Abstract
Increasing evidence suggests that reactive oxygen species (ROS), such as superoxide and hydrogen peroxide, act as necessary signaling molecules in processes underlying cognition. Moreover, ROS have been shown to be necessary in molecular process underlying signal transduction, synaptic plasticity, and memory formation. Research from several laboratories suggests that NADPH oxidase is an important source of superoxide in the brain. Evidence is presented here to show that ROS are in fact important signaling molecules involved in synaptic plasticity and memory formation. Moreover, evidence that the NADPH oxidase complex is a key regulator of ROS generation in synaptic plasticity and memory formation is discussed. Understanding redox signaling in the brain, including the sources and molecular targets of ROS, are important for a full understanding of the signaling pathways that underlie synaptic plasticity and memory. Knowledge of ROS function in the brain also is critical for understanding aging and neurodegenerative diseases of the brain given that several of these disorders, including Alzheimer’s disease and Parkinson disease, may be exacerbated by the unregulated generation of ROS.
INTRODUCTION
Over the last several years, several studies have indicated that reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), are important signaling molecules underlying mammalian learning and memory. Previously, ROS had been described as a class of destructive molecules that hinder neuronal function and have been implicated in degenerative processes underlying Alzheimer’s and Parkinson diseases (1, 70, 104, 124), as well as processes thought to underlie the general aging-associated decline of cognitive function (15, 35, 100). However, recent work has shown that ROS are required for normal cognitive function at cellular and behavioral levels of analyses. Specifically, ROS have been shown to be required for a form of synaptic plasticity called long-term potentiation (LTP), learning and memory, and for biochemical signal transduction cascades that are believed to underlie LTP and memory formation. Thus, the source of ROS responsible for these brain functions and how these small highly reactive molecules are regulated are important questions that this review will attempt to address.
Positive correlations have been made between LTP and learning. One such correlation is that signal transduction cascades that are activated during LTP have been shown to be required for memory formation. For instance, inhibition of NMDA receptors can block LTP and can interfere with certain types of memory formation (112). Furthermore, deficiencies in memory formation are observed in mice with gene specific mutations in loci coding for signal transduction elements that are critical for LTP (7, 12, 72, 81). Many of these signal transduction elements, such as small messenger molecules and protein kinases, become active once NMDA receptors are activated, thereby permitting the influx of Ca2+ into the postsynaptic terminal. Thus, NMDA receptor-dependent LTP in acute hippocampal slices has been used as an in vitro model to study synaptic plasticity and the cellular mechanisms underlying memory formation.
In the hippocampus, high-frequency stimulation (HFS) of CA3 Schaffer collaterals can induce LTP at synapses in area CA1. HFS induces the activation of postsynaptic NMDA receptors, resulting in the influx of Ca2+ into the postsynaptic terminal. The transient rise in Ca2+ results in the production of small messenger molecules, such as cyclic adenosine monophosphate (cAMP), nitric oxide, arachidonic acid, and ROS (106). These small messenger molecules in turn activate kinases such as protein kinase (PKC) and extracellular signal-regulated kinase (ERK) and inhibit phosphatases such as calcineurin (protein phosphatase 2B or PP2B) (107, 117). Many of these signaling molecules and enzymes are involved in the induction and expression of LTP, as well as memory formation (107, 117, 121). In addition to these well-studied signaling pathways, NMDA receptor activation has been shown to produce ROS (31), which also are required for LTP and hippocampus-dependent memory (56, 67, 71, 111). Until very recently, the source of ROS required for LTP and memory formation was unclear.
NADPH oxidase has been shown in multiple cell types to generate superoxide as a response to specific extracellular and intracellular stimuli (76, 91). The best known of these responses is the phagocytic oxidative burst (76). This burst generates large amounts of superoxide rapidly, transiently, and in a well-controlled manner. This pool of superoxide is used to break down phagocytosed material and as a signal to initiate signal transduction cascades underlying the bactericidal response (76). Furthermore, in several cell types, NADPH oxidase production of superoxide has been implicated in signaling required for transcriptional activation and cell proliferation (38, 59, 96, 98, 102).
In this review we will briefly discuss the evidence that ROS are important signaling molecules underlying LTP and memory, including known biochemical targets of ROS signaling in the brain. We also will discuss some of the hypothesized sources of ROS, focusing on recent evidence pointing to the role that NADPH oxidase plays in the generation of ROS that are required for NMDA receptor-dependent signal transduction, synaptic plasticity, and memory formation.
ROS ARE CRITICAL SIGNALING MOLECULES IN SYNAPTIC PLASTICITY
Synaptic plasticity is the physiological process that is thought to underlie learning and memory at the cellular level. One form of plasticity that has been commonly studied is LTP. Many of the molecular processes underlying LTP also are required for learning and memory (67, 103). Thus, LTP has become a popular model to study the mechanisms that underlie learning and memory behavior.
Investigation into the role of ROS in LTP has revealed an interesting, yet complex role for oxidative species such as superoxide and H2O2 in the molecular processes that lead to changes in synaptic strength. The role of ROS is specific to the identity of the oxidative molecule involved (i.e., superoxide or H2O2) (43, 56) and the concentration of ROS during the process (41, 42, 56).
The use of transgenic animals and pharmacological approaches to block the activity of ROS has been useful in identifying the necessity of these signaling molecules in synaptic plasticity and memory formation. LTP studies in the rodent hippocampus have revealed that scavenging superoxide blocks LTP induced with high-frequency stimulation (HFS-LTP) (48, 51), suggesting that superoxide is required for HFS-LTP. On the other hand, overproduction of H2O2 as a byproduct of superoxide dismutase (SOD) activity also was shown to inhibit LTP (29). To better understand the specific role of ROS, investigators have also applied exogenous sources of either superoxide or H2O2 to various in vitro systems and have observed interesting effects on cellular plasticity.
Xanthine-xanthine oxidase (X/XO) is often used to generate superoxide in vitro (55). Exogenous application of X/XO to hippocampal slices resulted in a short-term depression of synaptic transmission that eventually potentiated in an LTP-like manner that was inhibited by SOD (55). The role of H2O2 has also been investigated using the application of exogenous H2O2 to study the effect of this ROS on the molecular mechanisms underlying cellular plasticity. Kamsler and colleagues have shown that H2O2 could either potentiate or depress HFS-LTP in a concentration-dependent manner (41). Also, low concentrations of H2O2 potentiate or depress intracellular Ca2+ levels (63, 123).
Many of the studies implicating ROS involvement in the molecular mechanisms underlying synaptic plasticity and memory investigate a narrow range of superoxide or H2O2 concentrations. However, very interesting results were obtained when a range of ROS concentrations were tested for their effects on synaptic plasticity (41, 42, 55, 56). For example, high concentrations of superoxide or H2O2 resulted in the depression of excitatory postsynaptic field potentials (fEP-SPs) measured in hippocampal area CA1, whereas lower concentrations resulted in a potentiation of the fEPSP (41, 55).
