Abstract
Previous studies have shown that N-methyl-d-aspartate (NMDA) receptor activation results in production of reactive oxygen species (ROS) and activation of extracellular signal-regulated kinase (ERK) in hippocampal area CA1. In addition, application of ROS to hippocampal slices has been shown to result in activation of ERK in area CA1. To determine whether these events were linked causally, we investigated whether ROS are required for NMDA receptor-dependent activation of ERK. In agreement with previous studies, we found that treatment of hippocampal slices with NMDA resulted in activation of ERK in area CA1. The NMDA receptor-dependent activation of ERK was either blocked or attenuated by a number of antioxidants, including the general antioxidant N-acetyl-l-cysteine (L-NAC), the superoxide-scavenging enzyme superoxide dismutase (SOD), the membrane-permeable SOD mimetic Mn(III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), the hydrogen peroxide-scavenging enzyme catalase, and the catalase mimetic ebselen. The NMDA receptor-dependent activation of ERK also was blocked by the NADPH oxidase inhibitor diphenylene iodonium (DPI) and was absent in mice that lacked p47phox, one of the required protein components of NADPH oxidase. Taken together, our results suggest that ROS production, especially superoxide production via NADPH oxidase, is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1.
Keywords: learning and memory, long-term potentiation, oxygen species, reactive, superoxide
Activation of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor is required for several forms of synaptic plasticity in the hippocampus (Dineley et al. 2001; Shapiro 2001). NMDA receptor activation stimulates the production of a number of small messenger molecules including cyclic AMP, nitric oxide, and arachidonic acid (Medina and Izquierdo 1995; Sweatt 2001). These small messenger molecules in turn can activate, either directly or indirectly, a number of protein kinases, including cAMP-dependent protein kinase, protein kinase C (PKC), and extracellular signal-regulated kinase (ERK; Sweatt 2001). Another class of small messenger molecules produced by NMDA receptor activation are reactive oxygen species (ROS; Gunasekar et al. 1995), which include superoxide and typically have been studied with respect to their role in neurotoxicity. However, physiological stimulation results in transient elevations in ROS, which impact signaling pathways in a number of neuronal and non-neuronal cells. In non-neuronal cells, ROS have been shown to be required for activation of ERK (Jackson et al. 2004), and application of ROS to hippocampal slices results inactivation of ERK (Kanterewicz et al. 1998). Both ERK (English and Sweatt 1997) and ROS (Klann 1998; Knapp and Klann 2002) are required for NMDA receptor-dependent hippocampal long-term potentiation (LTP), which suggests the possibility that ROS are required for the activation of ERK during NMDA receptor-dependent LTP. However, it is not known whether NMDA receptor-dependent activation of ERK requires ROS and, if so, what the source of the ROS might be.
An enzymatic source of ROS that might be involved in NMDA receptor-dependent activation of ERK is the superoxide-generating NADPH oxidase complex (Seno et al. 2001; Sorescu et al. 2001). NADPH oxidase has been shown to be involved in regulation of ERK in T-cells (Jackson et al. 2004). Recently, we have found that a functional NADPH oxidase is present in hippocampal neurons at synaptic locations (Tejada-Simon et al. 2005). In the present study, we found that NMDA receptor-dependent activation of ERK was blocked by a number of antioxidants, including scavengers of superoxide. In addition, we observed that NMDA receptor-dependent activation of ERK was blocked by inhibiting NADPH oxidase and was absent in hippocampal slices prepared from mice that lack the NADPH oxidase protein p47phox. Our findings indicate that superoxide produced via NADPH oxidase is required for the NMDA receptor-dependent activation of ERK and suggest that NADPH oxidase might be involved in activation of ERK during hippocampal LTP.
Materials and methods
Materials
All reagents were purchased from Sigma Aldrich Inc. (St Louis, MO, USA) unless otherwise stated.
