Abstract
Idiopathic Intracranial Hypertension (IIH) is a disease of unknown etiology typically affecting young, obese women, producing a syndrome of increased intracranial pressure without identifiable cause. Despite a large number of hypotheses and publications over the past decade, the etiology is still unknown. Vitamin A metabolism, adipose tissue as an actively secreting endocrine tissue, and cerebral venous abnormalities are areas of active study regarding IIH’s pathophysiology. There continues to be no evidence-based consensus or formal guidelines regarding management and treatment of the disease. Treatment studies show that the diagnostic lumbar puncture is a valuable intervention beyond its diagnostic importance, and that weight management is critical. However, open questions remain regarding the efficacy of acetazolamide, cerebrospinal fluid shunting procedures, and cerebral transverse venous sinus stenting.
Keywords: Idiopathic intracranial hypertension, pseudotumor cerebri, papilledema, cerebral venous stenosis, cerebral venous stenting, obesity
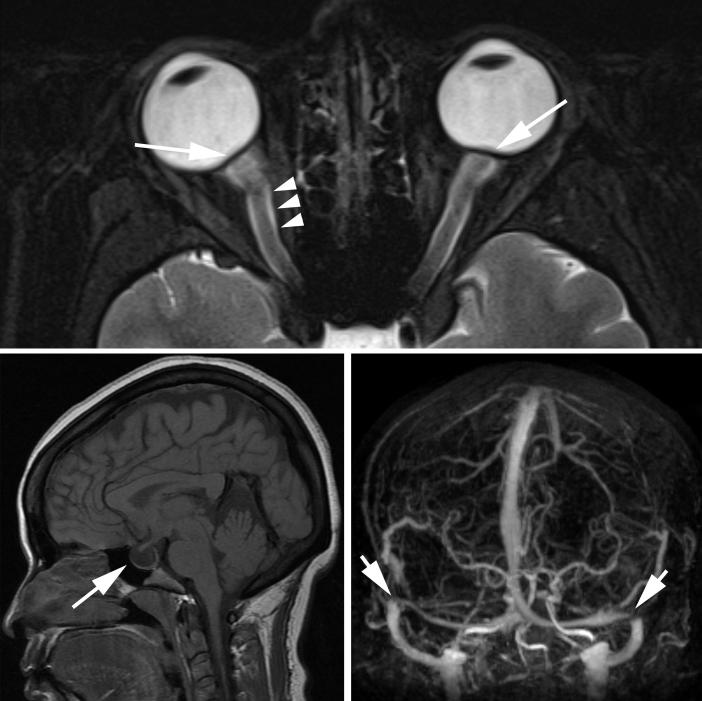
Idiopathic intracranial hypertension (IIH) is a syndrome characterized by increased intracranial pressure (ICP) of unknown cause (Table 1). By definition, the term “IIH” describes patients with isolated raised ICP that is not related to an intracranial disorder, a meningeal process, or cerebral venous thrombosis.[1] However, patients who develop a syndrome of raised ICP secondary to certain medications or who are found to have cerebral transverse venous sinus stenoses (not thrombosis) are still conventionally classified as having “IIH” (Figure 1). Therefore, although imperfect, the term “IIH” is currently the preferred designation for this disorder in the English literature, to the exclusion of “pseudotumor cerebri” (often including patients with other causes of raised ICP such as cerebral venous thrombosis) and “benign intracranial hypertension” (erroneously reassuring considering that a number of IIH patients irreversibly lose vision).[1-6] Although it has previously been emphasized that brain imaging should be normal in IIH patients, progress in non-invasive brain and vascular imaging has changed the radiological description of increased ICP, including IIH (Table 2, Figure 2).[7-11]
TABLE 1.
Modified Dandy criteria for the diagnosis of idiopathic intracranial hypertension
| 1- Signs and symptoms of increased intracranial pressure (headaches, nausea, vomiting, transient visual obscurations, papilledema). |
| 2- No localizing, focal neurologic signs, except unilateral or bilateral sixth nerve paresis. |
| 3- Cerebrospinal fluid opening pressure ≥ 25 cm of water*, but without cytologic or chemical abnormalities. |
| 4- Normal neuro-imaging adequate to exclude cerebral venous thrombosis, i.e., magnetic resonance imaging of the brain, often with additional sequences (computed tomography or magnetic resonance venography). |
The number of 25 cm of water is not an absolute cutoff, especially in children in whom cerebrospinal fluid opening pressure as high as 28 cm of water have been documented to be normal [20].
