Abstract
This review traces the evolution of the concept of the blood-aqueous barrier (BAB) during the past 20 years. The classical model simply stipulated that the tight junctions of the iris vasculature and ciliary epithelium excluded plasma proteins from the aqueous humor (AH). It failed to reconcile the presence of AH protein levels equal to 1% of that found in plasma. Moreover, models of barrier kinetics assumed that the processes of AH secretion and plasma protein entry were directly linked. Thus, elevations of AH protein levels could only be explained by a pathological breakdown of the BAB. Over the last 20 years it has been shown that the plasma proteins in normal AH by-pass the posterior chamber entirely. Instead, these proteins diffuse from the capillaries of ciliary body stroma, into the iris stroma and then into the anterior chamber. This creates a reservoir of plasma-proteins in the iris stroma that is not derived from the iris vessels. This reservoir is prevented from diffusing posteriorly by tight junctions in the posterior iris epithelium. The one-way valve created by the pupil resting on the anterior lens capsule, combined with the continuous, forward flow of AH through the pupil, prevents protein reflux into the posterior chamber. Importantly, in the new paradigm, secretion of AH and the entry of plasma proteins into AH, are semi-independent events. This opens the possibility that AH protein levels could increase in the absence of breakdown of the BAB. Clinical consequences of this new paradigm of the BAB are discussed.
1. Introduction
The notion that certain tissues of the body do not reach equilibrium with all constituents of plasma originated in the late 1880’s (Ehrlich, 1885) and more definitively in the early 1900’s when the vital dye Trypan Blue was injected intravenously and found to permeate virtually all of the body tissues except the brain (Goldmann, 1913). With time, as additional tissues were examined in greater detail, others were found to exhibit significant restrictions in the extent to which plasma constituents were permitted to reach equilibrium with the extracellular environment of the tissues being served by those vessels. Among these was the eye.
Traditionally, two barriers have been described in the eye – a blood-aqueous barrier and a blood-retinal barrier. The blood-retinal barrier is commonly presented as having an “inner” and outer” component. The “inner” component is provided by the interendothelial tight junctions of the intraretinal vasculature and the “outer” component of the barrier is provided by the tight junctions of the retinal pigmented epithelium. Several high caliber reviews of the blood-retinal barrier are available elsewhere and thus this discussion will be limited to the blood-aqueous barrier (BAB) and an integral concept of how the two barriers are inter-related (Cuhna-Vaz, 2004, Cuhna-Vaz, Bernardes and Lobo, 2011).
2. Early Studies of the Blood-Aqueous Barrier
The earliest studies of the BAB were biochemical comparisons between the ionic and molecular constituents of the aqueous humor (AH) and plasma (Davson, H, 1953, 1956, 1969). One of the most significant differences found between plasma and aliquots of aqueous humor obtained by paracentesis from the anterior chamber, was that the plasma-derived protein concentration in AH was about 1% of that found in plasma (Tripathi, Millard and Tripathi, 1989). The answers to the questions of how and where that small amount of protein entered the aqueous humor have changed over the years, but answering those two questions has been central to the assumptions used in models developed to interpret physiological and clinical data in both the normal and abnormal eye.
As electron microscopy came to the fore in the late 1960’s and early 1970’s, those interested in the question of where and how the protein entered the AH focused on the structure of the blood vessels within the anterior segment of the eye, looking at thin-sectioned material. Varying planes of section, and interspecies differences left open the question of whether the interendothelial junctions of iris blood vessels were continuous or discontinuous (Shakib and Cuhna-Vaz, 1966, Vegge and Ringvold, 1969; Saari, 1975; Hogan, Alvarado and Weddell, 1971) (Figure #1, inset).
Figure #1.
TOP- Transmission electron micrograph. Black, HRP reaction product fills the ciliary body stroma (*). The tracer has diffused between adjacent pigmented ciliary epithelial cells (PCE) and into the cleft between the apical surfaces of the adjoined pigmented and non-pigmented ciliary epithelium (NPCE). Attempting to diffuse between adjacent non-pigmented ciliary epithelial cells, the HRP is stopped abruptly by the presence of a tight junction joining the apico-lateral surfaces of these cells. (curved arrows) (X20,000). BOTTOM – Freeze-fracture electron micrograph shows branching and anastomosing strands of continuous tight junction joining the apico-lateral surfaces of non-pigmented ciliary epithelial cells.(X57,500). INSET – Transmission electron micrograph shows fusion points of tight junction joining iris vascular endothelial cells. L – lumen. (X130,000). With permission from Morrison, J.C., VanBuskirk, E.M., Freddo, T., 1989. Anatomy, microcirculation and ultrastructure of the ciliary body. In: Ritch, R., Shields, M.B. and Krupin, T., eds. The Glaucomas. C.V. Mosby Co., St. Louis.
A major breakthrough came with development of horseradish peroxidase as a vascular tracer that could be visualized at the light and electron microscopic level (Reese and Karnovsky, 1967). With this tracer, which behaved and distributed much like serum albumin, it became possible to probe the permeability of vascular systems to macromolecules under normal and abnormal conditions. Notwithstanding the early EM studies that left open the possibility that the iris vessels and ciliary epithelium were “leaky”, a host of studies, completed in several species, showed that iris vessels and the non-pigmented ciliary epithelium were impermeable to horseradish peroxidase. (Vegge, 1971a,b, Smith, 1971, Smith and Rudt, 1973; Uusitalo, Palkama and Stjernschantz, 1973; Raviola, 1974, Pederson, 1974; Freddo and Raviola, 1982a) (Figure 1 – top). The outcomes of these studies established that the anatomical equivalents of the BAB, as it was originally described, were the tight junctions of the iris vasculature and the non-pigmented ciliary epithelium (Raviola, 1977, Bill, 1986). Still the notion persisted that junctions of the iris vessels and the ciliary epithelium were leaky. The issue was resolved with the advent of freeze-fracture electron microscopy. Although regional variations in tight junction complexity were found, the literature was consistent in stating that both the non-pigmented ciliary epithelial cells and the endothelial cells of the iris vasculature were joined by continuous tight junctions (Raviola, 1974, Freddo and Raviola, 1982b, Noske, Stamm and Hirsch, 1994, Hirsch, Montcourrier, et al 1995)(Figure #1 – bottom). These direct views of tight junction structure were consistent with the vascular tracer studies showing that neither the ciliary epithelium, nor the iris vascular endothelium leaks plasma –proteins under normal conditions.
As more and more became known of the structural components of the BAB, it became increasingly difficult to reconcile these results with the notion that plasma derived proteins were consistently found in aliquots of AH taken from the anterior chamber of the eye by paracentesis, or when measured by noninvasive means. With the inability to directly examine kinetics in the posterior chamber of the eye, the methods available to probe the kinetics of solute distribution had to rely upon assumptions to underpin computational models of the barrier to predict unseen events in the posterior chamber. The models of kinetics in use until the mid-1990s made the assumption that the AH, at least regarding its plasma protein content, was the same in the posterior and anterior chambers.
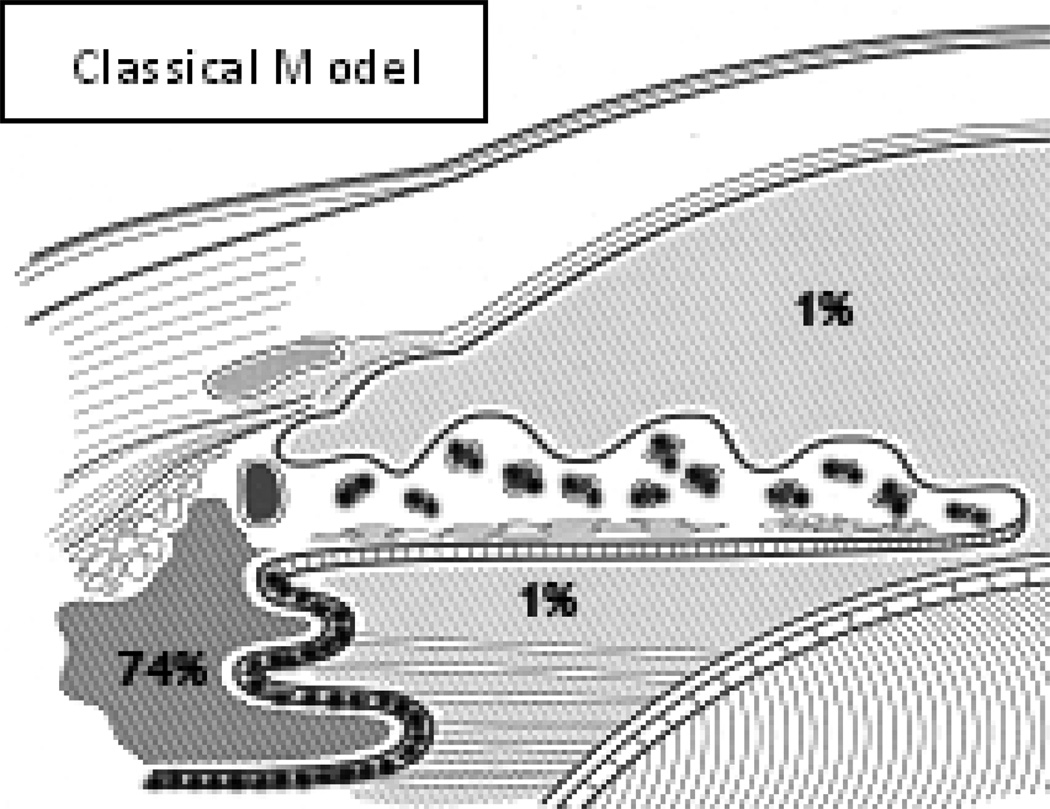
3. The Classical Model of the BAB
The Classical Model of the BAB that was in use until the mid to late-1990s included the tight junctions of the iris vascular endothelium as one critical element (FIGURE #2). This was presumed to be critical because the anterior surface of the iris is not covered by an epithelium and therefore aqueous humor freely permeates the iris stroma. The tight junctions of the iris vasculature kept plasma proteins from leaking into the iris stroma. The apico-lateral tight junctions of the non-pigmented ciliary epithelium were the other major element identified. These junctions prevented the plasma protein content of the ciliary body stroma from entering the posterior chamber. The source of this protein was the fenestrated capillaries of the ciliary body stroma and some estimates put the plasma protein content of the ciliary body stroma as high as 74% of that found in plasma itself (Bill, 1968). The AH in the anterior and posterior chambers was assumed to have the same plasma protein content, even if the source and point of entry of that protein remained uncertain.