Hippocampal slice preparation and pharmacological treatment Four hundred-micrometer hippocampal slices were prepared from 6- to 8-week-old C57Bl/6 mice using a vibratome tissue-slicer. Hippocampal sections were dissected in ice-cold cutting solution (110 mm sucrose, 60 mm NaCl, 3 mm KCl, 1.25 mm NaH2PO4, 25 mm NaHCO3, 0.5 mm CaCl2, 7 mm MgCl2, 5 mm glucose, and 0.6 mm ascorbate), transferred to cutting/artificial cerebrospinal fluid (ACSF) solution (mixed 1: 1) and allowed to recover for at least 15 min. The sections then were transferred to 100% ACSF (125 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 25 mm NaHCO3, 2 mm CaCl2, 1 mm MgCl2, and 25 mm glucose) and incubated for 1 h at 22 °C. Then the slices were incubated in ACSF for 2 h at 32 °C. The slices then were incubated with either the vehicle (control), an antioxidant, or an inhibitor for 10 min followed by a 3-min incubation with 100 lm NMDA. Experimental and control slices were frozen (two to three slices per treatment), area CA1 was microdissected and pooled, and the CA1 tissue was homogenized and stored at) 80 °C until assayed as previously described (Banko et al. 2004).
Western blot analysis
CA1 subregions were homogenized in buffer containing 10 mm HEPES, 150 mm NaCl, 50 mm NaF, 1 mm EDTA, 1 mm EGTA, 10 mm Na2P2O7, and 1 × dilution of phosphatase inhibitor cocktails (PIC) 1 and 2 from Sigma (PIC1: cantharidin, bromotetramisole, and microcystin LR; PIC2: sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole). Total protein concentrations were determined by the Bradford method (Bradford 1976). Samples containing equivalent amounts of protein were loaded onto 10% sodium dodecyl sulfate polyacrylamide gels (SDS–PAGE) and resolved by standard electrophoresis. Separated proteins were transferred from the SDS-PAGE gels to Immobilon-P membranes (Millipore, Bedford, MA, USA) using a transfer tank maintained at 4 °C. Once transferred, the membranes were incubated in blocking solution containing 0.02% I-Block, 50 mm Tris–HCl pH 7.5, 150 mm NaCl, and 0.05% Tween-20, at room temperature for approximately 1 h. The membranes were incubated at room temperature for 1 h with primary antibody (rabbit anti-dually phosphorylated ERK; Promega, Madison, WI, USA) that was diluted 1: 5000 in the blocking solution. The blots then were washed three times for 15 min in Tris-buffered saline (50 mm Tris–Hcl, pH 7.5, 150 mm NaCl, 0.05% Tween-20). The blots then were incubated at room temperature for 1 h with secondary antibody (horseradish peroxidase-conjugated anti-rabbit) that was diluted 1: 10 000 in the blocking solution. The blots were then washed three times for 15 min in Tris-buffered saline, and exposed to Kodak film using ECL chemiluminescence (Kodak, Rochester, NY, USA). Finally, the blots were stripped and probed for total ERK (1: 5000; rabbit anti-total ERK, Promega) following a protocol similar to that described above.
Quantification and statistical analysis
The bands from the western blots were quantified on film with densitometry using a desktop scanner and NIH image software to determine the levels of immunoreactivity. Dually phosphorylated ERK (pp-ERK) immunoreactivity was normalized to total ERK immunoreactivity and expressed as percentage of control. All drug treatments under comparison were performed on western blot bands generated within individual experiments directly comparing the particular treatment in question; furthermore, all pp-ERK to total ERK normalizations were made from data collected from the same western blot membrane in order to minimize the variability inherent to these types of experiments. GraphPad Prism 3.2 software was used for graph production and statistical analysis (GraphPad, San Diego, CA, USA).