The role of ROS in aging-related changes in synaptic plasticity and cognitive performance is an interesting and thriving area of research; we refer the reader to the review of Hu et al. presented in this forum. However, it should be mentioned that during aging there seems to be a shift in the role of ROS in synaptic plasticity and memory that may be dependent on the changes during aging that result from the accumulation of oxidative damage or changes in oxidant regulation via enzymatic antioxidants (100).
ROS ARE REQUIRED FOR LEARNING AND MEMORY
SOD mutant mice have been critical models for determining the role of ROS in learning and memory. SOD scavenges superoxide and dismutates the molecule to H2O2, which can then be rapidly degraded by catalase. There are three SOD isozymes: cytoplasmic-Cu/Zn-SOD (SOD-1), manganese-containing mitochondrial SOD (SOD-2 or MnSOD), and extracellular-SOD (EC-SOD), which also is a Cu/Zn-containing SOD. Mice that either overexpress SOD or express a dysfunctional SOD have been analyzed using various behavioral paradigms with surprising results.
The earliest evidence that ROS are required for learning and memory came from mice that overexpress SOD-1. Behavioral analyses of the SOD-1 overexpressing mice indicated that the mice displayed altered behavior in open field analysis, and more interestingly, showed decreased escape latencies in the hippocampus- and NMDA receptor-dependent Morris water maze paradigm as compared to wild-type mice (29, 66, 112). These data were the first to suggest that scavenging of superoxide impairs learning and memory. Consistent with a requirement of superoxide in learning and memory, Levin et al. showed that mice that either overexpress EC-SOD or are genetically deficient for the EC-SOD gene had impaired performance in the win-shift 8-arm radial maze (60), also a hippocampus-dependent task (80). Further analysis of the EC-SOD overexpressing mice revealed that these mice were impaired in hippocampus-dependent contextual fear conditioning (85, 86), but were normal for cue-dependent fear conditioning (35, 85, 111).
Hippocampus-dependent learning and memory formation has been a useful model in determining the role of ROS in cognition; however, behavioral analysis of SOD overexpressing mice has revealed potential roles for ROS in cognitive performance associated with other brain regions as well. EC-SOD overexpressing mice displayed alterations in performance on the 8-arm radial maze task that was dependent on motivational state induced by food restriction (60, 62). Under low motivational state, EC-SOD overexpressing mice showed impairments in learning; however, under a high motivational state EC-SOD overexpressing mice were able to learn, though at a slower rate, and express long-term memory (62).
Taken as a whole, the data generated from the analysis of SOD mutant mice suggest that ROS are critically involved in the molecular processes involved in cognition, particularly processes underlying learning and memory formation.
ROS ARE CRITICAL SIGNALING MOLECULES THAT MODULATE SIGNALING PATHWAYS INVOLVED IN LTP AND MEMORY
The molecular mechanisms underlying synaptic plasticity and memory have been intensely studied (107). Investigations of signal transduction cascades involved in synaptic plasticity have revealed a wide range of molecular players, including protein kinases (52), phosphatases (52, 95), transcription factors (40), translation factors (50), GTPases (116), and other Ca2+-dependent enzymes (88, 121). Interestingly, ROS have been shown to be important modulators of many of these pathways (43), including some evidence of direct modification of these signaling enzymes by ROS. As mentioned earlier, NMDA receptor-dependent signaling is one critical pathway that is thought to underlie synaptic plasticity and long-term memory formation that has been shown to contain several signaling molecules that are directly affected by ROS.
Glutamatergic synaptic transmission results in the activation of AMPA receptors and voltage-dependent calcium channels during fast synaptic transmission. Given the appropriate pattern of stimulation, NMDA receptors can become activated, which results in a transient spike in postsynaptic calcium levels that leads to the activation of numerous signal transduction cascades. Among the signaling events that occur following NMDA receptor activation are the activation of various protein kinases, including calcium/calmodulin-dependent protein kinase II (CaMKII) (121), protein kinase C (PKC) (49, 51), and extracellular signal-regulated kinase (ERK) (7, 24, 25), as well as the inhibition of protein phosphatases such as calcineurin and protein phosphatase 1 (6, 68, 79, 118). NMDA receptor stimulation also has been shown to result in the production of ROS (13). Following ROS generation, several target proteins become oxidatively modulated. For example, NMDA receptor activation was shown to result in the oxidation of neurogranin (NG) in a time- and dose-dependent manner that is sensitive to NMDA receptor antagonists (64). Neurogranin is a postsynaptic PKC substrate that binds to and sequesters calmodulin (CaM) (64); either oxidation or phosphorylation (36, 49) of neurogranin promotes the release of CaM, thereby promoting Ca2+/CaM signaling via the activation of CaMKII. This signaling module is likely to trigger the induction of LTP (121). NMDA receptor-mediated oxidation of neurogranin occurs within 3-5 min of stimulation and quickly returns to basal oxidation levels (64) and H2O2 can directly cause the oxidation of NG (64). In addition, the generation of superoxide by X/XO increases the phosphorylation of neurogranin by the activation of autonomous PKC (49). ROS also can directly oxidize and activate PKC (will be discussed below). Taken together, these reports suggest that neurogranin is an important target of NMDA receptor-induced ROS production, and when either oxidized or phosphorylated by PKC, promotes LTP and possibly long-term memory formation (64, 72, 119, 120).
Another signaling enzyme that is an important modulator of LTP and memory formation is PKC. LTP-inducing stimulation resulted in the activation of PKC that is NMDA receptor-dependent (51, 55, 109). Interestingly, the exogenous application of SOD and catalase inhibited not only LTP, but also the LTP-induced activation of PKC (51, 55), suggesting that superoxide and H2O2 are necessary for PKC activation. In addition, direct application of the superoxide-generating system X/XO to hippocampal slices induced not only an LTP-like potentiation, but also the persistent activation of PKC (54). SOD, but not catalase, was shown to block the X/XO-induced activation of PKC (54, 55). Furthermore, the PKC inhibitor bisindomaleimide blocked X/XO-induced LTP and HFS-induced LTP (55), suggesting a common pathway involving PKC for both HFS-LTP and X/XO-induced LTP. The mechanism of PKC activation via oxidation seems to be mediated by direct modification of the zinc finger region of the kinase; ZnCl2 blocked the X/XO-induced activation of PKC, whereas the zinc chelator (TPEN) activated PKC (54). Furthermore, X/XO stimulated the release of zinc from PKC (54). Interestingly, peroxynitrite, a strong oxidant that is generated via the reaction of superoxide and NO (92), also can modulate PKC activity in a concentration-dependent manner. Low concentrations of peroxynitrite activated PKC, whereas high concentrations of peroxynitrite inhibited PKC (53). At all concentrations tested, peroxynitrite increased the nitration of PKC (53). Although the activation of PKC by peroxynitrite was inhibited by reducing agents, peroxynitrite-induced inhibition of PKC activity was resistant to reducing agents (53). It remains to be determined whether peroxynitrite-induced modulation of PKC occurs during LTP.