Figure #2.
In the classical model of the blood-aqueous barrier, key tight junctions (•••••) are present joining iris vascular endothelial cells and non-pigmented ciliary epithelial cells. The concentration of plasma protein in the ciliary body stroma is assumed to be approximately 74% and that within the aqueous humor, at all points, is assumed to be 1%.
Unable to reconcile the source and point of entry of the unexplained plasma protein in normal AH, investigators were forced to continue using the assumption that anterior and posterior chamber aqueous protein levels were the same. And yet this conclusion could not be made consistent with the available models of aqueous production, which maintained that the tight junctions of the non-pigmented ciliary epithelium excluded all plasma-derived protein from the AH. Indeed, the exclusion of protein by ciliary epithelial tight junctions was central to the creation of the standing osmotic gradient presumed to be a major driving force in aqueous secretion (Cole, 1977).
Possibly one of the most important constraints of the Classical Model was that plasma-derived protein was assumed to be a part of AH at all points in the anterior and posterior chamber and in the same concentration throughout. Thus, when using this model to interpret kinetic data, the only conclusion one could reach when AH protein levels were found to be elevated was that BAB permeability had increased. Using this model, elevations in AH protein levels could not occur without alteration of the integrity of the tight junctions in the iris or ciliary body such as those shown to occur in anterior uveitis (Freddo, 1987, Freddo and Sacks-Wilner, 1989).
4. The Original Two-Compartment Model
When presented as a computational model, a two compartment model was used in which solutes moved directly from the blood into the AH, governed by a single barrier transfer coefficient (Goldmann, 1951, Nagataki, 1975) (FIGURE #3). Diffusional losses to the corneal stroma were also considered. In efforts to collect non-invasive data on BAB kinetics using such methods as fluorophotometry, several investigators began to notice that the two compartment model failed to adequately predict the time course of fluorescein entry from the blood into the anterior chamber (Walker, Brubaker and Nagataki, 1982). The actual process ramped up less slowly than predicted but then accelerated in a way that was inconsistent with the predictions of a two compartment model. Wilson and Barany (1983) noted an unexpected time-delay in the transfer of intravascular iodohippurate from the iris vessels into the AH of the anterior chamber. Their conclusion was that the time required for the iodohippurate to leak from the iris vessels, accumulate within and traverse the iris stroma to its anterior border layer, could account for the time-delay in the process of transfer between the two compartments. They modified the two-compartment model, adding a time-delay into the process of the transfer between the blood and the AH.
Figure #3.
Traditional two-compartment model of the anterior segment in which tracers diffuse directly from the plasma into the anterior chamber, with secondary diffusional losses to the corneal stroma. Va- Volume of anterior chamber, Vc-Volume of cornea, kd-blood-aqueous barrier transfer coefficient, kfoutflow transfer coefficient, kc.ca – aqueous to cornea transfer coefficient. With permission from Academic Press. McLaren, J., Ziai, N. and Brubaker, R.F., 1993. A simple three-compartment model of anterior segment kinetics. Exp. Eye Res. 56: 355–66.
A time delay was also found in the entry of higher molecular weight solutes such as proteins. In a direct attempt to determine the source and pathway taken by the small amount of plasma protein present in normal AH, Freddo et al (1990) used fluoresceinated horseradish peroxidase (F-HRP) as an intravenous tracer. They were able to measure the temporal kinetics of its entry into the anterior chamber by fluorophotometry and then sacrifice the animals to microscopically localize the source(s) contributing to the F-HRP they had measured in the AH of the anterior chamber over time. They noted an 8–10 min delay between the time of tracer injection and its detection in the AH of the anterior chamber. Extensive computational modeling by research team members Kamm and Barsotti suggested that the temporal kinetics of the fluorophotometric data were inconsistent with the tracer having come into the anterior chamber through the pupil, as an integral part aqueous flow. Similar results were found in both rabbit and monkey eyes. (Freddo, et al, 1990, Barsotti, et al, 1992)
The earlier of these two studies included a series of experiments in which HRP was intravenously injected into rabbits (Freddo, et al, 1990). Animals were sacrificed at 1, 3, 8 and 10 min after HRP injection, attempting to follow the HRP from its source to wherever it entered the anterior chamber during the 10 minute time delay before it could be measured by fluorophotometry. What these studies showed (FIGURE #4) was that the source of this protein was the fenestrated capillaries of the ciliary body stroma. From this source, each later time point (1,3 and 8 min time points shown) showed movement of the HRP through the ciliary body stroma and into the iris stroma, ultimately reaching the surface of the iris at the 8 minute time point. This was consistent with the time delay to initial detection of tracer during fluorophotometry. Additional computational modeling demonstrated that this pathway best described the kinetics of protein entry.
Figure #4.
(a) One minute after HRP injection, the tracer fills the vessels of the iris, ciliary body and conjunctiva. Early leakage is present in the latter two locations. C, cornea; AC, anterior chamber; PC, posterior chamber. (b) Three min after HRP injection, filling of the ciliary body stroma is more evident and migration of HRP can be followed toward the iris. (c) Eight min after HRP injection, the ciliary body stroma is filled and HRP has reached the anterior chamber at the root of the iris (arrow). With permission from The Assoc. for Research in Vision and Ophthalmology. Freddo, T.F., Bartels, S.P., Barsotti, M.F. and Kamm, R.D., 1990. The source of proteins in the aqueous humor of the normal rabbit. Invest. Ophthalmol. Vis Sci. 31: 125–137.
5. The Three-Compartment Model
Following up on these studies, McLaren, Ziai and Brubaker (1993) completed a series of studies in both rabbits and in normal human subjects exploring whether replacing the “time-delay” concept of Wilson and Barany (1983) with a defined third compartment would provide a better match with the actual kinetics of fluorescein entry from the blood into the AH of the anterior chamber. When the actual data were plotted along the predicted curves for both the two compartment and three compartment models, the agreement with the three compartment model was striking (FIGURE #5). The new third compartment, between the blood and the aqueous, was labeled the “iris”, signifying that solutes leaked first into the stroma of the ciliary body and diffused into the iris stroma (FIGURE #6). From there, the solutes partitioned into the AH of the anterior chamber across the glycocalyx that is known to be present at the iris surface. For each step (blood to iris and iris to aqueous) a separate transfer coefficient was determined.
Figure #5.
Fluorescein concentration in a rabbit anterior chamber after intravenous fluorescein injection. The three-compartment model (solid line) matched the measured concentrations (•) considerably better than the two-compartment model. With permission from Academic Press. McLaren, J., Ziai, N. and Brubaker, R.F., 1993. A simple three-compartment model of anterior segment kinetics. Exp. Eye Res. 56: 355–66.
Figure #6.
Three-compartment model of the anterior segment. Tracers must pass through the iris enroute from the plasma to the anterior chamber. Va- Volume of anterior chamber, Vc-Volume of cornea, Vi – Volume of the iris, ki.ip - iris to plasma transfer coefficient referred to the volume of the iris, ki.ia – iris to anterior chamber transfer coefficient referred to the volume of the iris, kf - outflow transfer coefficient, kc.ca – aqueous to cornea transfer coefficient. With permission from Academic Press. McLaren, J., Ziai, N. and Brubaker, R.F., 1993. A simple three-compartment model of anterior segment kinetics. Exp. Eye Res. 56: 355–66.
An additional and somewhat surprising conclusion from all of these studies was that the iris stroma contains significant amounts of plasma-derived proteins that have not come from the iris blood vessels. The presence of this soluble protein was confirmed by Kuchle, Vinores and Green (1995) who immunohistochemically localized albumin within the iris stroma of 36 normal human eyes. Indeed, Raviola and Butler (1985) demonstrated that not only did iris blood vessels not leak plasma proteins, they were capable of resorbing plasma proteins – but only those that were negatively charged. The same phenomenon has been shown following perfusion of the anterior chamber with fluorescein (Green et al, 1977; Sherman, Green and Laties, 1978) and horseradish peroxidase (Raviola and Butler, 1983).
If the models of aqueous humor production and the vascular tracer studies mentioned earlier are accurate, there is no non-specific diffusion of plasma-derived proteins into the AH of the posterior chamber. But if it is the intent to maintain the posterior chamber free of plasma-derived proteins, how is the depot of plasma proteins in the iris stroma prevented from diffusing into the posterior chamber? In the Classical model of the BAB, in which the protein content of the anterior and posterior chambers was assumed to be the same, there would be no need to separate these two environments. But, in fact, it has been shown that the posterior pigmented epithelium of the iris has well-developed tight junctions capable of preventing proteins in the iris stroma from diffusing into the posterior chamber, just like the non-pigmented ciliary epithelium with which this layer is continuous (Freddo, 1984). An additional component of the system, which separates the environments of the anterior and posterior chamber, is the continuous flow of AH forward through the pupil. This forward flow, combined with the one-way valve created by the apposition of the pupil and the lens capsule, ensures that plasma-proteins admitted into the AH of the anterior chamber do not backwash into the posterior chamber. But is this the case in-vivo?