Electrophysiological analysis of NMDA receptor-mediated fEPSPs
Acute hippocampal slices were prepared as described above. Slices were transferred to an interface recording chamber and allowed to recover. Field excitatory post-synaptic potentials (fEPSPs) were elicited such that baseline responses were 50% of the maximal fEPSP response for a given slice and were collected once every 20 s; six fEPSPs were averaged every 2 min. NMDA receptor-mediated fEPSPs were isolated by adding to the bath solution 0 mm MgCl2, 4 mm CaCl2, and 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). fEPSP responses were recorded for 20 min in 0 mm MgCl2 and 4 mm CaCl2 before slices were perfused for 20 min with CNQX. Once NMDA receptor-mediated fEPSPs were isolated, 10 μm DPI was added for 20 min. The fEPSPs recorded in the presence of CNQX with or without DPI under these conditions were NMDA receptor-dependent as they were blocked completely by 100 μm D-2-amino-5-phosphonopentanoic acid (APV).
Results
NMDA receptor-dependent activation of ERK requires ROS
We hypothesized that ROS are required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. Therefore, we applied 100 μm NMDA to hippocampal slices for 3 min in the presence of the general antioxidant N-acetyl-L-cysteine (L-NAC) and measured the levels of pp-ERK2. In agreement with previous studies, we found that NMDA increased the dual phosphorylation of ERK (Figs 1a, c and e), which was blocked by either 100 μm APV (an NMDA receptor antagonist) or 10 μm MK-801 (an NMDA receptor channel blocker) (data not shown). The NMDA receptor-dependent activation of ERK also was blocked by the general antioxidant L-NAC (Fig. 1a). These results indicate that NMDA receptor-dependent activation of ERK requires an oxidatively permissive redox state.
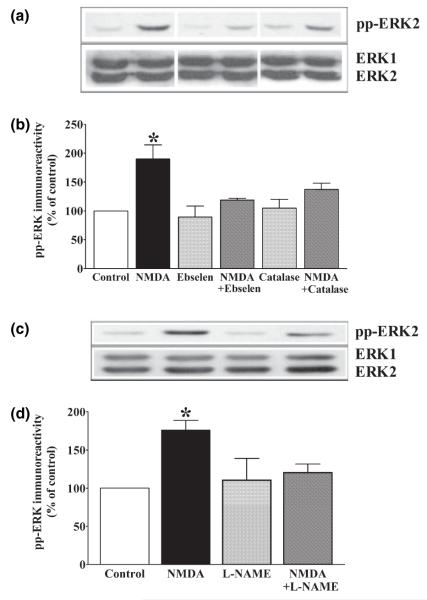
Fig. 1.
NMDA receptor-dependent ERK activation requires superoxide. Quantitative western blot analysis of hippocampal area CA1 from slices exposed to 100 μm NMDA for 3 min, with and without 20 mm L-NAC (a and b), 121 U/mL SOD (c and d), and 100 μm MnTBAP (e and f). Upper panels (a, c, and e) are representative blots depicting changes in pp-ERK2 immunoreactivity (pp-ERK2) and total ERK immunoreactivity (ERK1 and ERK2). Lower panels (b, d, and f) illustrate quantification of pp-ERK2 immunoreactivity normalized to total ERK2. Data are expressed as percent of control (mean ± SEM). *Indicates statistical significance (p < 0.05) determined by one-way anova with a Newman–Keuls multiple comparison test (b, d: n = 4; f: n = 5). Please note that in panel (e) the western blot image was adjusted by repositioning the NMDA + MnTBAP bands with Adobe Photoshop (Adobe, Mountain View, CA, USA). This was done for presentation purposes only. All of the bands shown came from the same membrane, and all densitometric analyses were done on bands from the same membranes.
NMDA receptor-dependent activation of ERK requires superoxide
NMDA receptor activation has been shown to result in superoxide production in area CA1 (Bindokas et al. 1996). Therefore, we hypothesized that superoxide is one of the critical ROS involved in NMDA receptor-dependent activation of ERK. To test this hypothesis, we applied NMDA to slices in the presence of either purified superoxide dismutase (SOD) or MnTBAP, both of which are scavengers of superoxide. Each of these superoxide scavengers was able to block the NMDA receptor-dependent activation of ERK in area CA1 (Figs 1c and e). These data indicate that superoxide is necessary for NMDA receptor-dependent activation of ERK in hippocampal area CA1.