Activation of NMDA receptors, either pharmacologically or electrically with LTP-inducing HFS, activated ERK (47, 65, 99). Consistent with the importance of ROS involvement in NMDA receptor-dependent signaling, superoxide and to a lesser extent H2O2, were required for NMDA receptor-dependent activation of ERK (47). In this study, NMDA receptor-dependent activation of ERK in acute hippocampal slices was blocked by exogenously added SOD, SOD mimetics, and catalase. Furthermore, it was shown that application of either X/XO or H2O2 to hippocampal slices resulted in the activation of ERK, which was inhibited by the general antioxidant N-acetyl-cysteine (44). Thus, the activation of ERK during NMDA receptor-dependent LTP and long-term memory may require ROS.
Protein phosphatases also have been shown to be important modulators of synaptic plasticity (117) that are regulated by ROS. For example calcineurin (protein-phosphatase 2B; PP2B) is thought to suppress LTP and long-term memory formation by opposing the effect of LTP-inducing kinases such as CaMKII and PKC (117). Calcineurin is highly sensitive to redox modification by ROS (75). For example, basal calcineurin activity was reduced by either X/XO or H2O2, but was enhanced by the addition of SOD. Furthermore, strong oxidizing agents inhibited calcineurin, whereas reducing agents enhanced calcineurin activity, possibly through direct oxidative modification of calcineurin protein (75). Consistent with the idea that a redox-sensitive calcineurin might play a role in LTP, FK506 (a calcineurin inhibitor) blocked H2O2-mediated enhancement of LTP and restored LTP in slices treated with concentrations of H2O2 that normally inhibited LTP (41). Interestingly, aged wild-type mice showed increased phosphatase activity that was similar to the levels of phosphatase activity observed in young wild-type mice that had been treated with exogenously applied H2O2. Thus, modulation of calcineurin by ROS may promote the expression of LTP following NMDA receptor activation.
Another potential target of ROS-mediated signal transduction during hippocampal synaptic plasticity and memory is the redox-sensitive transcription factor NF-κB. Several lines of evidence suggest that LTP and long-term memory may involve redox dependent activation of NF-κB. It has been observed that NF-κB becomes activated following LTP induction (4, 27) and that NF-κB can be activated by redox-mediated signaling (30, 32, 40). Furthermore, NF-κB has been implicated in the formation of long-term memory in nonmammalian organisms (26, 122) as well as in the consolidation of fear memories in rodents (122). Taken together, these findings suggest that NF-κB may be an important target of redox mediated signaling during synaptic plasticity and normal cognitive function. However, further studies are required to directly demonstrate that ROS-dependent activation NF-κB occurs during synaptic plasticity and memory.
Regulation of intracellular Ca2+ is an important function that controls synaptic plasticity and memory (88). ROS-mediated signaling could modulate intracellular Ca2+ via the oxidative modification of Ca2+ channels, thereby altering the Ca2+ response during plasticity-inducing stimuli. As mentioned previously, ROS can directly modulate voltage-dependent Ca2+ channels (63); however, there is also evidence that oxidative regulation of ryanodine receptors could be involved in redox-mediated modulation of intracellular Ca2+. Ryanodine receptors are very sensitive to redox modulation, which results in alterations in channel function (34). This is intriguing because mutant mice that lack one form of the ryanodine receptor, RyR3, have been shown to express alterations in LTP and spatial memory (9, 28). Thus, ROS-mediated alteration of ryanodine receptor function may be an important step during the molecular events involved in the modulation of intracellular Ca2+ that underlies LTP and memory.
It seems clear that ROS are important modulators of signal transduction cascades that underlie synaptic plasticity and memory formation. Further work to identify other targets of ROS signaling will be important to better understand the significance of redox signaling in the brain. Another important goal will be to identify the sources of ROS that are involved in modulating in these redox-sensitive signaling pathways. Uncontrolled ROS production would quickly be come unhealthy for the neural tissue producing it; thus a mechanism that yields a potent, yet controlled source of ROS should be identifiable. There are several candidate sources of ROS that meet these criteria that will be discussed in the next section.
POTENTIAL SOURCES OF ROS INVOLVED IN LTP AND MEMORY
Although ROS have been shown to be critical signaling molecules that are required in the molecular events that underlie synaptic plasticity and memory formation, the source of ROS during these events has yet to be determined. Several sources have been hypothesized and evidence showing the plausibility of ROS generation from these sources has been provided; however, experiments determining the distinct physiological significance of these potential sources of ROS have been elusive. Among the hypothesized sources of ROS are mitochondria, monoamine oxidase, cyclooxygenase, nitric oxide synthase, and NADPH oxidase. Each of these potential sources is known to generate ROS under pathophysiological conditions, but the physiological role of the ROS produced by these sources has not been determined. Experiments designed to determine the physiological significance of each of these sources of ROS, especially with respect to a role in synaptic plasticity and memory formation will be an important goal for understanding the role of ROS signaling in the brain.
Mitochondria
Mitochondria produce superoxide as a metabolic byproduct of the electron transport chain and oxidative phosphorylation. Typically, mitochondrial production of superoxide is studied in models of oxidative stress, apoptosis, and neurodegeneration; however, there is evidence that suggest that mitochondria may be a source of ROS that is stimulated by appropriate physiological stimuli. For instance, elevating Ca2+ and Na+ is sufficient to produce free radicals from isolated rat mitochondria (23). Also, NMDA receptor activation via glutamate application to cultured forebrain neurons induced a localized generation of ROS that was blocked by MK-801 (94). Interestingly, glutamate application caused intracellular pH to decrease in a Ca2+-dependent manner (94), which suggests that NOS could play a role in superoxide generation as well (33). NMDA receptor stimulation also was shown to result in the production of superoxide that occluded superoxide production induced by the uncoupling of protein transport with FCCP (13) The authors of this study suggested that this was evidence for mitochondrial production of superoxide; however, these data do not rule out the possibility that FCCP affects proton uncoupling on NADPH oxidase (2). Dugan et al. showed that NMDA-induced dihydrorhodamine (DHR) oxidation may be caused by ROS generated by mitochondria because the oxidation was inhibited by mitochondrial complex I (rotenone) and III (antimycin A) inhibitors (22). Interestingly, activation of NMDA receptors through the application of glutamate seemed to be necessary; however, FCCP treatment in the presence of MK-801 and NBQX was sufficient to cause the oxidation of DHR (22). Although these reports suggest that the mitochondrial electron transport chain may be a potent source of ROS during increased Ca2+ signaling, none of these reports distinguish the effects of high Ca2+ and ROS generation during the induction of synaptic plasticity from those underlying neurotoxicity and apoptotic signaling. Moreover, mice that overexpress SOD-2, which should scavenge superoxide produced by mitochondria, exhibit normal hippocampal LTP and memory (Hu and Klann, unpublished observations). Finally, there is much evidence suggesting that mitochondrial production of ROS is tightly regulated and that increased ROS production by the mitochondria quickly produces oxidative disease states, including the induction of apoptosis and neurodegeneration. Thus, at this time, the evidence suggests that mitochondria are not a likely source of ROS signaling during synaptic plasticity and memory.