6. In-Vivo Demonstration of the Anterior Diffusional Pathway
The computational modeling, fluorophotometric and morphological tracer studies all pointed toward the source of the small amount of plasma-derived protein present in the AH by-passing the posterior chamber and entering the AH of the anterior chamber from the iris root. But the actual events occurring in the posterior chamber of the eye had to be inferred from the kinetics of tracer entry into the anterior chamber using computational modeling. Kolodny, et al, (1996) were able to provide the first in-vivo studies of barrier kinetics in the posterior chamber using contrast-enhanced magnetic resonance imaging. In these studies, rabbits received an intravenous injection of the contrast agent gadolinium DTPA and its arrival and diffusion into the anterior segment of the eye was documented over time. The imaging was able to clearly resolve the posterior chamber and signal intensity measurements taken from critical locations in the anterior segment were possible. What they found was that the ciliary body demonstrated rapid enhancement, which gradually decreased over the 90 minute time period of the studies. After a time delay of 10–12 minutes, a gradual increase in signal intensity was seen in the anterior chamber but no significant change in signal intensity was observed in the posterior chamber throughout the duration of the study.
While these data supported the existence of an anterior protein pathway in-vivo, a lingering concern remained regarding the fact that the blood-aqueous barrier of the rabbit is notoriously fragile (Bito, 1984). Partially for this reason, a single owl monkey was also examined in the study, and this study gave the same result. (FIGURE #7).
Figure #7.
Mean percent signal enhancement as a function of time after administration of gadolinium contrast material for the ciliary body and the anterior and posterior chambers. With permission from The Assoc. for Research in Vision and Ophthalmology. Kolodny, N., Freddo, T.F., Lawrence, B., Suarez, C., and Bartels, S.P., 1996. Contrast-enhanced MRI confirmation of an anterior protein pathway in the normal rabbit eye. Invest. Ophthalmol. Vis Sci. 37: 1602–1607.
With the ability to document that solutes were by-passing the posterior chamber using MRI, it became possible to directly explore whether the anterior protein pathway also existed in the normal human eye invivo. Bert et al, (2006) using a flexible circular surface coil and 1.5 Tesla clinical MRI system, infused seven normal male and female subjects, ranging in age from 27–59 with the standard clinical contrast agent, gadopentetate dimeglumine (MW 938, 469 mg/mL, 0.4 mL/kg body weight). As seen previously in the rabbit and monkey, they documented rapid enhancement of the ciliary body, followed by a more gradual enhancement of the anterior chamber in all cases. No enhancement of the posterior chamber was seen (Figure #8). Enhancement in the anterior chamber began at the anterior chamber angle. Convection then gradually stirred the tracer to the center of the anterior chamber.
Figure #8.
A: Pre-contrast image shows details of tissues in the anterior segment of the eye, including iris, ciliary body and both the anterior and posterior chambers. B: Within two minutes of contrast infusion, there is clear enhancement of the ciliary body and the choroid, but the anterior chamber, posterior chamber and vitreous body show no enhancement. C: After 90 minutes, the enhancement in the ciliary body and choroid has begun to diminish. There is clear enhancement in the anterior chamber but the posterior chamber and the vitreous remain unchanged from Figures A and B.
The importance of the iris epithelium in this new view of the BAB was further demonstrated by this same research team in unpublished studies of an individual with pigment dispersion syndrome. Individuals with pigment dispersion syndrome commonly develop radial, spoke-like transillumination defects in the iris epithelium due to posterior bowing of the iris and constant rubbing on the zonules. A 56 y/o Caucasian male known to have pigment dispersion syndrome, without glaucoma, was examined. Both eyes exhibited prominent transillumination defects. He also had prominent Krukenberg spindles (vertical lines of corneal endothelial pigment) consistent with the diagnosis. Using the previously published MRI protocol, baseline images were obtained. The subject then received a bolus intravenous injection of 0.4 mg/kg Gadolinium dimeglumine i.v. (Magnevist, Berlex Imaging, Wayne, NJ). Post contrast, T1-weighted, spin-echo MR images of one eye were obtained every 15 min for 90 minutes. The images below show the eye of this individual before contrast (A) and 49 minutes after injection of contrast (B). As can be seen comparing the two images, in this subject with mid-peripheral transillumination defects of the nasal and inferior iris, gadolinium enhanced the AC as expected, but by 49 min it had entered the PC through the transillumination defects of the posterior iris epithelial barrier on the nasal side. These unpublished findings suggest that the iris epithelium does play an important role in preventing the protein that is shunted from the ciliary body into the iris stroma and AC, from reaching the posterior chamber and the tissues behind the iris - consistent with our new paradigm. Clearly, studies on additional individuals are required (FIGURE #9).
Figure #9.
T1- weighted MRI images (TR/TE=1400/15ms, acquisition time 4 min 45 sec) of 56 year-old male with pigment dispersion syndrome and no glaucoma. A: baseline pre-constrast image clearly shows the iris, ciliary body and anterior and posterior chambers. B: Image shows same eye 49 minutes after intravenous infusion of 0.4 mg/kg Gadolinium dimeglumine i.v (Magnevist, Berlex Imaging, Wayne, NJ). In this subject with mid-peripheral transillumination defects of the nasal and inferior iris, gadolinium has enhanced the AC as expected, but has secondarily entered the PC on the nasal side through the iris epithelial defects (arrow). T= temporal.
7. The New Model of the Blood-Aqueous Barrier
The New Model of the BAB differs in several important respects from the Classical Model. Most of these differences are depicted in FIGURE #10.
Figure #10.
A: In the Classical model, the tight junctions of the non-pigmented ciliary epithelium and of the iris vascular endothelium are the key elements. The iris stroma is presumed to be free of plasma proteins and the concentration of plasma proteins in the aqueous is uniform throughout the anterior and posterior chambers. Elevation of aqueous humor plasma protein concentrations can be explained only by an increase in blood-aqueous barrier permeability. B: In the new paradigm, the small amount of plasmaderived protein present in aqueous humor diffuses from the ciliary body stroma, to the root of the iris, accumulates in the iris stroma and is then released into the aqueous humor of the anterior chamber (arrows). The posterior chamber is essentially plasma-protein-free. Some of the protein delivered to the iris root immediately enters the trabecular outflow pathways (arrows). The tight junctions of the nonpigmented ciliary epithelium and of the iris vasculature endothelium remain key elements (•••••). An additional key element becomes the tight junctions of the posterior iris epithelium (•••••), which prevent the protein in the iris stroma from diffusing posteriorly, when combined with the one way valve created by the pupil resting on the anterior lens capsule, and the continuous forward flow of aqueous humor through the pupil. The entry of aqueous humor and the plasma proteins contained within it are semi-independent variables. As such, plasma protein concentrations in the aqueous humor can increase in the absence of an increase in blood-aqueous barrier permeability.
Like the Classical model, the tight junctions of the non-pigmented ciliary epithelium and of the iris vascular endothelium are key elements. Under normal conditions they prevent plasma-derived proteins from entering the aqueous humor of the anterior and posterior chambers. One of the things that has changed is that we now know the source and the pathway taken by the small amount of plasma-derived protein present in aqueous humor. This protein diffuses from the ciliary body stroma, to the root of the iris, accumulates in the iris stroma and is then released into the aqueous humor of the anterior chamber. This process is most conveniently described by the three-compartment model – the blood, the iris/ciliary body stromal compartment and the AH of the anterior chamber. (Freddo, et al, 1990, McLaren, Ziai and Brubaker, 1993) The entry of this protein into the anterior chamber angle, by-passing the posterior chamber, now makes the model consistent with existing models of AH production, suggesting that AH as it is secreted, is a plasma protein-free fluid, except for those proteins that reach higher concentrations in aqueous than in plasma due to active transport (e.g. transferrin).
The new model is also consistent with the known presence of soluble plasma proteins in the iris stroma, plasma-derived proteins which have not come from the iris vasculature. While in the iris stroma, evidence suggests that negatively charged proteins may be reabsorbed by the iris vessels. All of this protein is prevented from secondarily entering the posterior chamber by tight junctions joining the posterior pigmented epithelial cells of the iris and by the one-way valve created by the apposition of the pupillary margin and the anterior lens capsule, combined with the continuous, forward flow of aqueous humor through the pupil.
Possibly the greatest departure from the Classical model is that the secretion of AH and the entry of non-transported, plasma-derived proteins into the AH are semi-independent events. This important change opens the possibility that changes in the concentration of AH protein could occur that would not require breakdown of the BAB.
Additional evidence pointing to the semi-independence of aqueous secretion and the entry of plasma-proteins into aqueous comes from studies demonstrating daily cyclical changes in aqueous humor protein levels. These studies have consistently shown such a variation in both diurnal and nocturnal species. (Anjou, 1961a; Anjou, 1961b; Oshika, Araie and Masuda, 1988; McLaren, et al, 1990; Zhou and Liu, 2006). Whether the species is diurnal or nocturnal, the higher aqueous humor protein concentration appears when aqueous secretion is low, usually during sleep. Indeed, one set of studies in rabbits gave more direct evidence, showing that a high aqueous humor protein concentration is related to a slow aqueous humor flow rate or low IOP (Takahashi, et al, 1995). Using the previous model of the blood aqueous barrier these outcomes are inexplicable, except by suggesting that there was a diurnal variability in blood-aqueous barrier permeability (Oshika, Sakuri and Araie, 1993). In the revised model, however, these outcomes are logical, predictable and do not require a change in BAB permeability. As aqueous production was reduced during sleep, the entry of protein continued via the pathway described and the protein concentration rose through a simple concentration effect.
