Abstract
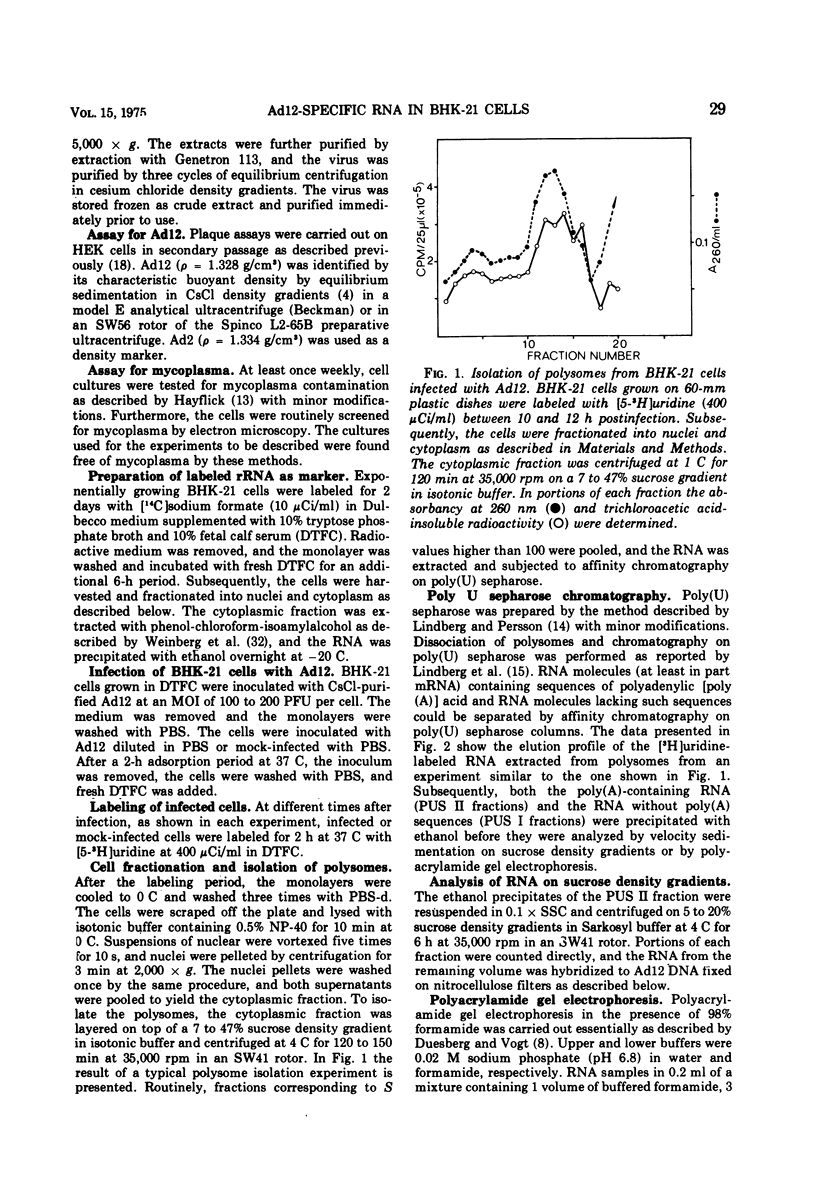
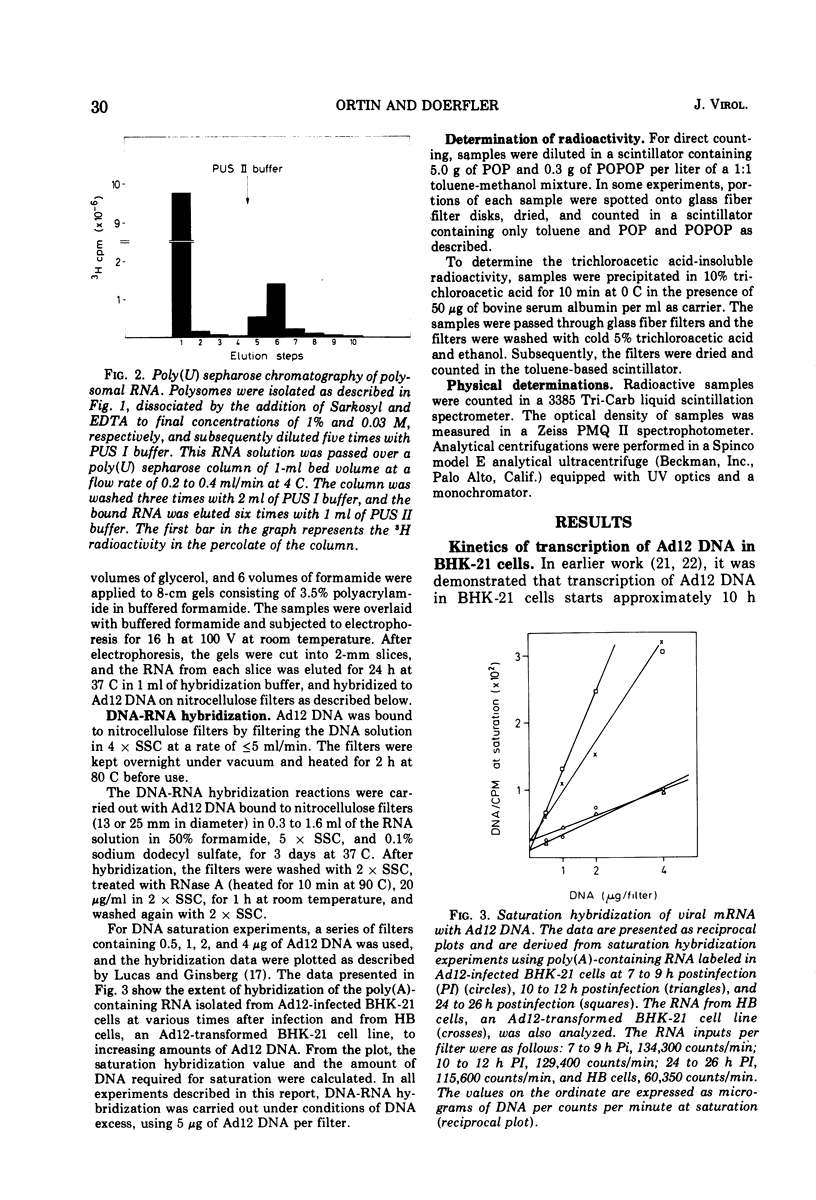
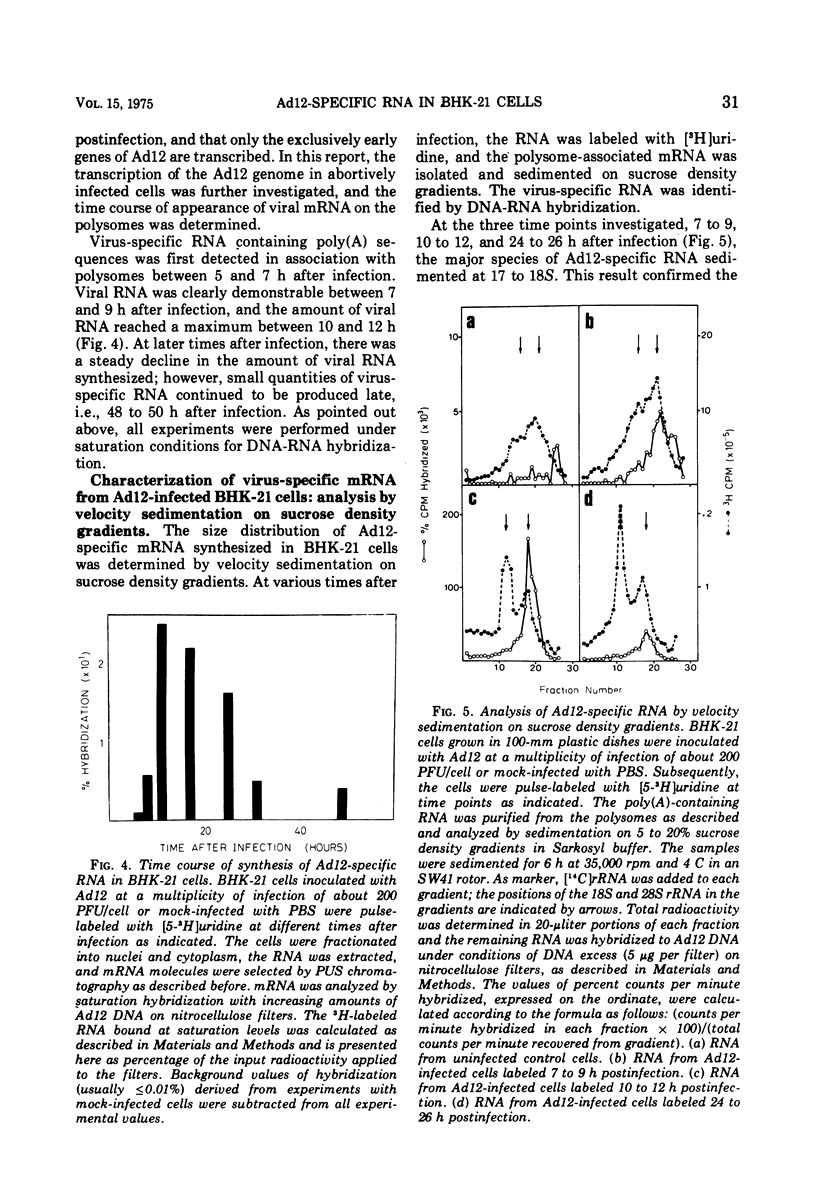
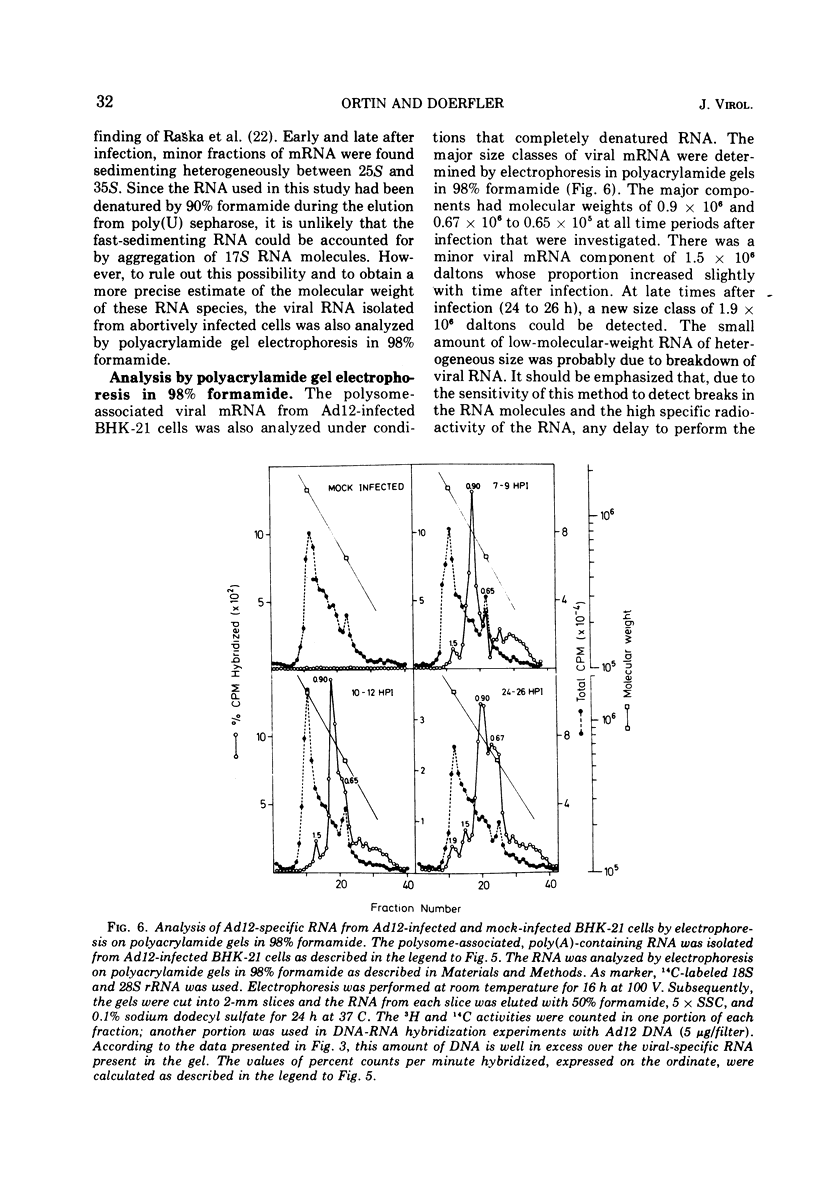
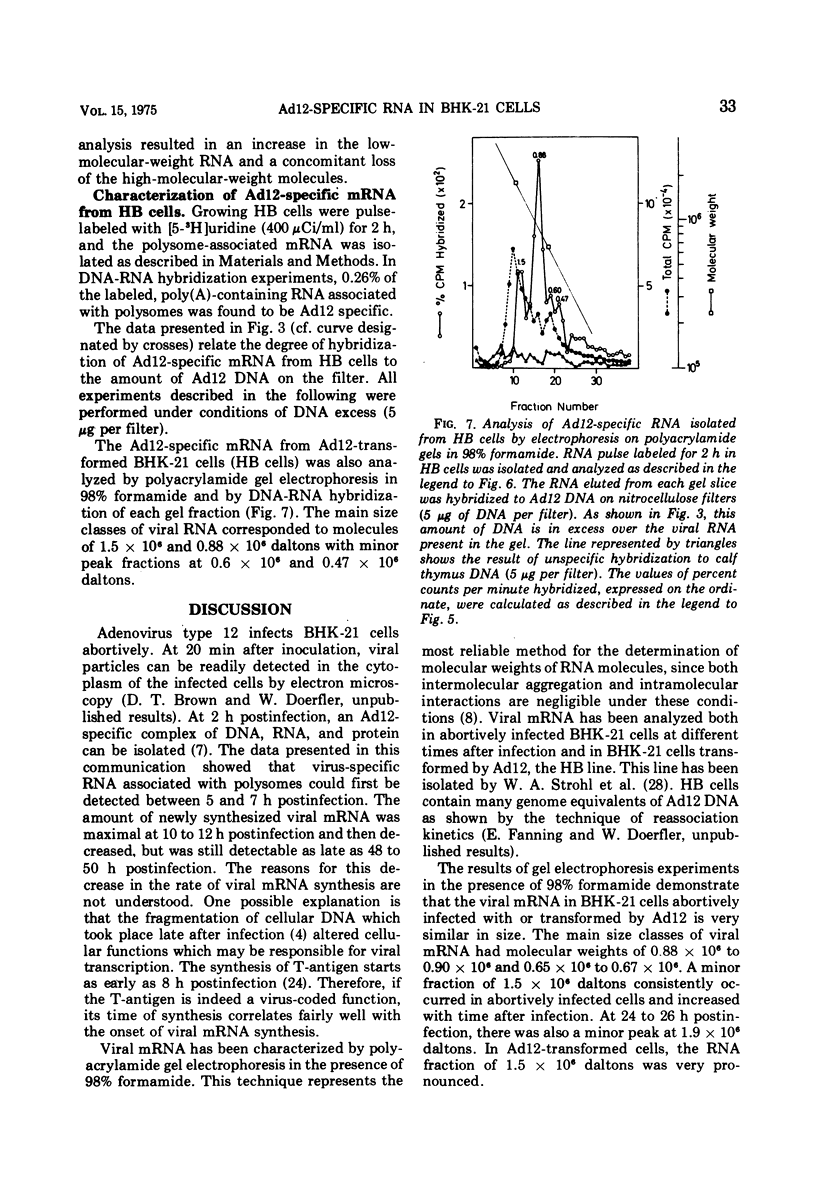
In baby hamster kidney (BKH-21) cells abortively infected with adenovirus type 12, polysome-associated, virus-specific RNA could be detected starting 5 to 7 h after infection. The amount of this RNA reached a maximum between 10 to 12 h after infection and continued to be synthesized at a reduced level until late in infection (48 to 50 h.). In BHK-21 cells transformed by adenovirus type 12 (HB cells), 0.26% of the polysome-associated mRNA was virus specific. The size of the virus-specific mRNA isolated from polysomes of BHK-21 cells abortively infected with, or transformed by adenovirus