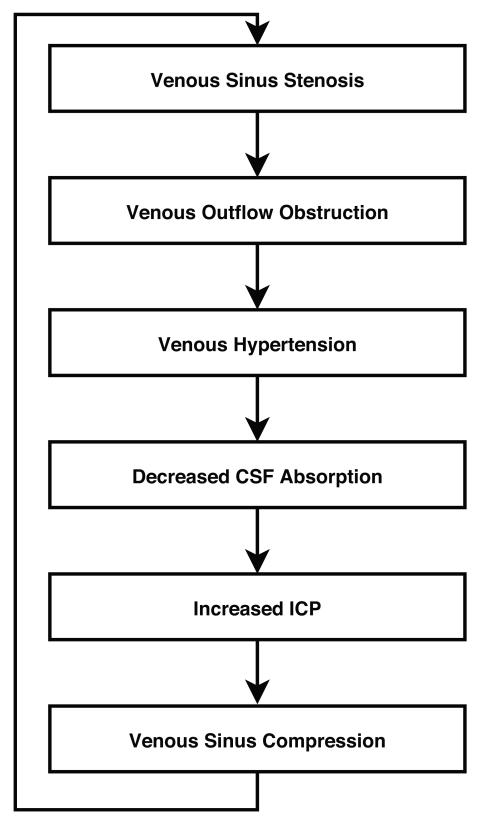
Figure 1.
Mechanism by which transverse sinus stenosis leads to increase intracranial pressure.
TABLE 2.
Brain and vascular imaging findings in patients with idiopathic intracranial hypertension
| Flattening of the posterior pole of the eyes |
| Dilation and tortuosity of the optic nerve sheaths |
| Empty sella turcica |
| Stenosis of one or both transverse cerebral venous sinuses |
Although common in IIH, these radiologic findings are not specific to IIH and are also found in patients with other causes of raised ICP. Additionally, patients with normal ICP are occasionally incidentally found to have one or all of the above radiologic findings.
Figure 2.
Magnetic resonance imaging findings in idiopathic intracranial hypertension.
Although a relatively rare disease, IIH is becoming an important area of clinical research and activity, mostly because of the rising prevalence of obesity.[3-5] Indeed, it was recently suggested that the economic costs of IIH in the United-States (US) exceed $444 million dollars per year, mostly because of frequent hospital admissions, unsatisfactory treatment options, and lost productivity of young patients.[12] The rising incidence of obesity in the world will likely make the prevalence of IIH increase, resulting in further increased IIH-related expenses.
There are multiple theories regarding the pathophysiology of this disorder, but there is no unifying hypothesis.[2-5] There continues to be no evidence-based consensus or formal guidelines regarding management and treatment of the disease, although a clinical trial is currently ongoing in the US (http://www.nordicclinicaltrials.com/). This review emphasizes recent studies regarding the epidemiology, pathophysiology, and management of IIH.
EPIDEMIOLOGIC DATA RELEVANT TO THE PATHOPHYSIOLOGY OF IIH
IIH occurs most frequently among obese women of childbearing age
A recent multicenter case-control study of newly-diagnosed women with IIH compared to women with other neuro-ophthalmologic disorders showed a dose relationship of higher body mass index (BMI) associated with a greater risk of IIH.[13] Interestingly, this study also showed that even non-obese patients (BMI<30) were at greater risk for IIH if they had a recent moderate weight gain (5 to 15% of their weight). Another recent study also showed that a group of 26 women with recurrent IIH had a greater BMI at the time of recurrence compared to their BMI at the time of initial diagnosis.[14] This study also demonstrated a greater degree of weight gain between initial resolution and recurrence compared to a group of 24 IIH women without recurrence. Furthermore, patients without recurrence had stable weights, while patients with recurrence had a 6% weight gain, suggesting that even moderate weight gain might be a risk factor for recurrence. Additionnally, in a more recent study, increasing degrees of obesity were associated with an increased risk of severe visual loss.[15] A study from Israel suggested that gynecoid (lower body) adiposity might be more associated with IIH than the more common visceral (abdominal or central) obesity, which is associated with cardiovascular diseases and diabetes.[16] Although not all 40 obese women evaluated in this study had lower body adiposity, fat distribution in these IIH patients was clearly shifted to the lower body compared with age-matched obese women living in the same country. The exact relationship between obesity and IIH remains poorly understood, with several etiologic hypotheses proposed, including increased central venous pressure and various hormonal and metabolic changes commonly found in obese patients.[17] Although there is no doubt that obesity plays a major role in the occurrence, and likely, the pathophysiology of IIH, many non-obese patients of either gender also develop a syndrome of isolated intracranial hypertension similar to IIH.[18,19] IIH is rare in pre-pubertal children and has characteristics distinct from the adult form, including no apparent predilection for obese girls. After puberty, however, the rate of obesity and the gender predilection is similar to that in the adult IIH population.[20] A recent large series confirmed that only about 10% of IIH patients are men.[19] While affected men have a similar BMI when compared to affected women, they are, on average, about a decade older than women at the time of presentation.[19] Although race does not seem to influence the incidence of IIH, a recent study questioned whether the association between obesity and development of IIH among Asians is as strong as in other populations.[21] Instead of affecting prevalence, race appears to be an important determining factor of a patients’ visual prognosis, worse in black patients compared with whites living in the US,[22] and worse in white US IIH patients compared with white French IIH patients.[23] Similarly, although men develop IIH less frequently than women, their visual prognosis is worse, perhaps because they have less headaches to alert them to the problem.[19]