8. The Relationship Between the New Model of the Blood-Aqueous Barrier and the Blood-Retinal Barrier
Taken out of isolation and viewed in the broader context of the blood-ocular barriers generally, the new model of the BAB changes the relationship between the blood-aqueous and blood-retinal barriers. In the new model, the BAB is less a barrier between the blood and the aqueous than it is a separation between the more restrictive environment behind the iris and the more permissive environment required by the metabolic needs of the avascular tissues facing the anterior chamber. The tight junctions of the ciliary epithelium play a role in the secretion of aqueous humor as a plasma-protein free fluid and in doing so, help to maintain the pristine environment created by the anatomical components of the blood-retinal barrier. The tight junctions of the posterior iris epithelium then carry the blood-retinal, blood-vitreous barrier forward, all the way to the pupillary margin, while the one-way valve of iris apposition to the lens, combined with the continuous forward flow of aqueous humor, ensure against backwash. Intriguingly, this leaves the metabolically active epithelium on the anterior surface of the lens exposed to plasma-proteins, growth factors and other nutrients available in the more permissive environment of the anterior chamber. But this new view of the barrier also sequesters the equatorial lens epithelium, the portion that undergoes cell division and lens fiber differentiation, in the more pristine environment behind the iris plane.
9. Potential Ramifications of this New Model
a. Possible Ramifications on Aqueous Outflow
Clearly, the anterior diffusional pathway for plasma-derived proteins and other macromolecules enters the anterior chamber at the anterior chamber angle. At this location, it would be reasonable to predict that some fraction of the solutes delivered to the surfaces of the iris and the ciliary body band is immediately carried into the aqueous outflow pathways through the trabecular meshwork (FIGURE #11). Computational modeling suggested that the amount of plasma-derived protein reaching the outflow pathways could be as high as 10% of that found in plasma, but the actual amounts remain unknown. (Freddo, et al, 1990, Sit, et al, 1997) Following up on the potential significance of this projection,Johnson et al. (1993) demonstrated that they could significantly reduce the rate of outflow pathway washout in the enucleated bovine eye by supplementing the perfusate with 10–15% serum. Similar observations were made in the living primate eye as well, using 5% autologous serum (Kee, et al. 1996). The wash-out effect is a phenomenon encountered in the perfusion of enucleated eyes in which the resistance to outflow decreases with the volume of fluid perfused. (Bárány, 1964) The assumption has been that this phenomenon resulted from something “washing-out” with perfusion time/volume and hence the name. Johnson, et al. (1993) hypothesized that if the nonspecific addition of protein to aqueous, just as it entered the trabecular meshwork, was responsible for sustaining outflow resistance, then depletion of this depot during perfusions with protein-free perfusion fluids, in eyes without a continuing blood supply, could account for the progressive decrease in outflow resistance seen in these perfusions. In this way, depletion of this protein depot might account for “washout.” (Gong and Freddo, 2009). In the studies of Johnson et al, (1993) a statistically significant correlation was found between aqueous outflow resistance and soluble protein concentration. Additional studies determined that these effects on outflow were not due to either albumin or c-globulin alone (Sit, et al 1997). When these outflow data were modeled, the results suggested that the correlation between total soluble protein concentration and outflow resistance could be due to the intrinsic outflow resistance actually setting the flow rate. This, in turn would determine the protein concentration of the effluent being measured. When all of these data were taken together, the conclusion was that the reduction in washout was not due to a simple mass-effect of the added protein but was more likely due to interactions with particular, unidentified proteins instead.
Figure #11.
Diagram of the ciliary body, iris and anterior chamber angle demonstrates pathway taken by plasma proteins entering the aqueous of the normal eye (large arrow). Additional protein, beyond that measurable in the circulating aqueous humor, is likely shunted directly into the trabecular outflow pathway (small arrow). With permission from The Mosby Publishers. Morrison, J and Freddo, T.F. Anatomy, microcirculation and ultrastructure of the ciliary body. In: The Glaucomas, 2nd ed, Vol. I, Ritch, R., Shields, M.B. and Krupin, T., 1996. Cptr 6, pp. 125–138.
Following up on the initial report of Johnson, et al (1993), other investigators began to explore whether smaller plasma-derived proteins (termed “fines”) might contribute to normal aqueous outflow resistance (Doss, Ward and Koretz, 1998; Russell, Koretz and Epstein, 1993). While this work provided general support for the possibility that certain proteins could influence outflow resistance, additional work on the role of proteins being added to the aqueous humor just prior to the outflow pathway is warranted.
Most recently, Fautsch, et al (2005) explored whether addition of aqueous humor as a supplement to standard cell culture media altered trabecular meshwork cell behavior and protein expression profiles when compared to cells grown in standard culture conditions supplemented with 10% fetal bovine serum. Of interest, they found that cells grown in media supplemented with aqueous humor (including its endogenously secreted and plasma-derived proteins) showed decreased cell proliferation, and altered cell morphology. Cells grown in aqueous humor supplemented media also demonstrated alteration of their intracellular protein expression. Specifically, myocilin expression was increased and TIMP-1 expression was decreased. None of these changes were found when the aqueous humor was heat-denatured prior to use, suggesting that it was the proteins in aqueous that were directing the changes that were found. Given that most of the growth factors found in aqueous humor are plasma-derived, it is clear that the most relevant data from cells in culture is likely to be obtained from cultures supplemented with aqueous humor, once confluency has been reached in-vitro using fetal bovine serum.
b. Do Oxygen and Plasma-Proteins Follow the Same Pathway?
It has been shown that vitrectomy increases the risk of developing open-angle glaucoma. This risk is greatly enhanced if the patient has also had cataract surgery (Chang, 2006, Luk, et al., 2009). There has been speculation that once the oxygen demand of the lens has been removed after surgery, additional oxygen remains available in the AH at levels that are toxic to the avascular trabecular meshwork (Chang, 2006).
Seeking to explore this theory, Siegfried, et al. (2010) sought to measure oxygen levels in the eyes of patients who were undergoing cataract removal subsequent to a vitrectomy. In these studies, they found that vitrectomy alone increased pO2 in the posterior chamber, with an additional small increase in the pO2 in the anterior chamber angle. A similar increase was not found in the central anterior chamber near the lens. But after both vitrectomy and subsequent cataract surgery, pO2 in the posterior chamber increased even more, but the most dramatic change was the pO2 in the anterior chamber angle, which nearly doubled.
In previous studies of oxygen distribution within the rabbit eye, these same authors had documented that decreasing oxygen levels in the blood, decreased pO2 in the anterior chamber angle, but not near the central cornea (Shui, et al., 2006). This led them to postulate that O2 levels in the angle were more closely linked to oxygen levels in the blood. Their intriguing conclusion from these studies was that a significant amount of the oxygen reaching the anterior chamber angle follows the same pathway as that taken by plasma derived proteins (FIGURE #12). Knowing that vitrectomy and cataract surgery increase oxygen delivery to the outflow pathway, what mechanisms might link this finding to the increased risk of developing open-angle glaucoma?
Figure #12.
Oxygen distribution in the nonsurgical eye and the proposed effect of vitrectomy and/or cataract surgery on oxygen delivery to the anterior chamber angle. (A) In the nonsurgical eye, oxygen enters from the retinal vasculature through the vitreous, across the ciliary epithelium from the ciliary body vasculature, and into the anterior chamber across the cornea. Oxygen is consumed by the lens and the ciliary epithelium. A small amount of oxygen enters the anterior chamber angle by diffusing across the ciliary body and iris stroma (curved red arrow). This is the same pathway as that of the plasma proteins (gold arrow). (B) After vitrectomy, more oxygen reaches the posterior chamber. This supplies more oxygen to the “aqueous surface” of the ciliary epithelium, reducing the amount of oxygen that the ciliary epithelium removes from the blood and slightly increasing the amount of oxygen available to enter the anterior chamber angle from the ciliary body stroma. (C) Cataract surgery reduces oxygen consumption by the lens, thereby increasing the pO2 anterior to the lens and in the posterior chamber. The increased oxygen on the aqueous surface of the ciliary epithelium reduces the amount of oxygen that the ciliary epithelium removes from the blood, thereby slightly increasing the amount of oxygen available to enter the anterior chamber angle from the ciliary body stroma. (D) After vitrectomy and cataract surgery, significantly more oxygen is available on the aqueous surface of the ciliary epithelium, resulting in the removal of significantly less oxygen from the blood. This process increases the amount of oxygen available to diffuse from the ciliary body stroma, across the iris stroma, and into the anterior chamber angle, exposing the outflow system to a large excess of oxygen and/or oxygen metabolites. With permission from The Assoc. for Research in Vision and Ophthalmology. Siegfried, C.J., Shui, Y-B., Holekamp, N.M., Bai, F. and Beebe, D.C., 2010. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Invest. Ophthalmol. Vis. Sci.51, 5731–5738.
There is a long history of investigations in the meshwork and in the lens, exploring oxygen toxicity as contributors to both cataract and glaucoma (Spector, et al., 1998, Li, et al., 2007, Shui Y-B, 2009, Babizhayev, 2012,). Chang (2006) has proposed that exposure of the trabecular meshwork to higher oxygen tensions in the AH could result in elevation of toxic oxygen metabolites, including hydrogen peroxide. Defining the biochemical link between the development of glaucoma and the documented increase in oxygen tension in the angle after vitrectomy and cataract removal, merits further study. Indeed development of a post-vitrectomy and post-lensectomy animal model could provide a useful means of exploring the consequence of increasing oxygen levels in the meshwork in vivo.
c. Does a Flow of Non-secreted Fluid Accompany Plasma-Proteins Towards the Anterior Chamber?
Studies involving in vitro perfused preparations of bovine eyes suggest that as much as 40% of aqueous humor formation is ouabain-insensitive (Shahidullah et al., 2003). If these studies accurately reflect the in-vivo situation, this percentage of aqueous production would be attributed to ultrafiltration, but the authors themselves argue that this may represent an overestimate. Attempting to identify alternative sources of flow that could reconcile these percentages, Candia has begun to explore whether a non-secreted fluid flow, along the pathway we have described for protein movement into the anterior chamber angle, could account for a portion of the fluid entering the anterior chamber (Candia and Alvarez, 2008).