type 12 was determined by electrophoresis in polyacrylamide gels in 98% formamide, i.e., under conditions which eliminated secondary structure or aggregation of RNA. In abortively infected hamster cells viral mRNA size classes of molecular weights 0.9 times 10-6 and 0.65 times 10-6 to 0.67 times 10-6 were predominant. A minor fraction of 1.5 times 10-6 daltons was consistently found and increased with time after infection. Late after infection (24 to 26 h), viral mRNA of 1.9 times 10-6 daltons was also observed. The size distribution of adenovirus type 12-specific mRNA from transformed hamster cells (HB line) was very similar to that in abortively infected cells, except that the relative amount of the viral mRNA fraction of 1.5 times 10-6 daltons was much higher. It is uncertain whether the viral mRNA of high-molecular-weight represents mixed transcripts derived from integrated viral genomes and adjacent host genes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U. Absence of replication of the DNA of adenovirus type 12 in BHK21 cells. Virology. 1970 Mar;40(3):754–757. doi: 10.1016/0042-6822(70)90222-9. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm U., Doerfler W. Temperature-sensitive mutants of human adenovirus type 12. Virology. 1971 Sep;45(3):827–829. doi: 10.1016/0042-6822(71)90206-6. [DOI] [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska K., Jr, Strohl W. A., Holowczak J. A., Zimmerman J. The response of BHK21 cells to infection with type 12 adenovirus. V. Stimulation of cellular RNA synthesis and evidence for transcription of the viral genome. Virology. 1971 May;44(2):296–306. doi: 10.1016/0042-6822(71)90261-3. [DOI] [PubMed] [Google Scholar]