While various medications have been proposed to cause or, more likely, to precipitate IIH (such as tetracycline and its derivatives, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen),[3] there are compelling test-retest data only in a few individual patients (such as in patients treated with tetracyclines, particularly minocycline).[24-26]
Obstructive sleep apnea (OSA) is also associated with IIH.[3] It is unclear, however, whether obesity is the common pathophysiological link, or whether OSA is another factor capable of triggering IIH in predisposed patients. It is important to emphasize that OSA appears to provide a worse prognosis for IIH patients.[19,27] Routine screening of IIH patients with the Berlin questionnaire for OSA (brief and validated screening questionnaire which includes questions about snoring, daytime somnolence, BMI and hypertension) has been shown to be a reliable metric in the young population commonly affected by IIH.[28] Overnight polysomnography is usually obtained when the clinical suspicion for OSA is high in IIH patients.[27,28]
PATHOPHYSIOLOGY OF IIH
The pathophysiologic mechanisms underlying the raised ICP in IIH remain unclear, but those proposed classically include increased brain water content, excess cerebrospinal fluid (CSF) production, reduced CSF absorption, and increased cerebral venous pressure. As emphasized, any pathophysiogic theory must ultimately account for the remarkable predilection IIH has for obese young women, as well as the few epidemiologic observations described above. Multiple coexisting mechanisms are likely necessary to produce the syndrome of isolated intracranial hypertension. It seems that some patients in particular (such as obese patients, especially young women, and those with anomalous distal transverse sinuses resulting in bilateral transverse sinus stenoses (TSS)) are predisposed to developing raised ICP, which might be triggered by specific events or situations, such as weight gain, endocrine changes, hypercoagulable states, specific medications, and OSA.
One long-standing hypothesis for the pathogenesis of IIH involves abnormal vitamin A metabolism. While early studies evaluating serum levels of vitamin A found conflicting evidence for the role of vitamin A,[29] two studies have shown elevated retinol levels in the CSF of patients with IIH.[30,31] One of these studies also demonstrated that patients with IIH have higher levels of serum, but lower levels of CSF, retinol binding protein.[31] However, despite a large number of publications in the field of IIH over the past few years, we could not find any major study regarding vitamin A metabolism published since 2007. The ongoing US-IIH trial (http://www.nordicclinicaltrials.com/) will likely provide useful information on this topic.
These observations regarding vitamin A may be linked to another area of emerging interest in endocrinology and IIH,[17] the nature of adipose tissue as an actively secreting endocrine tissue.[32] In particular, adipose tissue-derived retinol binding protein is released from adipose tissue and acts as a modulator of insulin sensitivity.[29] Other adipose-produced cytokines, such as leptin, have been implicated in the pathophysiology of IIH but their role remains unclear.[17] Because the hormonal secretions and biological functions of adipose tissue are highly dependent on its regional distribution in the body,[32] fat distribution may ultimately be as important as total adiposity in the pathogenesis of IIH.[16] However, the finding of predominant lower body adiposity in IIH is somewhat surprising given that IIH has been potentially associated with conditions related to increased intra-abdominal, visceral fat, such as elevated levels of adipose tissue-derived retinol binding protein,[30] polycystic ovarian disease in women,[33] and androgen deficiency in men.[34] Another recent area of research includes the brain water channel aquaporin-4, which mediates rapid rapid transmembrane osmotic movement of water and is critically involved in brain water homeostasis. So far, results of studies investigating aquaporin-4 have been negative.[35,36] Proteomics are also a promising area of study and aim to identify new candidate markers that may be involved in the pathophysiology of IIH.[37,38]
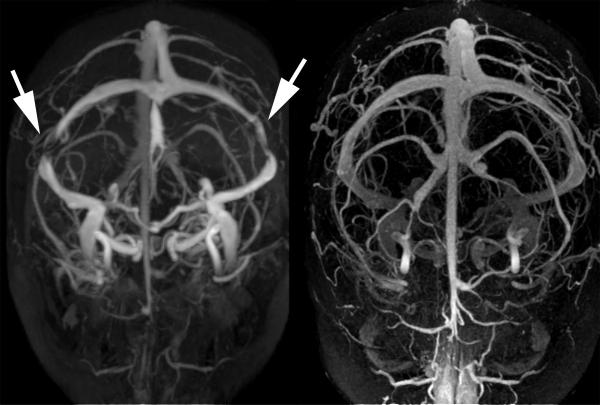