Candia makes an a priori argument that the solute content of any fluid entering the anterior chamber via the anterior chamber angle would have a solute content more reflective of plasma than does secreted aqueous humor (Candia and Alvarez, 2008). One implication would be that such fluid would not contain the 25–50% higher levels of ascorbate that results from active transport by the ciliary epithelium during secretion of AH into the posterior chamber (Kinsey, 1953; To et al., 2002). Certainly the presence of a gradient in ascorbate concentration from the posterior chamber to the anterior chamber documented by Kinsey (1953) would be consistent with the notion of dilution of ascorbate enriched aqueous with fluid containing lower, plasma-like levels of ascorbate. The future challenge is, of course, to account for what percentage of the reduction in ascorbate levels from posterior to anterior chamber results from its depletion by reacting with toxic oxygen species to produce hydrogen peroxide. But Kinsey (1953) also found a similar gradient for bicarbonate ion in rabbits. Kinsey attributed this concentration difference to passage of bicarbonate ions into iris blood vessels, following their concentration gradient, but experimental support for this explanation is lacking. Candia (Candia and Alvarez, 2008) currently favors entry of fluid along the anterior diffusional pathway for protein to account for these changes. A fluid flow anteriorly from the ciliary body stroma to the anterior chamber would certainly be countered by the posterior flow of uveoscleral outflow, which is known to pass into the face of the ciliary body band (Bill, 1971). But fluid movements in the recess of the anterior chamber angle are undoubtedly complex as the scleral spur divides flow destined for Schlemm’s canal from the flow into the uveoscleral pathway. Further study of this subject is certainly warranted.
d. Ramifications Regarding the Presence of Clinical Flare
1. Aqueous Suppressants
Over the years, a number of clinical scenarios have been reported in which patients develop a flare response to certain medications, most especially medications that suppress aqueous formation such as timolol and miotics such as pilocarpine. Flare is the clinical term used to describe the scattering of light from a slit lamp caused by elevations in aqueous humor protein levels in the anterior chamber.
Not long after timolol was approved for topical use in the treatment of glaucoma, concerns arose as practitioners noted the development of clinically significant flare in these patients (Beardsley and Shields, 1983). At that time, using the model of the BAB available, the only conclusion that could be reached was that the medication caused a modest breakdown of the BAB. Given that glaucoma is a chronic disease and patients were likely to be on this medication for a lifetime, even a modest compromise in the integrity of the BAB over such a period of time loomed as a potentially problematic side-effect.
If one accepts the new model of the BAB, however, an alternate explanation for the flare associated with use of aqueous suppressants comes into view. Timolol maleate has been shown to reduce aqueous inflow by 50% (Yablonski et al., 1978, Schenker, et al., 1981, Yablonski, et al., 1987, Larsson, 2001) and its effect is primarily on the secretory apparatus of the ciliary epithelium, rather than on the ciliary body vasculature. In this scenario, it is entirely likely that the movement of plasma-derived proteins from the ciliary body vasculature is uninterrupted. If we assume that the protein pathway continues to deliver the same amount of protein to the iris root, while the amount of diluent being produced through aqueous secretion is cut in half, there will be an inevitable concentration effect. The result will be an increase in AH protein concentration without needing to propose an increase in barrier permeability properties to explain why the patient has flare in their anterior chamber. This possibility has been previously considered by Stur, et al (1983). Indeed, in follow-up studies, these same authors provided indirect support for this theory by comparing AH protein levels of patients undergoing cataract surgery who were or were not taking timolol (Stur et al 1986). A direct assessment of this postulate remains to be tested but its conclusions are consistent with the finding of diurnal variations in aqueous protein concentration that parallel the marked sleep-related reductions in aqueous secretion.
2. Miotics
It has been well documented that miotics give rise to flare (Wessley, 1900; Seidel, 1920; Stocker, 1947; Abraham, 1959; Mori et al., 1992; Freddo et al., 2006). The first of the miotics demonstrated to give rise to flare was the muscarinic agent pilocaprine (Wessley, 1900). So certain were early investigators that the flare produced by pilocarpine was pathological, one of them even referred to the phenomenon as miotic iridocyclitis (Abraham 1959). Much more recently, in an attempt to unravel the source of the flare produced by topical instillation of pilocarpine, Mori, et al (1992) completed combined fluorophotometric and cell-flare meter studies on rabbits using clinically relevant doses of topical pilocarpine. As advanced as these methods are, even in the hands of skilled investigators, these methods are unable to directly examine events in the posterior chamber of the eye in-vivo. As such, these events had to be inferred using a model of BAB kinetics based on the classical model of the BAB. The conclusion reached was that pilocarpine produced a dose-dependent breakdown of the BAB.
Using the MRI contrast methods described earlier, and with the ability to directly and non-invasively assess kinetics on the posterior chamber, Freddo, et al, (2006) reassessed the effects of topical pilocarpine on BAB permeability in the normal human eye. Subjects received a single drop of 3% pilocarpine in one eye. Sixty minutes after administration of the pilocarpine, (the time at which pilocarpine-related flare is reported to peak, Mori, et al.,1992), sequential MRI images were taken over a 90 minute period after administration of the intravenous contrast material. The sequential time studies showed that the ciliary body signal intensity increased rapidly after infusion of the contrast agent. After 10–20 minutes, an increasing contrast signal was seen in the anterior chamber, in both treated and untreated eyes. During this same period, however, the baseline contrast signal observed in the posterior chamber remained constant and never enhanced in either eye (FIGURE #13). These direct studies of the posterior chamber documented that pilocarpine-induced “flare” was not the result of ciliary epithelial barrier breakdown. Unfortunately, the MRI technique was not sensitive enough to conclusively show that pilocarpine did not increase the permeability of the iris vasculature.
Figure #13.
T1-weighted spin-echo MR images (TR/TE = 1400/15 msec-image, each with an acquisition time of 4 min, 45 sec. LEFT: Baseline image acquired 45 min after instillation of 3% pilocarpine (note miotic iris) but prior to administration of intravenous gadolinium. RIGHT: Image obtained 60 min after gadolinium infusion shows enhancement in the anterior chamber but not in the posterior chamber or vitreous body. Insert compares split images of the anterior segments from the two time points shown in the main figure. With permission from Elsevier. Freddo, T.F., Patz, S and Arshanskiy, Y., 2006. Pilocarpine’s effects on the blood-aqueous barrier of the human eye as assessed by high-resolution, contrast magnetic resonance imaging. Exp. Eye Res. 82, 458–464.
In very recently reported studies, Freddo et al (2012) sought to determine whether the flare produced by pilocarpine could be the result of an increase in iris vascular permeability. To address this question, rabbits received one drop of 3% pilocarpine and baseline measurements were made of pupil size and anterior chamber flare. An hour later (the time at which the pilocaprine-induced flare response has been reported to peak, Mori, et al 1992) these measurements were repeated. As expected, flare increased significantly and pupil size decreased significantly in each pilocarpine treated eye. Each animal then received an intravenous injection of horseradish peroxidase (HRP). When uveal tissues were examined, no leakage of HRP from either the ciliary epithelium or the iris vasculature was seen by light or electron microscopy in either experimental or untreated control eyes. These findings confirmed that pilocarpine did not breakdown the BAB at the level of the ciliary epithelium and extended the earlier MRI findings in humans to demonstrate that pilocarpine did not increase the permeability of the iris blood vessels.
But if pilocarpine does not produce flare by increasing BAB permeability then what is the source of the flare? One hypothesis to consider was that the thinning of the iris stroma that occurs with strong miosis could displace some of the reservoir of plasma proteins from the porous iris stroma into the anterior chamber. To address this question, Freddo, et al (2012), treated rabbits with topical 3% pilocarpine in one eye. Measurements of pupil size and flare were made before instillation of pilocarpine and an hour after instillation. Again, pupil size decreased significantly and flare increased significantly in all treated eyes.
If the source of the pilocarpine-induced flare was extrusion of the plasma protein reservoir from the iris stroma, then the stroma of the miotic iris should contain significantly less elutable protein than the untreated contralateral iris. The anatomy of the rabbit and primate iris are quite different. In the rabbit there are extensions of the ciliary processes (termed iridal processes) onto the posterior surface of the peripheral iris and these contain leaky fenestrated vessels that become a secondary source of plasma protein leakage into the iris. The monkey and human iris stromas have no fenestrated vessels. To minimize the influence of the iridial processes upon the outcome of the experiments, only the portion of the iris closer to the pupil than the iridial processes was sampled to analyze the total elutable protein of the iris.
Iris tissues from the pilocarpine-treated eyes were found to have significantly less albumin / mg. iris tissue than fellow control eyes, suggesting that at least some of the pilocarpine-induced flare resulted from miosis-induced extrusion of soluble protein from the iris stroma and not from breakdown of the BAB. Lending additional support to this conclusion, there was a correlation between the magnitude of the change in pupil size and elutable protein levels in the pilocarpine-treated eyes. Thus, the stronger the miosis, the more protein was extruded from the iris stroma.
A modest additional contribution may result from the fact that pilocarpine reduces AH formation rate (Nagataki and Brubaker, 1982). Taken in context of the new model of the BAB, if the protein pathway kept delivering plasma-derived proteins to the anterior chamber, while aqueous secretion was reduced, the AH protein concentration would increase due to a concentration effect, just as is expected to occur with timolol and other aqueous suppressants, or with diurnal changes in aqueous secretion. Yet another contribution, potentially significant, may arise from the fact that by contracting the ciliary muscle, pilocarpine blocks the passage of colloids from the anterior chamber into the suprachoroidal space and the posterior uvea (Bill and Walinder, 1966). Assuming diffusion occurs equally in all directions from the source, in the absence of pilocarpine one would expect that a portion of the protein normally leaked from the fenestrated capillaries of the ciliary body would move posteriorly, away from the anterior chamber. But, in the presence of pilocarpine, virtually all of this protein would be redirected forward, toward the anterior chamber.