Hydrogen peroxide contributes to NMDA receptor-dependent activation of ERK
The chemistry of ROS is complex, with a number of studies suggesting that the roles for different ROS in regulating signaling pathways are tightly linked. For example, superoxide will dismutate into H2O2 either spontaneously or via the catalytic SOD enzymes. Interestingly, H2O2 is more stable than superoxide and is membrane-permeable; thus, H2O2 could act as a membrane-permeable second messenger during NMDA receptor-dependent signal transduction. Therefore, we applied NMDA in the presence of either ebselen, a membrane-permeable peroxidase mimetic, or catalase, a peroxide-scavenging enzyme. We observed that both ebselen and catalase attenuated NMDA receptor-dependent activation of ERK (Fig. 2b). Taken together, these findings suggest that H2O2 is required for the NMDA receptor-dependent activation of ERK.
Fig. 2.
NMDA receptor-dependent ERK activation requires hydrogen peroxide and nitric oxide synthase. Quantitative western blot analysis of hippocampal area CA1 from slices exposed to 100 μm NMDA for 3 min, with and without 10 μm ebselen (a and b), 260 U/mL catalase (a and b), and 100 μm L-NAME (c and d). Upper panels (a and c) are representative blots depicting changes in pp-ERK2 immunoreactivity (pp-ERK2) and total ERK immunoreactivity (ERK1 and ERK2). Lower panels (b and d) illustrate quantification of pp-ERK2 immunoreactivity normalized to total ERK2. Data are expressed as percentage of control (mean ± SEM). *Indicates statistical significance (p < 0.05) determined by one-way anova with a Newman–Keuls multiple comparison test (b: n = 4, d: n = 6). Please note that in panel (a) the western blot image was adjusted by repositioning the order of the bands with Adobe Photoshop. This was done for presentation purposes only. All of the bands shown came from the same membrane, and all densitometric analyses were done on bands from the same membranes.
NOS contributes to the NMDA receptor-dependent activation of ERK
Previous studies have shown that the reactive nitrogen species (RNS) nitric oxide (NO) can be produced in conjunction with superoxide via the nitric oxide synthase (NOS) enzyme subsequent to NMDA receptor activation (Gunasekar et al. 1995). Moreover, NO has been shown to be required for NMDA receptor-dependent activation of ERK in primary cortical neuronal cultures (Yun et al. 1999). Therefore, we examined whether NOS activity was required for the NMDA receptor-dependent activation of ERK in hippocampal area CA1 by applying NMDA to hippocampal slices in the presence of the NOS inhibitor, nitro-l-arginine methyl ester (L-NAME). We found that L-NAME significantly attenuated, NMDA-induced activation of ERK (Fig. 2c). These findings suggest that NOS is also required for NMDA receptor-dependent activation of ERK.
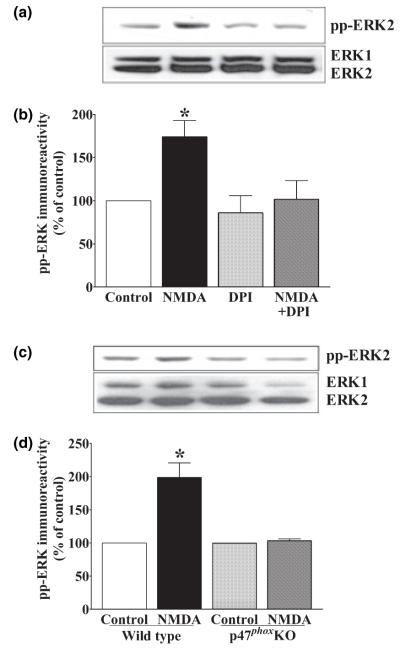
NADPH oxidase is required for NMDA receptor-dependent activation of ERK
The findings described above indicate that superoxide is required for the NMDA receptor-dependent activation of ERK. NADPH oxidase has been widely implicated in both neuronal and non-neuronal cell types as being a rapid and tightly controlled source of superoxide that is necessary for the activation of several signaling cascades, including the ERK-signaling cascade (Torres and Forman 1999; Jackson et al. 2004). To determine whether NADPH oxidase was required for NMDA receptor-dependent activation of ERK, we applied 100 μm NMDA to hippocampal slices in the presence of an NADPH oxidase inhibitor DPI. We found that 10 μm DPI completely inhibited the NMDA-induced activation of ERK (Fig. 3a), a finding consistent with the idea