- Raska K., Jr, Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. VI. Synthesis of virus-specific RNA. Virology. 1972 Mar;47(3):734–742. doi: 10.1016/0042-6822(72)90563-6. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. Alterations in hamster cell regulatory mechanisms resulting from abortive infection with an oncogenic adenovirus. Prog Exp Tumor Res. 1973;18:199–239. doi: 10.1159/000393168. [DOI] [PubMed] [Google Scholar]
- Strohl W. A., Rabson A. S., Rouse H. Adenovirus tumorigenesis: role of the viral genome in determining tumor morphology. Science. 1967 Jun 23;156(3782):1631–1633. doi: 10.1126/science.156.3782.1631. [DOI] [PubMed] [Google Scholar]
- Strohl W. A., Rouse H., Teets K., Schlesinger R. W. The response of BHK21 cells to infection with type 12 adenovirus. 3. Transformation and restricted replication of superinfecting type 2 adenovirus. Arch Gesamte Virusforsch. 1970;31(1):93–112. doi: 10.1007/BF01241669. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. 1. Cell killing and T antigen synthesis as correlated viral genome functions. Virology. 1969 Dec;39(4):642–652. doi: 10.1016/0042-6822(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology. 1969 Dec;39(4):653–665. doi: 10.1016/0042-6822(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Mak S. Synthesis of viral components in hybrids of differentially permissive cells infected with adenovirus type 12. Exp Cell Res. 1972 Oct;74(2):423–429. doi: 10.1016/0014-4827(72)90397-7. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



