: 10.1023/B:NEUR.0000021909.84327.e8. [DOI] [PubMed] [Google Scholar]
- 35.Osredkar D, Sall JW, Bickler PE, Ferriero DM. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis. 2010;38:259–65. doi: 10.1016/j.nbd.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–53. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 37.Ning B, Zhang A, Song H, Gong W, Ding Y, Guo S, et al. Recombinant human erythropoietin prevents motor neuron apoptosis in a rat model of cervical sub-acute spinal cord compression. Neurosci Lett. 2011;490:57–62. doi: 10.1016/j.neulet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, et al. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93:412–21. doi: 10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11:S37–44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 40.Kim SM, Song J, Kim S, Han C, Park MH, Koh Y, et al. Identification of peripheral inflammatory markers between normal control and Alzheimer's disease. BMC Neurol. 2011;11:51. doi: 10.1186/1471-2377-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]