Monoamine oxidase and cyclooxygenase
Monoamine oxidase and cyclooxygenase also are potential sources, albeit indirectly, of ROS that could be involved in synaptic plasticity and memory. In cerebellar granule cells a cyclooxygenase inhibitor (indomethican) and a monoamine oxidase inhibitor (nialamide) blocked NMDA- and kainic acid (KA)-induced ROS production, which suggests that the oxidative-metabolic activity of each of these enzymes may be responsible for the generation of ROS (14). Rotenone did not block either NMDA or KA-induced ROS production, which suggests that mitochondria are not the source of ROS as assessed in this system (14). In addition, the phorbol ester phorbol-12-myristate-13-acetate (PMA), which is a potent stimulator of NADPH oxidase, stimulated ROS production in a mechanism that was different than NMDA- or KA-induced ROS production because indomethican, nialamide, and rotenone were unable to block PMA-stimulated ROS production (14). These data indicate that there may be as many as three different sources of ROS in cerebellar granule cells. Further investigation into the potential role of monoamine oxidase in ROS generation during synaptic plasticity may lead to important insights regarding psychiatric disease, given the known effects of monoamine oxidase inhibitors on psychiatric disorders such as major depression (83).
Nitric oxide synthase
Nitric oxide synthase is well known for its role in generating nitric oxide (NO) gas, which has been observed to be a critical signaling molecule involved in synaptic plasticity and memory (97, 125). However, NOS may also be an important source of ROS for the molecular events required for LTP and memory formation. For example, purified NOS can generate superoxide that is dependent on Ca2+ and calmodulin (90). Interestingly, l-NAME, but not l-NMMA, could block the superoxide generation by NOS (90). In addition, it was shown that NOS could generate H2O2 under a variety of conditions (33). Specifically, low l-arginine and low pH each were shown to independently promote the generation of H2O2 production by purified NOS (33). On the other hand, H4-biopterin inhibited H2O2 and promoted NO production by NOS (33). Importantly, only certain NOS inhibitors inhibited NO and H2O2 formation. For instance, l-NNA, l-NAME, and l-NMMA all inhibited NO formation; however, only l-NNA and l-NAME also blocked H2O2 formation by NOS, and L-NMMA failed to block H2O2 formation (33, 89). Further evidence for NOS generation of superoxide has come from experiments using cultures of primary cerebellar granule neurons, where glutamate receptor stimulation induced NOS-dependent production of superoxide if the cultures were pre-treated with arginase (18).
NOS contains two enzymatic domains, one that generates NO and another that contains NADPH oxidase activity. NOS expression was shown to result in increased ERK activation that was inhibited by co-transfection of SOD (115), which suggested a role for either superoxide or H2O2 in NOS-mediated ERK activation. Interestingly, a mutation in NOS that rendered the NO synthase portion of the enzyme incompetent, yet retained NADPH oxidase activity, had no effect on ERK activation. In contrast, deletion of the NADPH-binding region of NOS blocked NOS-dependent ERK activation (115). These results suggest that NOS may generate superoxide that is critical for signal transduction cascades that is separable from NO generation.
Experiments that use various pharmacological agents to inhibit NOS activity often fail to discriminate between the specific roles that each of these domains play. For instance, l-NAME, which is often proposed to inhibit NO generation by NOS, is actually a very good inhibitor of NOS-mediated NADPH oxidation and superoxide generation (89). In addition, l-NMMA was shown to have little to no effect on the detection of the superoxide-dependent formation of DMPO-OOH, a superoxide-dependent electron spin-adduct, via neuronal NOS (89) suggesting that this pharmacological agent is good at dissecting the dual enzymatic function of NOS. Furthermore, diphenylene iodonium (DPI) is often used as an NADPH oxidase inhibitor (77); however, DPI also was shown to inhibit the effect of l-arginine inhibition of NOS-mediated superoxide formation (89), likely via interaction with the NADPH oxidase domain of the NOS enzyme. Unfortunately, many of the experiments that implicate generation of NO by NOS in synaptic plasticity use pharmacological inhibitors of NOS that fail to distinguish the enzymatic generation of NO from NOS from the generation of superoxide by NOS. There is substantial evidence that NO is an important signaling molecule during synaptic plasticity (125), but the potential role of superoxide generated by NOS is relatively unexplored.
Taken together, these reports suggest the possibility that NADPH oxidation by NOS could be an important source of ROS that is independent of NO generation during synaptic plasticity and memory formation. However, there is another enzyme complex whose primary enzymatic activity is the oxidation of NADPH and concomitant production of superoxide. The role of NADPH oxidase in the generation of superoxide will be the focus of the remainder of this review.
NADPH oxidase
The NADPH oxidase complex is a plausible source of ROS in synaptic plasticity and memory that previously has been considered primarily as a generator of superoxide in non-neuronal cells, including immune system cells (21, 76), endothelial cells (37), and glia (21, 124). NADPH oxidase has been shown to be well regulated, such that a burst of superoxide could be generated in response to particular stimuli, which could subsequently be turned off; thus, generation of ROS via the NADPH oxidase system is rapid, well controlled, and specific to particular signaling events (91). Interestingly, several of the activators and effectors of the NADPH oxidase complex also have been implicated in signal transduction mechanisms that underlie synaptic plasticity and memory formation. Moreover, recent evidence has been provided that directly support a role of NADPH oxidase-dependent superoxide generation during brain function, which may explain why human patients with mutations in genes encoding subunits in the NADPH oxidase complex display mild cognitive deficits (82).
