Abstract
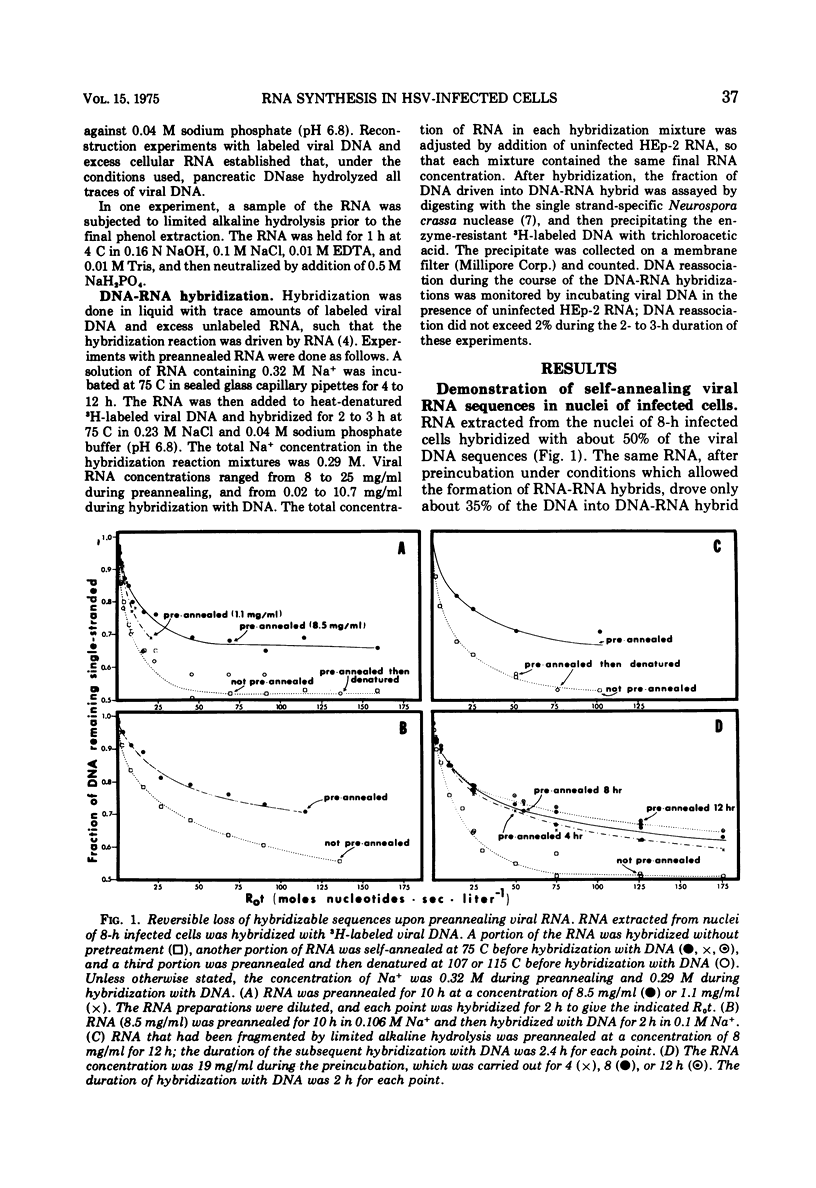
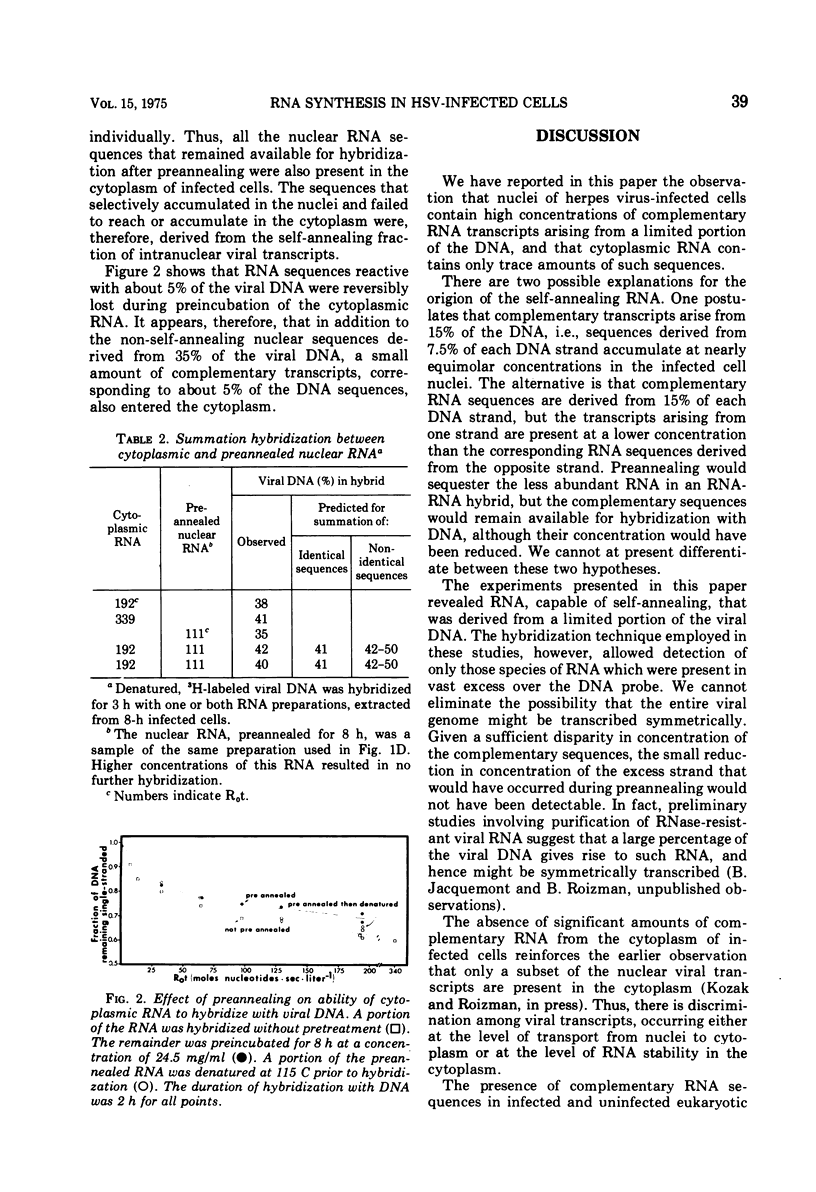
RNA extracted from nuclei of 8-h infected cells drove approximately 50% of herpes virus DNA into DNA-RNA hybrid. The same RNA, preannealed under conditions which allowed base pairing to take place, drove only 35% of the DNA into DNA-RNA hybrid; further annealing of the RNA did not diminish the amount of RNA sequences remaining available for subsequent hybridization with DNA. Upon denaturation of the preannealed RNA, the RNA sequences sequestered during preannealing became available again for hybridization with DNA. The base pairing that occurred during preincubation of the RNA was inter-molecular, since it was RNA concentration dependent and was not affected by limited alkaline hydrolysis. The nuclear viral transcripts that remained available for hybridization, after preannealing of the RNA, were subset of the RNA sequences that accumulated in the cytoplasm of infected cells. In addition, a small amount (derived from 5% or less of the viral DNA) of complementary transcripts was detected in the cytoplasm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Extensive symmetrical transcription of Simian Virus 40 DNA in virus-yielding cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2404–2409. doi: 10.1073/pnas.69.9.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bases R., Kaplan B. H. Double-stranded RNA from HeLa cell nuclei inhibits initiation of protein synthesis. Biochim Biophys Acta. 1973 Jul 13;312(3):574–580. doi: 10.1016/0005-2787(73)90455-3. [DOI] [PubMed] [Google Scholar]
- Colby C., Jurale C., Kates J. R. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J Virol. 1971 Jan;7(1):71–76. doi: 10.1128/jvi.7.1.71-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Rabin E. Z., Preiss B., Fraser M. J. A nuclease from Neurospora crassa conidia specific for single-stranded nucleic acids. Prep Biochem. 1971;1(4):283–307. doi: 10.1080/00327487108081946. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