that NADPH oxidase is a source of superoxide that is required for ERK activation. Hippocampal slices incubated with 20 μm NMDA also resulted in an increase in active ERK that was blocked by DPI (data not shown), indicating that inhibition of ERK activation by DPI was not dependent on the concentration of NMDA. Because DPI blocks NADPH oxidase by blocking reduction of flavin adenine dinucleotide (FAD) (Henderson et al. 1995), it is possible that DPI prevents the NMDA receptor-dependent activation of ERK by inhibiting a critical FAD-containing enzyme other than NADPH oxidase. Therefore, we determined whether NMDA receptor activation was able to induce ERK activation in slices from genetically modified mice that lack p47phox, a cytosolic component of the NADPH oxidase complex that is required for superoxide production (Jackson et al. 1995; van der Veen et al. 2000; Lavigne et al. 2001; Chabrashvili et al. 2002). In contrast to slices from wild-type littermates, we found that NMDA could not induce ERK activation in area CA1 of hippocampal slices prepared from p47phox mutant mice (Fig. 3c). Taken together, these findings indicate that NADPH oxidase activity is required for NMDA receptor-dependent activation of ERK, and that the activated NADPH oxidase complex may be a source of superoxide that regulates the ERK-signaling cascade in hippocampal area CA1.
Fig. 3.
NMDA receptor-dependent ERK activation requires NADPH oxidase. Quantitative western blot analysis of hippocampal area CA1 from slices exposed to 100 μm NMDA for 3 min, with and without 10 μm DPI (a and b) or the p47phox subunit of the NADPH oxidase complex (c and d). Upper panels (a and c) are representative blots depicting changes in pp-ERK2 immunoreactivity (pp-ERK2) and total ERK immunoreactivity (ERK1 and ERK2). Lower panels (b and e) illustrate quantification of pp-ERK2 immunoreactivity normalized to total ERK2. Data are expressed as percentage of control (mean ± SEM). *Indicates statistical significance (p < 0.05) determined by one-way anova with a Newman–Keuls multiple comparison test (b, d: n = 4).
DPI does not affect NMDA receptor-mediated fEPSPs in hippocampal area CA1
It is possible that the inhibition of the NMDA receptor-dependent activation of ERK by DPI occurs via direct effects on the NMDA receptor. We have shown previously that SOD and MnTBAP do not affect NMDA receptor-mediated fEPSPs (Klann 1998; Thiels et al. 2000). However, whether either DPI or the loss of p47phox alters NMDA receptor-mediated fEPSPs has not been investigated. To determine whether DPI directly affects NMDA receptor function, we isolated NMDA receptor-mediated excitatory fEPSPs in area CA1 of hippocampal slices (Figs 4a and b). Isolated NMDA receptor-mediated fEPSPs were not altered by 10 μm DPI (Fig. 4). In addition, NMDA receptor-mediated fEPSPs in p47phox knockout mice were not different from those in wild-type mice (data not shown). Taken together, these data suggest that neither pharmacological nor genetic inhibition of NADPH oxidase alters NMDA receptor function in hippocampal area CA1.
Fig. 4.

DPI does not affect NMDA receptor-mediated fEPSPs in hippocampal area CA1. Isolation of NMDA receptor-mediated fEPSPs recorded in hippocampal area CA1. (a) The change in fEPSP slope was monitored over time and plotted as a percentage of the baseline fEPSP. (b) Representative traces collected at the corresponding time points (1–5) in panel (a) b1: baseline fEPSP; b2: fEPSP in the presence of 4 mm CaCl2 and 0 mm MgCl2; b3: fEPSP after the addition of 20 μm CNQX; b4: fEPSP after 20 min of 10 μm DPI, and b5: fEPSP after the addition of 100 μm APV. n = 4 for each experimental condition.