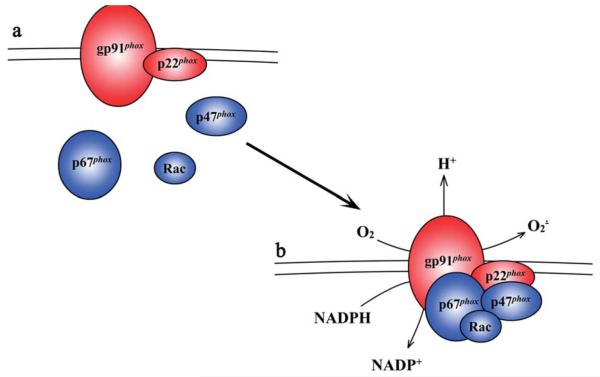
The structure and regulation of the NADPH oxidase have been well studied and extensively reviewed elsewhere (105). Briefly, the NADPH oxidase complex consists of five subunits, three cytosolic (p67phox, p47phox, and rac), and two membrane-spanning (gp91phox and p22phox). The membrane-spanning components exist as a heterodimer; gp91phox is the catalytic subunit that is responsible for the transfer of electrons between NADPH to molecular oxygen, as well as the H+ conductance that has been associated with this process. The regulation of NADPH oxidase activity is mediated through complex interactions between the cytosolic and membrane-associated components. Translocation of p67phox and GTP-bound active Rac to the membrane are essential for the activation of gp91phox-mediated electron transfer. p47phox, once phosphorylated, acts as an “organizer” of the complex and mediates correct positioning and association of the p67phox “activator” subunit to the gp91phox and p22phox heterodimer. Thus, translocation of all the cytosolic subunits in response to specific stimuli is required for the full activation of the NADPH oxidase complex. A model of NADPH oxidase is shown in Fig. 1.
FIG. 1. Subunit composition and activation of the NADPH oxidase complex.
(A) The NADPH oxidase complex consists of two membrane bound subunits (gp91phox and p22phox) and three cytosolic subunits (p67phox, rac, and p47phox), which upon activation translocate and associate with the membrane bound subunits (B). Upon activation, the NADPH oxidase complex transfers electrons from NADPH substrate to molecular oxygen, thus producing superoxide. During this process NADPH oxidase also pumps protons across the membrane.
Interestingly, many of the signaling agents involved in LTP and memory formation also regulate NADPH oxidase activity. The transcription-dependent regulation of NADPH oxidase subunits implicates transcription factor components known to be important in the regulation of LTP and memory formation. Specifically, treatment of murine monocytic cell with lipopolysacharride (LPS) and interferon-γ(IFN-γ) resulted in the increased expression of gp91phox mRNA and protein that was dependent on NF-κB (5), which has been shown to be an important transcription factor involved in LTP and memory (4, 27, 122). More near term signaling events also have implicated a potential role for NADPH oxidase in the generation of ROS-mediated signaling in neurons.
The phorbol ester PMA is a widely used compound that induces NADPH oxidase activation via the phosphorylation of p47phox through activated PKC (19). In cerebellar granule cells, PMA stimulated ROS production (14) and in hippocampal slices, PMA treatment led to a LTP-like potentiation that is dependent on PKC activation (55). Furthermore, phospholipase A2 (PLA2)-dependent genesis of arachidonic acid also was shown to induce the activation of NADPH oxidase in intact neutrophils (69). Consistent with the possibility that this type of signaling might be involved in synaptic plasticity, hippocampal slices treated with arachidonic acid during a brief train of tetanization resulted in an LTP-like potentiation (78). Moreover, NMDA applied to cultured cerebellar granule cells led to the generation of superoxide that was mimicked by the application of arachidonic acid and inhibited by mepacrine, a PLA2 inhibitor (58). In addition, NMDA- and glutamate-induced oxidation of dichlorofluorescin (DCF) in cerebellar granule cells could be blocked via PLA2 inhibition (31). The PI3 kinase-Akt pathway is another kinase signaling pathway that plays a critical role in synaptic plasticity (45, 74, 114) and has been shown to be an important activator of NADPH oxidase in non-neuronal cells (16, 102).
Not only have upstream activators of NADPH oxidase been shown to be important regulators of plasticity and memory formation, but downstream effectors of NADPH oxidase-generated ROS in non-neuronal cells also parallel important signaling pathways involved in synaptic plasticity and memory. For instance, NADPH oxidase generated ROS have been shown in T-cells to regulate phosphorylation and activation of the ERK signal transduction pathway (38), which is a critical signaling pathway in LTP and memory formation. The evidence above is consistent with the hypothesis that NAPDPH oxidase is an important source of ROS in signal transduction pathways in the brain. More direct evidence recently has been provided that implicates NADPH oxidase in normal brain function.
NADPH OXIDASE EXPRESSION IN THE BRAIN
Consistent with an important function for the NADPH oxidase complex in the central nervous system, all components of the complex, including the various homologs of specific subunits are expressed in various regions throughout the brain (10, 17, 73, 93, 101, 108, 113). Serrano et al has shown that mouse hippocampi are immunoreactive for gp91, p47, p67, p40, and p22phox proteins (73, 101) and that p47phox and gp91phox immunoreactivity was observed in pyramidal neurons in area CA1 (101). Furthermore, the NADPH oxidase subunits gp91phox and p67phox also have been found in synaptosomal fractions prepared from the whole brain and the hippocampus (108), suggesting a localized distribution that is consistent with a role for NADPH oxidase in synaptic plasticity. Interestingly, gp91phox and p47phox proteins also were found in several other areas of the brain including the cortex, habenula, paraventricular thalamic nucleus, anterior and posterior basolateral nucleus, basomedial nucleus of the amygdala, and striatum (101).
As discussed earlier, gp91phox is the catalytic subunit of NADPH oxidase that is responsible for the transfer of electrons between NADPH to molecular oxygen. Recently, several homologs of gp91phox have been described, some of which also are expressed in the brain. In addition to gp91phox (also referred to as NOX-2), NOX-4 (17, 113), and NOX-5 (17) have been shown by rt-PCR to be expressed in the adult brain and NOX-4 was detected using in situ hybridization in the mouse cortex, cerebellum, and pyramidal cells of hippocampus (113). Furthermore, NOX-3 was shown to be highly expressed in the inner ear by rt-PCR and by in situ hybridization (10). p47phox and p67phox and their respective homologs also were detected in the brain using rt-PCR analysis (10) and by Northern blot analysis (73). Rao et al. also showed that NADPH oxidase is expressed and functional in lens epithelium (93). Thus, there are likely to be multiple homologs of gp91phox. Whether the other NOX proteins are regulated in a similar manner to gp91phox and whether they are critical for ROS signaling in the brain remains to be determined.
The expression pattern of NADPH oxidase suggests that it may be involved in ROS-dependent signaling throughout the brain. Consistent with this notion, it was shown in cultured hippocampal neurons that PMA could stimulate the redistribution of the cytosolic subunits of the NADPH oxidase complex to the membrane (108). Also in hippocampal slices, PMA induced the generation of superoxide that was inhibited by either DPI or AEBSF (108), two pharmacological inhibitors of the NADPH oxidase complex (20, 77). Furthermore, stimulation of the cellular prion protein (PrPc) was shown in a number of neuronal and non-neuronal cell lines to lead to the activation of NADPH oxidase, which induced the activation of the MEK-ERK pathway in an NADPH oxidase- and ROS-dependent manner (96). Thus, a functional NADPH oxidase is expressed in the brain, suggesting that this superoxide-generating complex may be involved in signaling, synaptic plasticity, and memory.