Increased intracranial venous pressure related to stenosis of the distal portion of the transverse cerebral sinuses is another proposed mechanism of IIH that has received substantial recent interest.[3-10,39-42] Since CSF is passively resorbed into the intracranial venous sinuses via the arachnoid granulations, a stenosis of a dominant transverse sinus or stenoses of both transverse sinuses can impair venous drainage, resulting in cerebral venous hypertension and impaired CSF absorption (Figure 1). Numerous recent publications have shown that such transverse sinus stenoses (TSS) are found in a large majority of patients with IIH. It is, however, not clear whether they are incidental, secondary to increased ICP, or causal.[40,43,44] Anatomic studies have suggested that these stenoses might be incidentally related to the presence of trabeculae, septae, or large arachnoid granulations in the transverse sinuses.[39] It has also been shown that such distal TSS can develop secondary to increased ICP because of external compression of the distal portion of the transverse venous sinuses. The observation of complete reversal of TSS after a lumbar puncture or a CSF shunting procedure supports this theory (Figure 3).[43,44] In addition, TSS are occasionally found in patients without intracranial hypertension, suggesting that they may have no functional significance in some patients.[7] Regardless of the cause of the TSS often observed in IIH patients, treatment with endovascular stenting usually results in decreased venous pressure gradient, thereby inducing improved CSF absorption and subsequent decrease in ICP (Figure).[43-45] It is still unclear, however, whether the presence of uni- or bilateral TSS has any direct consequence on the natural history and prognosis of IIH.
Figure 3.
Resolution of bilateral transverse sinus stenosis after lumboperitoneal shunt in a young obese woman with idiopathic intracranial hypertension.
Another venous mechanism proposed in the development of increased ICP in IIH patients is the occurrence of microthrombosis from thrombophilia within the cerebral veins, resulting in impaired CSF absorption.[46-48] While many would argue that the presence of any thrombophilia would make these cases ineligible for the diagnosis of IIH, the absence of true venous sinus thrombosis and the strength of the associations between underlying systemic hypercoagulability disorders and so-called IIH in these studies is provocating. However, routine hypercoagulation studies in presumed IIH patients are not warranted, unless there is a demonstrated clot within the cerebral venous sinuses, or a strong personal or family history of thrombophilia.[49]
TREATMENT OF IIH
The two goals of treatment in IIH are to alleviate symptoms of increased ICP, particularly headaches, and to preserve vision. General recommendations include evaluation and treatment of potential contributing factors (including weight gain and obesity, medication use, anemia, and obstructive sleep apnea), weight loss, and medical headache management. Immediate management is primarily based on the duration of symptoms, evaluation of visual function and patients’ characteristics (Table 3).
TABLE 3.
Factors independently associated with a worse visual outcome in idiopathic intracranial hypertension
| Male gender |
| Race (black patients) |
| Morbid obesity |
| Anemia |
| Obstructive sleep apnea |
| Acute onset of symptoms and signs of raised intracranial pressure (fulminant IIH) |
The treatment of raised ICP itself begins with the diagnostic lumbar puncture, which is often effective in transiently improving symptoms and signs. Interestingly, it is not uncommon to observe a lasting clinical remission following a single lumbar puncture in some IIH patients,[50] obviating the need for further medical or surgical treatment. This phenomenon cannot be simply explained by the amount of CSF drained, or by the hole made in the dura by the needle used for the lumbar puncture. Mathematical models of CSF hydraulics likely explain this phenomenon by the interaction of various factors, such as CSF formation, compliance, cerebral blood flow, and outflow resistance, which can lead to multiple stable and unstable equilibrium levels of ICP in IIH.[40] If a single lumbar puncture lowers the CSF pressure beyond the threshold level of an unstable equilibrium, the ICP must settle into a lower stable pressure state until other factors cause the pressure to exceed that threshold. This observation emphasizes the importance of the initial lumbar puncture as a therapeutic procedure in IIH in addition to its diagnostic importance. It also explains why some patients dramatically improve after two or three lumbar punctures or require episodic lumbar punctures to remain asymptomatic.
Patients with persistent symptoms and signs can be treated using medical and surgical approaches, although there are currently no results from large randomized controlled trials prospectively assessing and comparing these treatments.