10. What Are the Proteins that Enter the Anterior Chamber Via This Pathway and How Does This New Model of the BAB Alter the Ways In Which the Avascular Tissues of the Eye Interact With Posterior and Anterior Chamber Aqueous Humor?
As methods for protein analysis of small volumes of fluid have improved, it has become feasible to perform increasingly sophisticated protein analyses on aliquots of aqueous humor-all of which have been obtained from the anterior chamber. Progressing from 2-d gels (Tripathi, Millard and Tripathi, 1989) to mass spectrometry (Rohde, et al., 1998), to more complex proteomic analyses, it has become possible to identify a large number of proteins in AH. Some are produced by tissues within the eye and others enter from the plasma, either by diffusion or active transport. A host of important proteins are encoded for within the tissues of the anterior segment. Some of these are secreted and have known physiological effects. Indeed, an elegant and extensive set of cDNA libraries has been produced from the tissues of the ciliary body that have been critical to cloning and characterization of genes and gene products of these tissues (Coca-Prados, Escribano and Ortego 1999). Those gene products that are exported become especially important as non-plasma-derived proteins of posterior chamber AH.
Of equal importance are those proteins that diffuse into the AH of the anterior chamber from the plasma and would not be found in the posterior chamber AH under normal conditions. For many of the proteins that have been identified in AH it is not known whether they arise from the plasma or are produced locally within the eye, or both. But it is likely safe to assume that those proteins identified as being bound to albumin are most probably among the proteins entering the anterior but not the posterior chamber.
From the earliest studies of AH proteins, it has been known that the predominant protein in aliquots of anterior chamber AH, representing 50% of total protein, is albumin (Chowdhury et al, 2010). This large amount of albumin can mask the presence of other proteins of similar weight or in smaller concentrations. In an effort to overcome this challenge, Richardson, et al. (2009) pooled samples of anterior chamber AH from 12 patients undergoing cataract surgery and albumin-depleted a portion of the sample prior to analysis. They then subjected both the albumin-depleted and non-depleted fractions to multidimensional protein identification technology (MudPIT). Twelve proteins were identified with high confidence in the albumin-bound fraction and 50 proteins in the albumin-depleted fraction. Similarly, Chowdhury, et al. (2010) immunodepleted albumin, transferrin, antitrypsin, haploglobin, IgG and IgA from portions of their pooled samples of AH. These investigators identified over 676 proteins in human AH and estimated that 58% of them were also found in plasma.
To illustrate some of the known roles of the albumin-bound proteins in aqueous humor, Prostaglandin-H2 D-isomerase is believed to play an undefined role in regulation of intraocular pressure, possibly via uveoscleral outflow, and in maintenance of the the integrity of the blood-aqueous barrier (Gerashchenko, et al. 1998). Glutathione peroxidase has been studied for years exploring a role for it as an antioxidant to prevent toxic changes in both the lens and the trabecular meshwork (Huang et al, 1997).
What comes from this analysis is that in order for us to understand the functions of these proteins, as they relate to the various avascular tissues of the anterior segment, we need to know which proteins are present in the aqueous humor of the posterior chamber. Only then can we probe such questions as whether there is a consequence of allowing plasma-derived proteins in the anterior chamber to access to the posterior chamber. Much in the same way that confirmation of the source of plasma-derived proteins in AH had to await development of new methods that could visualize the posterior chamber, a fuller understanding of the biological implications of differences between anterior and posterior chamber AH will have to await a method to obtain clean, uncontaminated samples of posterior chamber AH.
11. Summary and Future Considerations
The new paradigm, if understood in its complete form, fundamentally changes the concept of the blood-aqueous barrier. Viewed in its full context, the BAB is less a barrier between blood and aqueous per se, than it is a complex system designed to separate the pristine environment behind the iris from the more permissive environment required to sustain the avascular tissues at, or anterior to, the iris plane including the cornea, trabecular meshwork and the metabolically-active anterior epithelium of the lens. The small amount of plasma-derived protein present in AH is added only in the anterior chamber and enters in close proximity to the aqueous outflow pathway. The question of whether a protein or proteins delivered to the meshwork via this pathway serves a role in sustaining or regulating normal outflow resistance, or prompts development of abnormal resistance that could raise intraocular pressure in glaucoma, remains a viable subject for further exploration.
The entry of plasma proteins via a pathway separate from that taken by secreted AH indicates that these processes are semi-independent. Applying this new reality to clinical situations it becomes clear that, contrary to long-held belief, not all anterior chamber flare is pathological. Plasma-protein concentration in the AH can increase transiently under certain circumstances, either through release of the reservoir within the iris stroma or through a concentration effect when aqueous production is suppressed. Not all clinically evident flare represents a breakdown of the tight junctions that form the anatomical basis of the barrier to protein diffusion.
The presence of tight junctions in the posterior iris epithelium, the one-way valve created by the pupil resting on the lens, and the forward flow of aqueous humor through the pupil all combine to ensure that the plasma-derived protein shunted into the anterior chamber is prevented from backwashing into the more pristine environment behind the iris. In this regard, it is worth noting that in virtually all situations where a discontinuity is introduced into the posterior iris epithelium, (transillumination defects in pigment dispersion, peripheral iridotomies, etc) cataract development appears to be accelerated (Lim, et al., 2005). From the case of pigment dispersion presented in this review, it is clear that such discontinuities permit the non-specific entry of anterior chamber plasma-proteins, including their associated growth factors, etc, into the posterior chamber.
There has been an ongoing debate regarding why laser iridotomies accelerate cataract development. Naturally, the assumption has been that the cause of this accelerated development was the laser energy itself, but total laser energy delivered seems not to correlate with cataract progression (Lim, et al., 2005). While laser energy may be the culprit for iridotomies that are inadvertently placed more centrally in the iris, where the iris and lens are in very close proximity, this new view of the BAB offers an alternative explanation that merits exploration.
An additional hypothesis arising from this new view of the BAB, is that there is likely a consequence of the anterior lens epithelium being exposed to a different aqueous humor than that normally encountered by the equatorial lens epithelium where cell division and lens fiber differentiation occur. Regional differences are known to exist between the anterior lens epithelium exposed to anterior chamber AH vs the equatorial epithelium, which is normally exposed to only the AH of the posterior chamber. As but one example, ADAMTS (disintegrin and metalloproteinase with thrombospondin-like motifs) are known to facilitate cell division, migration and differentiation in the lens. ADAMTS1 and 2 expression are most abundant in the equatorial lens epithelium and found to a much lesser extent in the anterior lens epithelium (Hodgkinson, et al, 2010). The question arises whether there is a negative consequence of exposing the dividing and differentiating equatorial lens epithelium to anterior chamber AH, with its added plama-proteins and their associated growth factors?
A final realization arising from this new view of the BAB is that the anterior chamber and the space behind the iris are intended to be functionally separate and unique. Exploration of the physiological and pathological consequences of allowing the more pristine environment of the posterior chamber to be exposed to the more permissive biological environment of the anterior chamber deserves further study as a mechanism contributing to conditions such as cataract, “after”-cataract and open angle glaucoma.
ARTICLE HIGHLIGHTS.
The source and route of plasma protein entry into aqueous humor is described.
The blood-aqueous barrier is not primarily a barrier between blood and aqueous.
The blood-aqueous barrier separates different environments in front of and behind the iris.
Not all clinically observable anterior chamber flare is pathological.
ACKNOWLEDGEMENTS
The work of the author cited in this article has been supported by NIH RO1 EY-04567, RO1 EY-13825 to TFF, by RO1 EY-09699 to Dr. Mark Johnson (MIT and Northwestern University), by The Whitaker Foundation, by National Glaucoma Research, a program of the American Health Assistance Foundation, by Fight for Sight in support of Nathan Neville, O.D., M.S. and The Massachusetts Lions Eye Research Fund, Inc. The long term loan of a Kowa Flare-Cell meter from Alcon Laboratories, Inc. and critical support of our Fluorotron Master fluorophotometer from Bruce Ishimoto of OcuMetrics, Inc. is gratefully acknowledged. Excellent technical assistance was provided by Rozanne Richman, M.S. This line of research would not have reached fruition without the expertise and insights of long-term collaborators Drs. Roger Kamm, Stephen Bartels, Nancy Kolodny, Haiyan Gong, Mark Johnson, Sam Patz and Robert Bert. The author would also like to thank Dr. Jay McLaren for his insightful suggestions and discussions over the past 20 years and Dr. Nicholas Delamere for his suggestions regarding the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest exists in the work of the author summarized in this review.