NADPH OXIDASE-MEDIATED SIGNALING IN THE BRAIN
NMDA receptor-dependent activation of ERK is well known to be involved in various forms of synaptic plasticity and memory (88, 106, 107). Consistent with a role for NADPH oxidase in mediating this type of signaling, DPI, an NADPH oxidase inhibitor, was shown to inhibit NMDA receptor-mediated ERK activation (47). Moreover, mice that lacked the p47phox subunit (39) also lacked the NMDA receptor-dependent activation of ERK (47). These findings are consistent with previous reports that have implicated NADPH oxidase activity with MEK-ERK signal transduction in non-neuronal cells (38, 96). However, it was unclear from these studies whether the response to NMDA receptor activation was one typical of synaptic plasticity or one typical of neurotoxicity. However, a recent series of studies with NADPH oxidase mutant mice indicate that this enzyme is indeed critical for synaptic plasticity.
A ROLE FOR NADPH OXIDASE IN HIPPOCAMPAL SYNAPTIC PLASTICITY AND MEMORY
Recent studies with pharmacological inhibitors of NADPH oxidase as well as studies with mutant mice that are genetically-deficient for either gp91phox (87) or p47phox (39) indicate that NADPH oxidase is involved in LTP. Two pharmacological inhibitors of the NADPH oxidase complex, DPI and apocycnin (77, 84), blocked early-phase LTP (E-LTP) and mutant mice that lacked either the gp91phox or p47phox subunits also expressed deficient E-LTP. Interestingly slices from gp91phox KO mice and slices from wild-type mice treated with DPI also expressed deficient post-tetanic potentiation (PTP),which is a form of NMDA receptor-independent short-term plasticity (46). Other forms of presynaptic plasticity were normal in both p47phox KO and gp91phox KO mice (46). Thus, NADPH oxidase appears to be required for E-LTP.
Behavioral studies with the NADPH oxidase mutant mice indicate that superoxide produced by this enzyme may have an important role in hippocampus-dependent learning and memory. Consistent with the idea that NADPH oxidase-generated superoxide is necessary for learning and memory, it was shown that gp91phox knockout mice displayed mild deficits in the Morris water maze, and that p47phox knockout mice displayed deficits in the contextual fear conditioning (46). Interestingly, these mice also showed differences in the accelerating rotating rod apparatus and in the open field analysis, suggesting that areas other than the hippocampus may be affected in these mutant animals (46). Thus, in addition to its role in hippocampal LTP, NADPH oxidase appears to play a role in several types of hippocampus-dependent memory.
CONCLUSIONS AND FUTURE DIRECTIONS
Here we have discussed evidence that ROS, including superoxide and H2O2, are important signaling molecules in a variety of neuronal and non-neuronal systems. Importantly, ROS have been shown to be critical signaling molecules underlying fundamental cognitive functions including learning and memory (Fig. 2). This is atypical of previous views that placed ROS in a class of oxidatively destructive molecules that when produced led to toxic processes underlying cellular degeneration and apoptosis (8). We also have presented evidence that NADPH oxidase is likely an important source of ROS in the brain. This is evidenced by the fact that NADPH oxidase has been shown to be required for biochemical signal transduction cascades, synaptic plasticity, and cognitive behaviors involved in the formation and expression of memory. The relatively small amount of research aimed at determining the role of NADPH oxidase in brain function already has uncovered interesting results that warrant further investigation.
FIG. 2. Schematic depicting the role of ROS in the molecular mechanisms underlying cognition.

(A) Low concentrations of ROS are required for signaling, synaptic plasticity, and memory formation; however, as the concentration of ROS increases, their function switches from a signaling molecule to an inhibitory or even toxic molecule. (B) On the left: superoxide and H2O2 activate protein kinase C (PKC) and extracellular-signal regulated kinase (ERK) and oxidize neurogranin (NG), which then releases calmodulin, resulting in the activation of calcium/calmodulin-dependent protein kinase II (CaMKII). On the right: superoxide and H2O2 also inhibit calcineurin (a.k.a. protein phosphatase 2B, PP2B). Activation of PKC, ERK, and CaMKII promote LTP, whereas the activity of PP2B tends to block LTP; thus activation of PKC, ERK, and CaMKII, along with the inhibition PP2B are all plausible, redox-sensitive, mechanisms by which ROS could promote synaptic plasticity in a concentration-dependent and cellular signaling state-dependent manner.
NADPH oxidase was shown to be required for hippocampus-dependent learning and memory, as well as for normal performance in behavioral paradigms that require other brain regions. This is consistent with the observation that ROS-dependent learning and memory has a motivational component (61) and that subunits of the NADPH oxidase complex are expressed in brain regions other than the hippocampus, including the cortex, cerebellum, striatum, and amygdala (101). Future research into the role that NADPH oxidase plays in cognitive function should address the role of ROS signaling in these other brain regions as well.
Interestingly, there are several homologs of the main catalytic subunit gp91phox that have been shown to be expressed in various regions of the brain, which include the cortex and the hippocampus. These other NADPH oxidase subunit homologs may be important for plasticity and cognition. Interestingly, it is known that each of these homologs require different upstream signals for oxidase activation. For example, NOX-5 contains EF-hand regions that respond directly to Ca2+ influx (11). Determining the role that the various NADPH oxidase subunit homologs play in synaptic plasticity and memory should be an important goal for future investigations.
Not only is it important to determine the role of NADPH oxidase in generating ROS involved in plasticity and cognition, but it will be equally important to determine the role of other sources of ROS in these processes. We have mentioned several other potential sources of ROS that have been implicated in signal transduction and synaptic plasticity. Future research should address the distinct roles each of these sources of ROS play in mediating the molecular signaling underlying synaptic plasticity and memory.
An important issue that has been addressed only sparingly is the identity of the relevant targets of ROS signaling during synaptic plasticity and memory. As summarized in Fig. 2, several studies point directly to activation and inactivation of various kinases and phosphatases (44, 52, 54, 55, 109, 110). Although there have been few reports in neuronal systems, direct oxidative modification of ion channels, including voltage-gated Ca2+ channels and the ryanodine receptor have been shown to regulate the levels of intracellular Ca2+ (28, 63). Also shown to be an important signaling target of ROS are redox-sensitive transcription factors such as NF-kB (40). The elucidation of all of the targets of ROS signaling, in addition to the sources of ROS responsible for redox regulation of these targets, will be critical in understanding the roles that these highly reactive molecules play in normal cognitive function, and importantly, how perturbation of these signaling systems could lead to alterations in cognition, including neurodegenerative conditions mediated by oxidative stress.