Weight loss is a critical part of the treatment of overweight and obese IIH patients. Only a modest degree of weight loss (about 5-10% of total body weight) is usually required for improvement in symptoms and signs,[51] although it is likely that aggressive weight control is an efficient way to improve the overall quality of life of obese IIH patients. A recent study of 25 obese women with IIH showed that weight loss effectively reduces not only headaches and papilledema, but also ICP.[52] Weight loss is often not an effective short-term treatment and, thus, usually must be initiated in association with other treatments, However, it remains essential to emphasize the importance of weight loss and the prevention of weight fluctuation in the long-term management of obese or overweight IIH patients in order to minimize the risk of recurrence.[14] Bariatric surgery can be considered in morbidly obese IIH patients in whom attempts at weight loss have been unsuccessful or in whom other medical morbidities of obesity already coexist, and two recent studies have specifically reviewed the positive effects of bariatric surgery on IIH.[53,54]
Carbonic anhydrase inhibitors, such as acetazolamide (1-2 g daily, most often divided in two doses), are the main medical treatment classically prescribed for IIH. Acetazolamide decreases the production of CSF in humans, and therefore, has always been consided the treatment of choice in IIH,[55] although no trial data are currently available to confirm its effectiveness. The preliminary results of the IIH Pilot Trial from Birmingham, UK, in which 50 patients were randomized to receive acetazolamide or no acetazolamide, emphasized the practical difficulties of performing such a study because of poor recruitment and medication side-effects (paresthesias, altered taste sensation, and lethargy).[56] A multicenter, double-blind, placebo-controlled clinical trial, called the IIHTT (the Idiopathic Intracranial Hypertension Treatment Trial), is currently enrolling patients in the US (http://www.nordicclinicaltrials.com/). This trial compares the efficacy of acetazolamide and placebo in the treatment of IIH patients with moderate visual field defects. All patients are also treated with a low-sodium diet and participate in a standardized weight loss program. This trial will hopefully clarify the efficacy of acetazolamide and weight loss in IIH patients with moderate visual loss.
Topiramate (which has weak carbonic anhydrase inhibition properties) has also been suggested for the treatment of IIH, particularly for the treatment of headaches.[57]
Oral steroids have been used as a treatment for IIH in the past, but are associated with significant long-term side effects, such as weight gain, obviously undesirable in this patient population, and therefore should not be prescribed. High-dose intravenous steroids are still occasionally used in patients with rapidly progressive visual loss from fulminant IIH while a more definitive treatment is organized.[58]
Surgery is required in patients with a fulminant onset of disease or when other treatments have failed to prevent progressive visual loss. More rarely, surgery may be performed for refractory headaches related to chronically elevated ICP. The choice of procedure depends on local resources, as well as the patient’s symptoms and signs. In patients with papilledema who have severe visual loss, but minimal or no headache, optic nerve sheath fenestration (ONSF) is often advised, while in those with visual loss, papilledema, and headache, a CSF diversion procedure, such as ventriculo-(VP) or lumbo-peritoneal (LP) shunting, is preferred. Aggressive management with CSF shunting is usually required to prevent catastrophic visual loss in those with acute and rapidly progressive visual loss.[58] These patients might benefit from a transient lumbar drain while awaiting a more definite surgical procedure. Although no prospective study has effectively compared LP shunts with VP shunts, a large number of recent publications have addressed surgical interventions of IIH.[59-65] Retrospective series suggest that both techniques are equally efficient in controlling clinical manifestations of IIH as well as reducing the intracranial pressure in the immediate post-operative period. In one series,[64] failure rates seemed to be slightly higher for VP shunts (14%) than LP shunts (11%). However, revision rates were higher with LP shunts (60%) than with VP shunts (30%). Despite the apparent high rate of complications (and failure), CSF shunting procedures remain the most widely performed surgical treatment for IIH and are usually very useful acutely to prevent or treat devastating visual loss in selected patients. It is important to emphasize that most IIH patients have a relatively “benign” and self-limited course, and never require any surgical treatment.
It is now well accepted that the stenting of transverse venous sinus stenoses reduces cerebral venous pressure, reduces ICP, and improves symptoms and signs in selected IIH patients.[43,45,66] However, endovascular venous sinus stenting can result in serious complications, such as stent migration, venous sinus perforation, in-stent thrombosis, subdural hemorrhage, and the development of recurrent stenoses immediately proximal to the stent. Until more data are available on the safety and long-term outcome of venous stenting in young IIH patients, this procedure should be limited to selected patients with bilateral TSS, and refractory symptoms and signs of increased ICP, who cannot undergo (or have failed) more conventional surgical treatments.
CONCLUSION
Many questions remain unanswered about IIH. Its association with female gender and obesity is striking. However, studies indicate that IIH can also occur in men, non-obese adults, older adults, and in pre-pubertal children. Identification of subgroups at high risk for irreversible visual loss, such as black patients, men, and patients with fulminant IIH (Table 3), helps determine management approaches and refine follow-up strategies. Ongoing pathophysiology studies, as well as clinical trials, are promising and should provide more insight into this relatively common, yet poorly understood, syndrome of isolated intracranial hypertension.
Acknowledgments
Disclosure: This study was supported in part by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, by core grant P30-EY06360 (Department of Ophthalmology). Dr. Bruce receives research support from the NIH/PHS (KL2-RR025009, UL1-RR025008), NIH/NEI (K23-EY019341), and the Knights Templar Eye Foundation; and received the American Academy of Neurology Practice Research Fellowship. Dr. Biousse receives research support from NIH/PHS (UL1-RR025008). Dr. Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award.