Discussion
The results presented in this study demonstrate that ROS are required for the NMDA receptor-dependent activation of ERK in hippocampal area CA1. We found that superoxide (Figs 1c and e), H2O2 (Fig. 2a), and NO (Fig. 2c) all are involved in the NMDA receptor-dependent activation of ERK. Taken together, these results suggest that superoxide production is required for NMDA receptor-dependent activation of ERK activation in hippocampal area CA1 and that H2O2 and NO also play an important role in promoting the full expression of ERK activation.
The relationship between superoxide and other ROS and RNS such as NO and peroxynitrite is complex. NMDA receptor activation can result in the parallel production of superoxide and NO (Gunasekar et al. 1995) and the formation of H2O2 and peroxynitrite under these conditions is possible (Rodenas et al. 1995). Furthermore, NOS activity can also produce both NO and superoxide given the appropriate cellular conditions (Culcasi et al. 1994), plausibly leading to the subsequent production of peroxynitrite. Any of these reactive species would then be able to modulate the ERK-signaling pathway. For instance, superoxide can be dismutated, either spontaneously or enzymatically, to H2O2 (Hoffstein et al. 1985), which is known to activate ERK in numerous cells and tissues (Guyton et al. 1996; Torres and Forman 1999; Lee and Esselman 2001; Song et al. 2005), including the hippocampus (Kanterewicz et al. 1998). Alternatively, superoxide can react with NO at near diffusion-limited rates to form peroxynitrite, a highly reactive RNS (Ortega and Amaya 2000). This could result in either the oxidation or nitration and subsequent activation of downstream elements such as MEK, as has been shown in rat lung myofibroblasts (Zhang et al. 2000). Previous studies also have shown that NOS is required for NMDA receptor-dependent activation of ERK in primary cortical neuronal cultures (Yun et al. 1999) and primary cerebellar neuronal cultures (Llansola et al. 2001). Given that NOS has been shown to produce both NO and superoxide (Culcasi et al. 1994) it is unclear whether these these species act separately in parallel signaling pathways to activate ERK, or whether they act together on a single upstream signaling target to trigger the activation of ERK. These possibilities remain to be determined.
The source(s) of ROS, specifically of superoxide, required for NMDA receptor-dependent activation of ERK in the hippocampus is an open question. NMDA receptor activation in hippocampal slices has been shown to result in increased production of superoxide via the mitochondrial electron transport chain (Bindokas et al. 1996). Additionally, in cultured hippocampal neurons mitochondria have been implicated as a source of superoxide that is necessary for activity-dependent increases in the phosphorylation of cAMP response element binding protein (CREB; Hongpaisan et al. 2003), a transcription factor known to be a downstream effector of ERK (Sweatt 2001). NADPH oxidase is another source of superoxide that could regulate ERK in the hippocampus. The NADPH oxidase complex, originally characterized in phagocytic neutrophils in the immune system, is known to produce large, localized quantities of superoxide (Quinn and Gauss 2004). In phagocytic cells, NADPH oxidase is a heterotetramer consisting of two cytosolic components (p47phox and p67phox) and two membrane-associated proteins (p22phox and gp91phox) that can be activated by the small G protein Rac (Quinn and Gauss 2004). Recent evidence indicates that components of the NADPH oxidase complex are localized to hippocampal neurons (Olenik et al. 1997; Mizuki et al. 1998; Serrano et al. 2003; Tejada-Simon et al. 2005). We found that inhibition of NADPH oxidase, either pharmacologically (Fig. 3a) or by genetic deletion of p47phox (Fig. 3c), abolished NMDA receptor-dependent activation of ERK.