We have argued that ROS signaling plays an important and necessary role in synaptic plasticity and memory formation. Moreover, NADPH oxidase is likely to be one of the important sources of ROS mediating these effects. Two key features make NADPH oxidase an attractive candidate for an ROS source in these physiological processes. First, the enzymatic complex generates large amounts of superoxide quickly, and second, can do so in a well-regulated manner. Dysfunction in either of these aspects could lead to neuronal dysfunction, as well as potential cognitive problems. Recent work indicates that not generating enough superoxide via NADPH oxidase leads to deficient synaptic plasticity and cognitive function in mice (46). Interestingly, human patients with mutations that render NADPH oxidase inactive also may express mild cognitive deficits (82). One can imagine that if NADPH oxidase regulatory mechanisms were altered, especially the mechanisms responsible for shutting down superoxide production, the well-known destructive role that ROS are known for in the brain could be fulfilled. Exuberant ROS generation could quickly lead to oxidative stress and subsequent neuronal dysfunction and damage. A fuller understanding of ROS signaling may lead to a better understanding of the degenerative mechanisms that underlie disorders such as Parkinson disease (57) and Alzheimer’s disease (1) that may be in part caused by aberrant ROS generation. Thus, understanding the regulatory mechanisms that underlie NADPH oxidase-mediated signaling in the brain, as well as the regulation of other potential sources of ROS, should be an imperative for future research.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants NS034007 (EK), NS047384 (EK), and NS047852 (KTK), and an Investigator-Initiated Research Grant from the Alzheimer’s Association (EK).
ABBREVIATIONS
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- CaM
Ca2+-calmodulin
- CaMKII
CaM-dependent kinase II
- DCF
dichlorofluorescin
- DHR
dihydrorhodamine
- DPI
diphenylene iodonium
- EC-SOD
extracellular superoxide dismutase
- E-LTP
early phase long-term potentiation
- ERK
extracellular-signal regulated kinase
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- fEPSPs
field-excitatory postsynaptic potentials
- H2O2
hydrogen peroxide
- HFS
high frequency stimulation
- IFN-γ
interferon-gamma
- KA
kainic acid
- l-NAME
l-N(G)-nitroarginine methyl ester
- l-NMMA
N-G-monomethyl-l-arginine acetate
- l-NNA
N-l-nitroarginine
- LPS
lippopolysacharride
- LTP
long-term potentiation
- NG
neurogranin
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthase
- PKC
protein kinase C
- PLA2
phospholipase A2
- PMA
phorbol 12-myristate 13-acetate
- PP2B
protein phosphatase 2B or calcineurin
- PrPc
cellular prion protein
- PTP
post-tetanic potentiation
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TPEN
N,N,N′,N′,-tetrakis(2-pyridylmethyl) ethylenediamine
- X/XO
xanthine/xanthine oxidase
REFERENCES
- 1.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742:81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlin A, De Boer M, Roos D, Leusen J, Smith CI, Sundin U, Rabbani H, Palmblad J, Elinder G. Prevalence, genetics and clinical presentation of chronic granulomatous disease in Sweden. Acta Paediatr. 1995;84:1386–1394. doi: 10.1111/j.1651-2227.1995.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 4.Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 6.Atkins CM, Davare MA, Oh MC, Derkach V, Soderling TR. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J Neurosci. 2005;25:5604–5610. doi: 10.1523/JNEUROSCI.5051-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 8.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497:1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 9.Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU, Sorrentino V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J. 1999;18:5264–5273. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 11.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 12.Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldyrev AA, Carpenter DO, Huentelman MJ, Peters CM, Johnson P. Sources of reactive oxygen species production in excitotoxin-stimulated cerebellar granule cells. Biochem Biophys Res Commun. 1999;256:320–324. doi: 10.1006/bbrc.1999.0325. [DOI] [PubMed] [Google Scholar]
- 15.Brewer GJ. Neuronal plasticity and stressor toxicity during aging. Exp Gerontol. 2000;35:1165–1183. doi: 10.1016/s0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, McLeish KR. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 18.Culcasi M, Lafon-Cazal M, Pietri S, Bockaert J. Glutamate receptors induce a burst of superoxide via activation of nitric oxide synthase in arginine-depleted neurons. J Biol Chem. 1994;269:12589–12593. [PubMed] [Google Scholar]
- 19.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 20.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- 21.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 22.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 24.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 25.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 26.Freudenthal R, Romano A. Participation of Rel/NF-kappaB transcription factors in long-term memory in the crab Chasmagnathus. Brain Res. 2000;855:274–281. doi: 10.1016/s0006-8993(99)02358-6. [DOI] [PubMed] [Google Scholar]
- 27.Freudenthal R, Romano A, Routtenberg A. Transcription factor NF-kappaB activation after in vivo perforant path LTP in mouse hippocampus. Hippocampus. 2004;14:677–683. doi: 10.1002/hipo.20020. [DOI] [PubMed] [Google Scholar]
- 28.Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- 29.Gahtan E, Auerbach JM, Groner Y, Segal M. Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci. 1998;10:538–544. doi: 10.1046/j.1460-9568.1998.00058.x. [DOI] [PubMed] [Google Scholar]
- 30.Ginn-Pease ME, Whisler RL. Redox signals and NF-kappaB activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 31.Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- 32.Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 33.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidalgo C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: cellular redox sensors? IUBMB Life. 2005;57:315–322. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- 35.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang KP, Huang FL, Li J, Schuck P, McPhie P. Calcium-sensitive interaction between calmodulin and modified forms of rat brain neurogranin/RC3. Biochemistry. 2000;39:7291–7299. doi: 10.1021/bi000336l. [DOI] [PubMed] [Google Scholar]
- 37.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 38.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 39.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 41.Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23:269–76. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamsler A, Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 44.Kanterewicz BI, Knapp LT, Klann E. Stimulation of p42 and p44 mitogen-activated protein kinases by reactive oxygen species and nitric oxide in hippocampus. J Neurochem. 1998;70:1009–1016. doi: 10.1046/j.1471-4159.1998.70031009.x. [DOI] [PubMed] [Google Scholar]
- 45.Karpova A, Sanna PP, Behnisch T. Involvement of multiple phosphatidylinositol 3-kinase-dependent pathways in the persistence of late-phase long term potentiation expression. Neuroscience. 2006;137:833–841. doi: 10.1016/j.neuroscience.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 49.Klann E, Chen SJ, Sweatt JD. Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci USA. 1993;90:8337–8341. doi: 10.1073/pnas.90.18.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 51.Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273:4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- 52.Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 53.Knapp LT, Kanterewicz BI, Hayes EL, Klann E. Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem Biophys Res Commun. 2001;286:764–770. doi: 10.1006/bbrc.2001.5448. [DOI] [PubMed] [Google Scholar]