REFERENCES
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol. 2004;24:138–145. doi: 10.1097/00041327-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28:593–617. doi: 10.1016/j.ncl.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Digre KB. Idiopathic intracranial hypertension. BMJ. 2010;341:c2836. doi: 10.1136/bmj.c2836. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor KG. More than meets the eye? Redefining idiopathic intracranial hypertension. Int J Neurosci. 2010;120:471–482. doi: 10.3109/00207451003760098. [DOI] [PubMed] [Google Scholar]
- 6.Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. 1996;46:198–202. doi: 10.1212/wnl.46.1.198. [DOI] [PubMed] [Google Scholar]
- 7.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–1424. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- 8.Degnan AJ, Levy LM. Pseudotumor Cerebri: Brief Review of Clinical Syndrome and Imaging Findings. AJNR Am J Neuroradiol. 2011 Jun 16; doi: 10.3174/ajnr.A2404. [Epub ahead of print] PubMed PMID: 21680652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohr AC, Riedel C, Fruehauf MC, et al. MR imaging findings in patients with secondary intracranial hypertension. AJNR Am J Neuroradiol. 2011;32:1021–1029. doi: 10.3174/ajnr.A2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor SE, Siddiqui MA, Stewart VR, O’Flynn EA. The relationship of transverse sinus stenosis to bony groove dimensions provides an insight into the aetiology of idiopathic intracranial hypertension. Neuroradiology. 2008;50:999–1004. doi: 10.1007/s00234-008-0431-5. [DOI] [PubMed] [Google Scholar]
- 11.Shofty B, Ben-Sira L, Constantini S, et al. Optic nerve sheath diameter on MR Imaging: Establishment of norms and comparison of pediatric patients with idiopathic intracranial hypertension with healthy controls. AJNR Am J Neuroradiol. 2011 Nov 24; doi: 10.3174/ajnr.A2779. [Epub ahead of print] PubMed PMID: 22116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friesner D, Rosenman R, Lobb BM, Tanne E. Idiopathic intracranial hypertension in the USA: the role of obesity in establishing prevalence and healthcare costs. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 13.Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri) Am J Ophthalmol. 2007;143:635–641. doi: 10.1016/j.ajo.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 14.Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology. 2011;76:1564–1567. doi: 10.1212/WNL.0b013e3182190f51. [DOI] [PubMed] [Google Scholar]
- 15.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol. doi: 10.1097/WNO.0b013e31823f852d. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesler A, Kliper E, Shenkerman G, Stern N. Idiopathic intracranial hypertension is associated with lower body adiposity. Ophthalmology. 2010;117:169–174. doi: 10.1016/j.ophtha.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Ooi LY, Walker BR, Bodkin PA, Whittle IR. Idiopathic intracranial hypertension: can studies of obesity provide the key to understanding pathogenesis? Br J Neurosurg. 2008;22:187–194. doi: 10.1080/02688690701827340. [DOI] [PubMed] [Google Scholar]
- 18.Bruce BB, Kedar S, Van Stavern GP, et al. Atypical idiopathic intracranial hypertension: normal BMI and older patients. Neurology. 2010;74:1827–1832. doi: 10.1212/WNL.0b013e3181e0f838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (pseudotumor cerebri) Horm Res Paediatr. 2010;74:381–389. doi: 10.1159/000321180. [DOI] [PubMed] [Google Scholar]
- 21.Kim TW, Choung HK, Khwarg SI, et al. Obesity may not be a risk factor for idiopathic intracranial hypertension in Asians. Eur J Neurol. 2008;15:876–879. doi: 10.1111/j.1468-1331.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 22.Bruce BB, Preechawat P, Newman NJ, et al. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–867. doi: 10.1212/01.wnl.0000304746.92913.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrejen S, Vignal C, Bruce BB, et al. Idiopathic intracranial hypertension: a comparison between French and North-American white patients. Rev Neurol (Paris) 2009;165:542–548. doi: 10.1016/j.neurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005;6:29–37. doi: 10.2165/00128071-200506010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand. 2004;110:408–411. doi: 10.1111/j.1600-0404.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocyclin treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116–121. doi: 10.1016/s0002-9394(98)00063-4. [DOI] [PubMed] [Google Scholar]