Uncovering the sources of ROS production and downstream effectors of ROS in the hippocampus is critical for understanding how ROS contribute to synaptic plasticity, memory formation, and general cognitive function. It previously was shown that ROS are required for NMDA receptor-dependent LTP in the hippocampus and hippocampus-dependent learning and memory (Thiels et al. 2000). In addition, the activation of ERK is required for NMDA receptor-dependent LTP (English and Sweatt 1997) and hippocampus-dependent learning and memory (Atkins et al. 1998). Finally, ERK activation in hippocampal slices can be stimulated by ROS (Kanterewicz et al. 1998). Our findings provide evidence that causally links NMDA receptor activation to ERK activation via production of ROS by NADPH oxidase consistent with the idea that ERK is an effector of NADPH oxidase-dependent ROS signaling during LTP and hippocampus-dependent memory. Interestingly, patients with chronic granulomatous disease (CGD), which is characterized by a deficient phagocytic NADPH oxidase (Holland 2003), have been reported to suffer from cognitive deficits (Pao et al. 2004). It will be of great interest to determine whether mice that model CGD, such as the p47phox deficient mice, exhibit LTP and/or hippocampus-dependent memory deficits. Finally, altered NADPH oxidase function may occur in neurological disorders such as Alzheimer’s disease (Zekry et al. 2003). Thus, understanding the normal physiological function of NADPH oxidase-dependent ROS production in the hippocampus may provide insight into the role of NADPH oxidase in the neuropathology of Alzheimer’s disease.
Acknowledgements
This work was supported by NIH grants N5034007 (E.K.) and N5047852 (K.T.K.).
Abbreviations used
- ACSP
artifical cerebrospinal fluid
- APV
d-2-amino-5-phosphonopentanoic acid
- CGD
chronic granulomatous disease
- CNQX
cyano-7-nitroquinoxaline-2,3-dione
- CREB
cAMP response element binding protein
- DPI
diphenylene iodonium
- ERK
extracellular signal-regulated kinase
- FAD
flavin adenine dinucleotide
- fEPSP
field excitatory post-synaptic potential
- L-NAC
N-acetyl-l-cysteine
- L-NAME
nitro-l-arginine methyl ester
- LTP
long-term potentiation
- MnTBAP
Mn(III) tetrakis (4-benzoic acid) porphyrin
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- PIC
phosphatase inhibitor concktails
- PKC
protein kinase C
- pp-ERK
dually phosphorylated ERK
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SDS–PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- SOD
superoxide dismutase.
References
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J. Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- Culcasi M, Lafon-Cazal M, Pietri S, Bockaert J. Glutamate receptors induce a burst of superoxide via activation of nitric oxide synthase in arginine-depleted neurons. J. Biol. Chem. 1994;269(12):589–12. 593. [PubMed] [Google Scholar]
- Dineley KT, Weeber EJ, Atkins C, Adams JP, Anderson AE, Sweatt JD. Leitmotifs in the biochemistry of LTP induction: amplification, integration and coordination. J. Neurochem. 2001;77:961–971. doi: 10.1046/j.1471-4159.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogenactivated protein kinase cascade in hippocampal long-term potentiation. J. Biol. Chem. 1997;272(19):103–19. 106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J. Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Henderson LM, Banting G, Chappell JB. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J. Biol. Chem. 1995;270:5909–5916. [PubMed] [Google Scholar]
- Hoffstein ST, Gennaro DE, Manzi RM. Neutrophils may directly synthesize both H2O2 and O2− since surface stimuli induce their release in stimulus-specific ratios. Inflammation. 1985;9:425–437. doi: 10.1007/BF00916342. [DOI] [PubMed] [Google Scholar]
- Holland SM. Update on phagocytic defects. Pediatr. Infect. Dis. J. 2003;22:87–88. doi: 10.1097/00006454-200301000-00020. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Winters CA, Andrews SB. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol. Cell Neurosci. 2003;24:1103–1115. doi: 10.1016/j.mcn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Kanterewicz BI, Knapp LT, Klann E. Stimulation of p42 and p44 mitogen-activated protein kinases by reactive oxygen species and nitric oxide in hippocampus. J. Neurochem. 1998;70:1009–1016. doi: 10.1046/j.1471-4159.1998.70031009.x. [DOI] [PubMed] [Google Scholar]