REFERENCES
- Abraham SV. Miotic iridocyclitis: Its role in the surgical treatment of glaucoma. Am. J. Ophthalmol. 1959;48:634–643. doi: 10.1016/0002-9394(59)90454-4. [DOI] [PubMed] [Google Scholar]
- Anjou CI. Physiological variations of the aqueous flare density in normal human eyes. Acta Ophthalmol. (Copenh.) 1961a;39:525–539. doi: 10.1111/j.1755-3768.1961.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Anjou CI. Influence of light on the 24-hour variation in aqueous flare density and intra-ocular pressure in normal rabbits' eyes. Acta Ophthalmol. (Copenh.) 1961b;39:852–873. doi: 10.1111/j.1755-3768.1961.tb07750.x. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: Challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundamental & Clin. Pharmacol. 2012;26:86–117. doi: 10.1111/j.1472-8206.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest. Ophthalmol. Vis. Sci. 1964;3:135–143. [PubMed] [Google Scholar]
- Barsotti MF, Bartels SP, Freddo TF, Kamm RD. The source of protein in the aqueous humor of the normal monkey eye. Invest. Ophthalmol. Vis. Sci. 1992;33:581–595. [PubMed] [Google Scholar]
- Beardsley TL, Shields MB. Effect of timolol on aqueous humor protein concentration in humans. Am. J. Ophthalmol. 1983;95:448–450. doi: 10.1016/0002-9394(83)90263-5. [DOI] [PubMed] [Google Scholar]
- Bert RJ, Caruthers SD, Jara H, Krejza J, Melhem ER, Kolodny NH, Patz S, Freddo TF. Demonstration of an anterior diffusional pathway for solutes in the normal human eye: High spatial resolution contrast-enhanced dynamic MR imaging. Invest. Ophthalmol. Vis. Sci. 2006a;47:5153–5162. doi: 10.1167/iovs.05-0372. [DOI] [PubMed] [Google Scholar]
- Bill A, Walinder P-E. The effects of pilocarpine on the dynamics of aqueous humor in a primate Macaca irus. Invest. Ophthalmol. Vis. Sci. 1966;5:170–175. [Google Scholar]
- Bill A. Capillary permeability to and extravascular dynamics of myoglobin, albumin and gammaglobulin in the uvea. Acta Physiol. Scand. 1968;73:204–219. doi: 10.1111/j.1748-1716.1968.tb04097.x. [DOI] [PubMed] [Google Scholar]
- Bill A. Uveoscleral drainage of aqueous humor in human eyes. Exp. Eye Res. 1971;12:275–278. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- Bill A. The blood-aqueous barrier. Trans. Ophthalmol. Soc. U.K. 1986;105:149–155. [PubMed] [Google Scholar]
- Bito LZ. Species differences in the responses of the eye to irritation and trauma: A hypothesis of divergence in ocular defense mechanisms, and the choice of experimental animals for eye research. Exp. Eye Res. 1984;39:807–829. doi: 10.1016/0014-4835(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Candia OA, Alvarez LJ. Fluid transport phenomena in ocular epithelia. Prog. in Retinal and Eye Res. 2008;27:197–212. doi: 10.1016/j.preteyeres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. LXII Edward Jackson Lecture: Open angle glaucoma after vitrectomy. Am. J. Ophthalmol. 2006;141:1033–1043. doi: 10.1016/j.ajo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Chowdhury UR, Madden BJ, Charlesworth MC, Fautsch MP. Proteome analysis of human aqueous humor. Invest. Ophthalmol. Vis. Sci. 2010;51:4921–4931. doi: 10.1167/iovs.10-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M, Escribano J, Ortego J. Differential gene expression in the human ciliary epithelium. Prog. In Ret. and Eye Res. 1999;18:403–429. doi: 10.1016/s1350-9462(98)00026-3. [DOI] [PubMed] [Google Scholar]
- Cole DF. Secretion of aqueous humor. Exp. Eye Res. (suppl) 1977;25:161–176. doi: 10.1016/s0014-4835(77)80015-8. [DOI] [PubMed] [Google Scholar]
- Cuhna-Vaz J. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004;78:715–721. doi: 10.1016/s0014-4835(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011;21(Suppl. 6):3–9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- Davson H. The penetration of large water-soluble molecules into the aqueous humour. J. Physiol (Lond.) 1953;122(suppl):10–11. [PubMed] [Google Scholar]
- Davson H. Physiology of the Ocular and Cerebrospinal Fluids. London: J. A. Churchill Ltd; 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H. The Eye. 2nd ed. vol. 1. New York and London: Vegetative physiology and biochemistry, Academic Press; 1969. The intraocular fluids; pp. 67–186. [Google Scholar]
- Doss EW, Ward KA, Koretz JF. Investigation of the “fines” hypothesis of primary open angle glaucoma; the possible role of alpha crystallin. Ophthalmic Res. 1998;30:142–156. doi: 10.1159/000055468. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Das Sauerstoff-Bedurfnis des Organismus. Berlin: Eine Farben-analytische studie; 1885. [Google Scholar]
- Fautsch MP, Howell KG, Vrabel AM, Charlesworth MC, Muddiman DC, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest. Ophthalmol. Vis. Sci. 2005;46:2848–2856. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- Freddo T, Raviola G. The homogeneous structure of blood vessels in the vascular tree of Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 1982a;22:279–291. [PubMed] [Google Scholar]
- Freddo T, Raviola G. Freeze-fracture analysis of the interendothelial junctions in blood vessels of the iris in Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 1982b;23:154–167. [PubMed] [Google Scholar]
- Freddo T. Intercellular junctions of the iris epithelia in Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 1984;25:1094–1104. [PubMed] [Google Scholar]
- Freddo TF. Intercellular junctions of the ciliary epithelium in anterior uveitis. Invest. Ophthalmol. Vis. Sci. 1987;28:320–329. [PubMed] [Google Scholar]
- Freddo TF, Sacks-Wilner R. Interendothelial junctions of the iris vasculature in anterior uveitis. Invest. Ophthalmol. Vis. Sci. 1989;30:1104–1111. [PubMed] [Google Scholar]
- Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest. Ophthalmol. Vis. Sci. 1990;31:125–137. [PubMed] [Google Scholar]
- Freddo TF, Patz S, Arshanskiy Y. Pilocarpine’s effects on the blood-aqueous barrier of the human eye as assessed by high-resolution, contrast magnetic resonance imaging. Exp. Eye Res. 2006;82:458–464. doi: 10.1016/j.exer.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Freddo TF, Gong H, Neville N. Pilocarpine-induced flare is physiological rather than pathological. Exp. Eye Res. 2012 doi: 10.1016/j.exer.2012.11.003. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko DY, Beuckmann CT, Marcheselli VL, Gordon WC, Kanaoka Y, Eguchi N, Urade Y, Hayaishi O, Bazan NG. Localization of lipocalin-type prostaglandin D synthase (beta-trace) in iris, ciliary body, and eye fluids. Invest. Ophthalmol. Vis. Sci. 1998;39:198–203. [PubMed] [Google Scholar]
- Goldmann EE. Vitalbarfung am Zentralnervensystem. Abhandl.Konigl. Preuss Akad. Wiss. 1913;1:1–60. [Google Scholar]
- Goldmann H. Abflussdruck Minutevolumen und Widerstrand der Kammerwasserstromung des Menschen. Doc. Ophthalmol. 1951;5:278–356. doi: 10.1007/BF00143664. [DOI] [PubMed] [Google Scholar]
- Gong H, Freddo TF. The washout phenomenon in aqueous outflow - Why does it matter? . Exp. Eye Res. 2009;88:729–737. doi: 10.1016/j.exer.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K, Sherman SH, Laties AM, Pederson JE, Gaasterland DE, MacLellan HM. Fate of anterior chamber tracers in the living rhesus monkey eye with evidence for uveo-vortex outflow. Trans. Ophthalmol. Soc. U.K. 1977;97:731–739. [PubMed] [Google Scholar]
- Hirsch M, Montcourrier P, Arguillere P, Keller N. The structure of tight junctions in the ciliary epithelium. Curr. Eye Res. 1995;4:493–501. doi: 10.3109/02713688509025166. [DOI] [PubMed] [Google Scholar]
- Hodgkinson LM, Wang Li-X, Duncan G, Edwards DR, Wormstone IM. ADAM and ADAMTS gene expression in native and wound healing human lens epithelial cells. Molec. Vis. 2010;16:2765–2776. [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye. Philadelphia: W.B. Saunders Company; 1971. [Google Scholar]
- Haung W, Koralewska-Makar A, Bauer B, Akesson B. Extracellular glutathione peroxidase and ascorbic acid in aqueous humor and serum of patients operated on for cataract. Clin. Chim. Acta. 1997;261:117–130. doi: 10.1016/s0009-8981(97)06520-0. [DOI] [PubMed] [Google Scholar]
- Johnson M, Gong H, Freddo TF, Ritter N, Kamm R. Serum proteins and aqueous outflow resistance in bovine eyes. Invest. Ophthalmol. Vis. Sci. 1993;34:3549–3557. [PubMed] [Google Scholar]
- Kee C, Gabelt BT, Croft MA, Gange SJ, Menage MJ, Kaufman PL. Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest. Ophthalmol. Vis. Sci. 1996;37:1840–1848. [PubMed] [Google Scholar]
- Kinsey VE. Comparative chemistry of aqueous humor in posterior and anterior chambers of rabbit eye, its physiologic significance. AMA Arch. Ophthalmol. 1953;50:401–417. doi: 10.1001/archopht.1953.00920030409001. [DOI] [PubMed] [Google Scholar]
- Kolodny NH, Freddo TF, Lawrence B, Suarez C, Bartels SP. Contrast-enhanced magnetic resonance imaging: Confirmation of an anterior protein pathway in normal rabbit eyes. Invest. Ophthalmol. Vis. Sci. 1996;37:1602–1607. [PubMed] [Google Scholar]
- Kuchle M, Vinores SA, Green WR. Immunohistochemical evaluation of the integrity of the blood-aqueous barrier in normal and rubeotic human eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995;233:414–420. doi: 10.1007/BF00180944. [DOI] [PubMed] [Google Scholar]
- Larsson L-I. Aqueous humor flow in normal human eyes treated with brimonidine and timolol, alone and in combination. Arch Ophthalmol. 2001;119:492–495. doi: 10.1001/archopht.119.4.492. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Husain R, Gazzard G, Seah SK, Aung T. Cataract progression after prophylactic laser peripheral iridotomy: potential implications for the prevention of glaucoma blindness. Ophthalmology. 2005;112:1355–1359. doi: 10.1016/j.ophtha.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Luk FO, Kwok AK, Lai TY, Lam DS. Presence of crystalline lens as a protective factor for the late development of open angle glaucoma. Retina. 2009;29:218–224. doi: 10.1097/IAE.0b013e31818ba9ca. [DOI] [PubMed] [Google Scholar]