- 27.Wall M, Purvin V. Idiopathic intracranial hypertension in men and the relationship to sleep apnea. Neurology. 2009;72:300–301. doi: 10.1212/01.wnl.0000336338.97703.fb. [DOI] [PubMed] [Google Scholar]
- 28.Thurtell MJ, Bruce BB, Rye DB, et al. The berlin questionnaire screens for obstructive sleep apnea in idiopathic intracranial hypertension. J Neuroophthalmol. 2011;31:316–319. doi: 10.1097/WNO.0b013e31821a4d54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libien J, Blaner WS. Retinol and retinol-binding protein in cerebrospinal fluid: can vitamin A take the “idiopathic” out of idiopathic intracranial hypertension? J Neuroophthalmol. 2007;27:253–257. doi: 10.1097/WNO.0b013e31815c44bc. [DOI] [PubMed] [Google Scholar]
- 30.Tabassi A, Salmasi AH, Jalali M. Serum and CSF vitamin A concentrations in idiopathic intracranial hypertension. Neurology. 2005;64:1893–1896. doi: 10.1212/01.WNL.0000163556.31080.98. [DOI] [PubMed] [Google Scholar]
- 31.Warner JE, Larson AJ, Bhosale P, et al. Retinol-binding protein and retinol analysis in cerebrospinal fluid and serum of patients with and without idiopathic intracranial hypertension. J Neuroophthalmol. 2007;27:258–262. doi: 10.1097/WNO.0b013e31815b9af0. [DOI] [PubMed] [Google Scholar]
- 32.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 33.Glueck CJ, Aregawi D, Goldenberg N, et al. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145:72–82. doi: 10.1016/j.lab.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Fraser JA, Bruce BB, Rucker J, et al. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010;290:86–89. doi: 10.1016/j.jns.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhungana S, Waters P, Ismail A, et al. Absence of aquaporin-4 antibodies in patients with idiopathic intracranial hypertension. J Neurol. 2010;257:1211–1212. doi: 10.1007/s00415-010-5499-2. [DOI] [PubMed] [Google Scholar]; J Neurol. 2010;257:1229–1230. Corrected and republished in: [Google Scholar]
- 36.Kerty E, Heuser K, Indahl UG, et al. Is the brain water channel aquaporin-4 a pathogenetic factor in idiopathic intracranial hypertension? Results from a combined clinical and genetic study in a Norwegian cohort. Acta Ophthalmol. 2011 Sep 13; doi: 10.1111/j.1755-3768.2011.02231.x. doi: 10.1111/j.1755-3768.2011.02231.x. [Epub ahead of print] PubMed PMID: 21914143. [DOI] [PubMed] [Google Scholar]
- 37.Brettschneider J, Hartmann N, Lehmensiek V, et al. Cerebrospinal fluid markers of idiopathic intracranial hypertension: is the renin-angiotensinogen system involved? Cephalalgia. 2011;31:116–121. doi: 10.1177/0333102410375726. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair AJ, Viant MR, Ball AK, et al. NMR-based metabolomic analysis of cerebrospinal fluid and serum in neurological disease – a diagnostic tool? NMR Biomed. 2010;23:123–132. doi: 10.1002/nbm.1428. [DOI] [PubMed] [Google Scholar]
- 39.Strydom MA, Briers N, Bosman MC, Steyn S. The anatomical basis of venographic filling defects of the transverse sinus. Clin Anat. 2010;23:153–159. doi: 10.1002/ca.20911. [DOI] [PubMed] [Google Scholar]
- 40.Bateman GA, Stevens SA, Stimpson J. A mathematical model of idiopathic intracranial hypertension incorporating increased arterial inflow and variable venous outflow collapsibility. J Neurosurg. 2009;110:446–456. doi: 10.3171/2008.6.17609. [DOI] [PubMed] [Google Scholar]
- 41.Sander K, Poppert H, Etgen T, et al. Dynamics of intracranial venous flow patterns in patients with idiopathic intracranial hypertension. Eur Neurol. 2011;66:334–338. doi: 10.1159/000331002. [DOI] [PubMed] [Google Scholar]
- 42.Rohr A, Bindeballe J, Riedel C, et al. The entire dural sinus tree is compressed in patients with idiopathic intracranial hypertension: a longitudinal, volumetric magnetic resonance imaging study. Neuroradiology. 2011 Feb 22; doi: 10.1007/s00234-011-0850-6. [Epub ahead of print] PubMed PMID: 21340576. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed R, Friedman DI, Halmagyi GM. Stenting of the transverse sinuses in idiopathic intracranial hypertension. J Neuroophthalmol. 2011;31:374–380. doi: 10.1097/WNO.0b013e318237eb73. [DOI] [PubMed] [Google Scholar]
- 44.Stienen A, Weinzierl M, Ludolph A, et al. Obstruction of cerebral venous sinus secondary to idiopathic intracranial hypertension. Eur J Neurol. 2008;15:1416–1418. doi: 10.1111/j.1468-1331.2008.02340.x. [DOI] [PubMed] [Google Scholar]