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J. Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J. Neurosci. 2002;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic requirement of p47phox for superoxide production by murine microglia. Faseb J. 2001;15:285–287. doi: 10.1096/fj.00-0608fje. [DOI] [PubMed] [Google Scholar]
- Lee K, Esselman WJ. cAMP potentiates H(2)O(2)-induced ERK1/2 phosphorylation without the requirement for MEK1/2 phosphorylation. Cell Signal. 2001;13:645–652. doi: 10.1016/s0898-6568(01)00178-4. [DOI] [PubMed] [Google Scholar]
- Llansola M, Saez R, Felipo V. NMDA-induced phosphorylation of the microtubule-associated protein MAP-2 is mediated by activation of nitric oxide synthase and MAP kinase. Eur. J. Neurosci. 2001;13:1283–1291. doi: 10.1046/j.0953-816x.2001.01497.x. [DOI] [PubMed] [Google Scholar]
- Medina JH, Izquierdo I. Retrograde messengers, longterm potentiation and memory. Brain Res. Brain Res. Rev. 1995;21:185–194. doi: 10.1016/0165-0173(95)00013-5. [DOI] [PubMed] [Google Scholar]
- Mizuki K, Kadomatsu K, Hata K, et al. Functional modules and expression of mouse p40(phox) and p67(phox), SH3-domain-containing proteins involved in the phagocyte NADPH oxidase complex. Eur. J. Biochem. 1998;251:573–582. doi: 10.1046/j.1432-1327.1998.2510573.x. [DOI] [PubMed] [Google Scholar]
- Olenik C, Barth H, Just I, Aktories K, Meyer DK. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Brain Res. Mol. Brain Res. 1997;52:263–269. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- Ortega MA, Amaya AA. Nitric oxide reactivity and mechanisms involved in its biological effects. Pharmacol. Res. 2000;42:421–427. doi: 10.1006/phrs.2000.0701. [DOI] [PubMed] [Google Scholar]
- Pao M, Wiggs EA, Anastacio MM, et al. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Rodenas J, Mitjavila MT, Carbonell T. Simultaneous generation of nitric oxide and superoxide by inflammatory cells in rats. Free Radic. Biol. Med. 1995;18:869–875. doi: 10.1016/0891-5849(94)00215-6. [DOI] [PubMed] [Google Scholar]
- Seno T, Inoue N, Gao D, et al. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb. Res. 2001;103:399–409. doi: 10.1016/s0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch. Neurol. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Song HJ, Lee TS, Jeong JH, Min YS, Shin CY, Sohn UD. Hydrogen peroxide-induced extracellular signal-regulated kinase activation in cultured feline ileal smooth muscle cells. J. Pharmacol. Exp. Ther. 2005;312:391–398. doi: 10.1124/jpet.104.074401. [DOI] [PubMed] [Google Scholar]
- Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic. Biol. Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon M, Serrano F, Villasana LEKBI, Wu G, Quinn MTAKE. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol. Cell. Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Forman HJ. Activation of several MAP kinases upon stimulation of rat alveolar macrophages: role of the NADPH oxidase. Arch. Biochem. Biophys. 1999;366:231–239. doi: 10.1006/abbi.1999.1225. [DOI] [PubMed] [Google Scholar]
- van der Veen RC, Dietlin TA, Hofman FM, Pen L, Segal BH, Holland SM. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J. Immunol. 2000;164:5177–5183. doi: 10.4049/jimmunol.164.10.5177. [DOI] [PubMed] [Google Scholar]
- Yun HY, Dawson VL, Dawson TM. Glutamate-stimulated calcium activation of Ras/Erk pathway mediated by nitric oxide. Diabetes Res. Clin. Pract. 1999;45:113–115. doi: 10.1016/s0168-8227(99)00039-x. [DOI] [PubMed] [Google Scholar]
- Zekry D, Epperson TK, Krause KH. A role for NOX NADPH oxidases in Alzheimer’s disease and other types of dementia? IUBMB Life. 2003;55:307–313. doi: 10.1080/1521654031000153049. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang YZ, Kagan E, Bonner JC. Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J. Biol. Chem. 2000;275(22):479–22. 486. doi: 10.1074/jbc.M910425199. [DOI] [PubMed] [Google Scholar]