- McLaren JW, Trocme SD, Relf S, Brubaker RF. Rate of flow of aqueous humor determined from measurements of aqueous flare. Invest. Ophthalmol. Vis. Sci. 1990;31:339–346. [PubMed] [Google Scholar]
- McLaren J, Ziai N, Brubaker R. A simple three-compartment model of anterior segment kinetics. Exp. Eye Res. 1993;56:355–366. doi: 10.1006/exer.1993.1046. [DOI] [PubMed] [Google Scholar]
- Mori M, Araie M, Sakurai M, Oshiko T. Effects of pilocarpine and tropicamide on bloodaqueous barrier permeability in man. Invest. Ophthalmol. Vis. Sci. 1992;33:416–423. [PubMed] [Google Scholar]
- Nagataki S. Aqueous humor dynamics of human eyes as studies using fluorescein. Jpn. J. Ophthalmol. 1975;19:235–249. [Google Scholar]
- Nagataki S, Brubaker RF. Effect of pilocarpine on aqueous humor formation in human beings. Arch. Ophthalmol. 1982;100:818–822. doi: 10.1001/archopht.1982.01030030822020. [DOI] [PubMed] [Google Scholar]
- Noske W, Stamm CC, Hirsch M. Tight junctions of the human ciliary epithelium: regional morphology and implications on transepithelial resistance. Exp. Eye Res. 1994;59:141–149. doi: 10.1006/exer.1994.1092. [DOI] [PubMed] [Google Scholar]
- Oshika T, Araie M, Masuda K. Diurnal variation of aqueous flare in normal human eyes measured with laser flare-cell meter. Jpn J Ophthalmol. 1988;32:143–150. [PubMed] [Google Scholar]
- Oshika T, Sakurai M, Araie M. A study on diurnal fluctuation of blood-aqueous barrier permeability to plasma proteins. Exp. Eye Res. 1993;56:129–133. doi: 10.1006/exer.1993.1019. [DOI] [PubMed] [Google Scholar]
- Pederson O. A light and electron microscopic study of the permeability of the rabbit iris blood vessels to horseradish peroxidase in experimental uveitis. Acta Ophthalmol. (Copen.) 1974;53:678–687. doi: 10.1111/j.1755-3768.1974.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Raviola G. Effects of paracentesis on the blood-aqueous barrier: An electron microscope study on Macaca mulatta using horseradish peroxidase as a tracer. Invest. Ophthalmol. 1974;13:8282–8858. [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp. Eye Res. 1977;25(suppl):27–64. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Raviola G, Butler JM. Unidirectional vesicular transport mechanism in retinal vessels. Invest. Ophthalmol. Vis. Sci. 1983;24:1465–1474. [PubMed] [Google Scholar]
- Raviola G, Butler JM. Morphological evidence for the transfer of anionic macromolecules from the interior of the eye to the blood stream. Curr. Eye Res. 1985;4:503–516. doi: 10.3109/02713688509025167. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural detail localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MR, Price MO, Price FW, Pardo JC, Grandin JC, Uou J, Wand M, Yoder MC. Proteomic analysis of human aqueous humor using multidimensional protein identification technology. Molec. Vis. 2009;15:2740–2750. [PMC free article] [PubMed] [Google Scholar]
- Rohde E, Tomlinson AJ, Johnson DH, Naylor S. Comparison of protein mixtures in aqueous humor by membrane preconcentration - capillary electrophoresis - mass spectrometry. Electrophoresis. 1998;19:2361–2370. doi: 10.1002/elps.1150191317. [DOI] [PubMed] [Google Scholar]
- Russell P, Koretz J, Epstein DL. Is primary open angle glaucoma caused by small proteins? . Med. Hypoth. 1993;41:455–458. doi: 10.1016/0306-9877(93)90125-a. [DOI] [PubMed] [Google Scholar]
- Saari M. Ultrastructure of the microvessels of the iris in mammals with special reference to their permeability. AlbvGraefes Arch. Ophthalmol. 1975;194:87–93. doi: 10.1007/BF00413372. [DOI] [PubMed] [Google Scholar]
- Schenker HI, Yablonski ME, Podos SM, et al. Fluorophotometric study of epinephrine and timolol in human subjects Arch. Ophthalmol. 1981;99:1212–1216. doi: 10.1001/archopht.1981.03930020086007. [DOI] [PubMed] [Google Scholar]
- Seidel E. Ueber den Vorgang der physiologischen Kammerwasserabsonderung und seine pharmakologische Beeinflussung. Arch. F. Ophth. 1920;102:366–371. [Google Scholar]
- Shakib M, Cuhna-Vaz JG. Studies of the permeability of the blood-retinal barrier. IV Junctional complexes of the retinal vessels and their role in permeability of the blood-retinal barrier. Exp. Eye Res. 1966;5:229–234. doi: 10.1016/s0014-4835(66)80011-8. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Wilson WS, Yap M, To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest. Ophthalmol. Vis. Sci. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- Sherman SH, Green K, Laties AM. The fate of anterior chamber fluorescein in the monkey eyeIThe anterior chamber outflow pathways. Exp. Eye Res. 1978;27:159–173. doi: 10.1016/0014-4835(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Shui Y-B, Fu J-J, Garcia C, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest. Ophthalmol. Vis. Sci. 2006;47:1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- Shui Y-B, Holekamp NM, Kramer BC, et al. The gel state of the vitreous and ascorbatedependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch. Ophthalmol. 2009;127:1–8. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried CJ, Shui Y-B, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Invest. Ophthalmol. Vis. Sci. 2010;51:5731–5738. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R, Johnson M. The role of soluble proteins in generating aqueous outflow resistance in the bovine and human eye. Exp. Eye Res. 1997;64:813–821. doi: 10.1006/exer.1997.0276. [DOI] [PubMed] [Google Scholar]
- Smith RS. Ultrastructural studies of the blood-aqueous barrier I. Transport of an electron dense tracer in the iris and the ciliary body of the mouse. Am. J. Ophthalmol. 1971;71:1066–1077. [PubMed] [Google Scholar]
- Smith RJ, Rudt LA. Ultrastructural studies of the blood-aqueous barrier II. The barrier to horseradish peroxidase in primates. Am. J. Ophthalmol. 1973;76:937–947. doi: 10.1016/0002-9394(73)90086-x. [DOI] [PubMed] [Google Scholar]
- Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2Invest. Ophthalmol. Vis. Sci. 1998;39:1188–1197. [PubMed] [Google Scholar]
- Stocker FW. Experimental studies on the blood-aqueous barrier; electrophotometric measurements of fluorescein content of aqueous after intravenous injection of fluorescein, the eye being under the influence of physostigmine, pilocarpine, neostigmine or atropine. Arch. Ophthal. 1947;37:583–590. [PubMed] [Google Scholar]
- Stur M, Grabner G, Dorda W, Zehetbauer G. The effect of timolol on the concentrations of albumin and IgG in the aqueous humor of the human eye. Am. J. Ophthalmol. 1983;96:726–729. doi: 10.1016/s0002-9394(14)71914-2. [DOI] [PubMed] [Google Scholar]
- Stur M, Brabner G, Huber-Spitzy V, Schreiner J, Haddad R. Effect of timolol on aqueous humor protein concentration in the human eye. Arch. Ophthalmol. 1986;104:899–900. doi: 10.1001/archopht.1986.01050180133045. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Inamochi K, Masuda K, Sawa M. Circadian rhythms in aqueous protein concentration and intraocular pressure in rabbits. Jpn. J. Ophthalmol. 1995;39:49–54. [PubMed] [Google Scholar]
- To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin. Exp. Opt. 2002;85:335–349. [PubMed] [Google Scholar]
- Tripathi RC, Millard CB, Tripathi BJ. Protein composition of human aqueous humor: SDSPAGE analysis of surgical and post-mortem samples. Exp. Eye Res. 1989;48:117–130. doi: 10.1016/0014-4835(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Uusitalo R, Palkama A, Stjernschantz J. An electron microscopical study of the bloodaqueous barrier in the ciliary body and iris of the rabbit. Exp. Eye Res. 1973;17:49–63. doi: 10.1016/0014-4835(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Vegge T, Ringvold A. Ultrastructure of the wall of human iris vessels. Z. Zellforsch. 1969;94:19–31. doi: 10.1007/BF00335184. [DOI] [PubMed] [Google Scholar]
- Vegge T. An epithelial blood-aqueous barrier to horseradish peroxidase in the ciliary processes of the vervet monkey. Z. Zellforsch. 1971a;114:309–320. doi: 10.1007/BF00331458. [DOI] [PubMed] [Google Scholar]
- Vegge T. An electron microscopic study of permeability of iris capillaries to horseradish peroxidase in the vervet monkey. Z. Zellforsch. 1971b;123:195–208. doi: 10.1007/BF00330918. [DOI] [PubMed] [Google Scholar]
- Walker SD, Brubaker RF, Nagataki S. Hypotony and aqueous humor dynamic in myotonic dystrophy. Invest Ophthalmol. Vis. Sci. 1982;22:744–751. [PubMed] [Google Scholar]
- Wessley K. Experimentalle Untersuchungen uber Reizubertrangung von einem Auge zum andern. von Graefe’s Arch. Ophthalmol. 1900;50:123. [Google Scholar]
- Wilson WS, Barany E. Iris delay, a neglected factor in aqueous humor dynamics. A study in the cynomolgus monkey (Macaca fascicularis) . Exp. Eye Res. 1983;37:293–301. doi: 10.1016/0014-4835(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Yablonski ME, Zimmerman TJ, Waltman SR, et al. Fluorophotometric study of the effect of topical timolol on aqueous humor dynamics. Exp. Eye Res. 1978;27:135–142. doi: 10.1016/0014-4835(78)90083-0. [DOI] [PubMed] [Google Scholar]
- Yablonski ME, Novack GD, Burke PJ, et al. The effect of levobunolol on aqueous humor dynamics Exp. Eye Res. 1987;44:49–54. doi: 10.1016/s0014-4835(87)80024-6. [DOI] [PubMed] [Google Scholar]
- Zhou LX, Liu JHK. Circadian variation of mouse aqueous humor protein. Molec.Vis. 2006;12:639–643. [PubMed] [Google Scholar]