- 45.Albuquerque FC, Dashti SR, Hu YC, et al. Intracranial venous sinus stenting for benign intracranial hypertension: clinical indications, technique, and preliminary results. World Neurosurg. 2011;75:648–652. doi: 10.1016/j.wneu.2010.11.012. discussion 592-5. [DOI] [PubMed] [Google Scholar]
- 46.Kesler A, Kliper E, Assayag EB, et al. Thrombophilic factors in idiopathic intracranial hypertension: a report of 51 patients and a meta-analysis. Blood Coagul Fibrinolysis. 2010;21:328–333. doi: 10.1097/MBC.0b013e328338ce12. [DOI] [PubMed] [Google Scholar]
- 47.Hannerz J, Antovic JP, Blombäck M, et al. Inflammatory and haemostatic markers in idiopathic intracranial hypertension. J Intern Med. 2011;270:496–499. doi: 10.1111/j.1365-2796.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- 48.Biousse V, Rucker JC, Vignal C, et al. Anemia and papilledema. Am J Ophthalmol. 2003;135:437–446. doi: 10.1016/s0002-9394(02)02062-7. [DOI] [PubMed] [Google Scholar]
- 49.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53:1537–1542. doi: 10.1212/wnl.53.7.1537. [DOI] [PubMed] [Google Scholar]
- 50.De Simone R, Marano E, Fiorillo C, et al. Sudden re-opening of collapsed transverse sinuses and longstanding clinical remission after a single lumbar puncture in a case of idiopathic intracranial hypertension. Pathogenetic implications. Neurol Sci. 2005;25:342–344. doi: 10.1007/s10072-004-0368-3. [DOI] [PubMed] [Google Scholar]
- 51.Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007;7:15. doi: 10.1186/1471-2415-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi: 10.1136/bmj.c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan RJ, Meredith HE, Coulston JE, et al. The effects of laparoscopic adjustable gastric banding on idiopathic intracranial hypertension. Obes Surg. 2011;21:161–166. doi: 10.1007/s11695-010-0307-8. [DOI] [PubMed] [Google Scholar]
- 54.Fridley J, Foroozan R, Sherman V, et al. D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2011;114:34–39. doi: 10.3171/2009.12.JNS09953. [DOI] [PubMed] [Google Scholar]
- 55.Rubin RC, Henderson ES, Ommaya AK, et al. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25:430–436. doi: 10.3171/jns.1966.25.4.0430. [DOI] [PubMed] [Google Scholar]
- 56.Ball AK, Howman A, Wheatley K, et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. J Neurol. 2011;258:874–881. doi: 10.1007/s00415-010-5861-4. [DOI] [PubMed] [Google Scholar]
- 57.Celebisoy N, Gokcay F, Sirin H, Akyurekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116:322–327. doi: 10.1111/j.1600-0404.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- 58.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–232. doi: 10.1212/01.wnl.0000251312.19452.ec. [DOI] [PubMed] [Google Scholar]
- 59.Sinclair AJ, Kuruvath S, Sen D, et al. Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia. 2011 Oct 3; doi: 10.1177/0333102411423305. [Epub ahead of print] PubMed PMID: 21968519. [DOI] [PubMed] [Google Scholar]
- 60.Ulivieri S, Oliveri G, Georgantzinou M, et al. Long-term effectiveness of lumboperitoneal flow-regulated shunt system for idiopathic intracranial hypertension. J Neurosurg Sci. 2009;53:107–111. [PubMed] [Google Scholar]
- 61.El-Saadany WF, Farhoud A, Zidan I. Lumboperitoneal shunt for idiopathic intracranial hypertension: patients’ selection and outcome. Neurosurg Rev. 2011 Sep 29; doi: 10.1007/s10143-011-0350-5. [Epub ahead of print] PubMed PMID: 21956361. [DOI] [PubMed] [Google Scholar]
- 62.Kandasamy J, Hayhurst C, Clark S, et al. Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurg. 2011;75:155–160. doi: 10.1016/j.wneu.2010.10.025. discussion 32-33. [DOI] [PubMed] [Google Scholar]
- 63.Tarnaris A, Toma AK, Watkins LD, Kitchen ND. Is there a difference in outcomes of patients with idiopathic intracranial hypertension with the choice of cerebrospinal fluid diversion site: a single centre experience. Clin Neurol Neurosurg. 2011;113:477–479. doi: 10.1016/j.clineuro.2011.02.008. Epub 2011 Mar 15. PubMed PMID: 21411220. [DOI] [PubMed] [Google Scholar]
- 64.Abubaker K, Ali Z, Raza K, et al. Idiopathic intracranial hypertension: lumboperitoneal shunts versus ventriculoperitoneal shunts--case series and literature review. Br J Neurosurg. 2011;25:94–99. doi: 10.3109/02688697.2010.544781. [DOI] [PubMed] [Google Scholar]
- 65.Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology. 2011;118:412–414. doi: 10.1016/j.ophtha.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32:1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]