- 54.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 55.Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 57.Kostrzewa RM, Kostrzewa JP, Brus R. Neuroprotective and neurotoxic roles of levodopa (L-DOPA) in neurodegenerative disorders relating to Parkinson’s disease. Amino Acids. 2002;23:57–63. doi: 10.1007/s00726-001-0110-x. [DOI] [PubMed] [Google Scholar]
- 58.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 59.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, Crapo JD. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28:381–390. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- 61.Levin ED, Brucato FH, Crapo JD. Molecular overexpression of extracellular superoxide dismutase increases the dependency of learning and memory performance on motivational state. Behav Genet. 2000;30:95–100. doi: 10.1023/a:1001947003299. [DOI] [PubMed] [Google Scholar]
- 62.Levin ED, Christopher NC, Lateef S, Elamir BM, Patel M, Liang LP, Crapo JD. Extracellular superoxide dismutase overexpression protects against aging-induced cognitive impairment in mice. Behav Genet. 2002;32:119–125. doi: 10.1023/a:1015201823417. [DOI] [PubMed] [Google Scholar]
- 63.Li A, Segui J, Heinemann SH, Hoshi T. Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J Neurosci. 1998;18:6740–6747. doi: 10.1523/JNEUROSCI.18-17-06740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Pak JH, Huang FL, Huang KP. N-methyl-d-aspartate induces neurogranin/RC3 oxidation in rat brain slices. J Biol Chem. 1999;274:1294–1300. doi: 10.1074/jbc.274.3.1294. [DOI] [PubMed] [Google Scholar]
- 65.Llansola M, Saez R, Felipo V. NMDA-induced phosphorylation of the microtubule-associated protein MAP-2 is mediated by activation of nitric oxide synthase and MAP kinase. Eur J Neurosci. 2001;13:1283–1291. doi: 10.1046/j.0953-816x.2001.01497.x. [DOI] [PubMed] [Google Scholar]
- 66.Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 67.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 68.Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 69.Maridonneau-Parini I, Tauber AI. Activation of NADPH-oxidase by arachidonic acid involves phospholipase A2 in intact human neutrophils but not in the cell-free system. Biochem Biophys Res Commun. 1986;138:1099–1105. doi: 10.1016/s0006-291x(86)80395-3. [DOI] [PubMed] [Google Scholar]
- 70.Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- 71.Medina JH, Izquierdo I. Retrograde messengers, long-term potentiation and memory. Brain Res Brain Res Rev. 1995;21:185–194. doi: 10.1016/0165-0173(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 72.Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 73.Mizuki K, Kadomatsu K, Hata K, Ito T, Fan QW, Kage Y, Fukumaki Y, Sakaki Y, Takeshige K, Sumimoto H. Functional modules and expression of mouse p40(phox) and p67(phox), SH3-domain-containing proteins involved in the phagocyte NADPH oxidase complex. Eur J Biochem. 1998;251:573–582. doi: 10.1046/j.1432-1327.1998.2510573.x. [DOI] [PubMed] [Google Scholar]
- 74.Mizuno M, Yamada K, Takei N, Tran MH, He J, Nakajima A, Nawa H, Nabeshima T. Phosphatidylinositol 3-kinase: a molecule mediating BDNF-dependent spatial memory formation. Mol Psychiatry. 2003;8:217–224. doi: 10.1038/sj.mp.4001215. [DOI] [PubMed] [Google Scholar]
- 75.Namgaladze D, Hofer HW, Ullrich V. Redox control of calcineurin by targeting the binuclear Fe(2+)-Zn(2+) center at the enzyme active site. J Biol Chem. 2002;277:5962–5969. doi: 10.1074/jbc.M111268200. [DOI] [PubMed] [Google Scholar]
- 76.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 77.O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Odell EW, Segal AW. Killing of pathogens associated with chronic granulomatous disease by the non-oxidative microbicidal mechanisms of human neutrophils. J Med Microbiol. 1991;34:129–135. doi: 10.1099/00222615-34-3-129. [DOI] [PubMed] [Google Scholar]
- 79.Onuma H, Lu YF, Tomizawa K, Moriwaki A, Tokuda M, Hatase O, Matsui H. A calcineurin inhibitor, FK506, blocks voltagegated calcium channel-dependent LTP in the hippocampus. Neurosci Res. 1998;30:313–319. doi: 10.1016/s0168-0102(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 80.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, Westphal H, Huang KP. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci USA. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pao M, Wiggs EA, Anastacio MM, Hyun J, DeCarlo ES, Miller JT, Anderson VL, Malech HL, Gallin JI, Holland SM. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- 83.Pare CM. New pharmacological developments in antidepressants. Psychopathology. 1986;19(Suppl 2):103–107. doi: 10.1159/000285140. [DOI] [PubMed] [Google Scholar]
- 84.Pearse DB, Dodd JM. Ischemia-reperfusion lung injury is prevented by apocynin, a novel inhibitor of leukocyte NADPH oxidase. Chest. 1999;116:55S–56S. doi: 10.1378/chest.116.suppl_1.55s. [DOI] [PubMed] [Google Scholar]
- 85.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 86.Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 88.Poser S, Storm DR. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. Int J Dev Neurosci. 2001;19:387–394. doi: 10.1016/s0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 89.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 90.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 91.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 92.Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 93.Rao PV, Maddala R, John F, Zigler JS., Jr Expression of non-phagocytic NADPH oxidase system in the ocular lens. Mol Vis. 2004;10:112–121. [PubMed] [Google Scholar]
- 94.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rusnak F, Reiter T. Sensing electrons: protein phosphatase redox regulation. Trends Biochem Sci. 2000;25:527–529. doi: 10.1016/s0968-0004(00)01659-5. [DOI] [PubMed] [Google Scholar]
- 96.Schneider B, Mutel V, Pietri M, Ermonval M, Mouillet-Richard S, Kellermann O. NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc Natl Acad Sci USA. 2003;100:13326–13331. doi: 10.1073/pnas.2235648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 98.Segal BH, Kuhns DB, Ding L, Gallin JI, Holland SM. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47(phox): complement, leukotrienes, and reactive oxidants in acute inflammation. J Leukoc Biol. 2002;71:410–416. [PubMed] [Google Scholar]
- 99.Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 102.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 103.Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch Neurol. 2001;58:874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- 104.Smythies J. Redox aspects of signaling by catecholamines and their metabolites. Antioxid Redox Signal. 2000;2:575–583. doi: 10.1089/15230860050192332. [DOI] [PubMed] [Google Scholar]
- 105.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 106.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 107.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 108.Tejada-Simon M, Serrano F, Villasana LE, Kanterewicz BI, Wu G, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neuroscience. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thiels E, Kanterewicz BI, Knapp LT, Barrionuevo G, Klann E. Protein phosphatase-mediated regulation of protein kinase C during long-term depression in the adult hippocampus in vivo. J Neurosci. 2000;20:7199–7207. doi: 10.1523/JNEUROSCI.20-19-07199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thiels E, Norman ED, Barrionuevo G, Klann E. Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience. 1998;86:1023–1029. doi: 10.1016/s0306-4522(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 111.Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 113.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 114.Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 115.Wang W, Wang S, Nishanian EV, Del Pilar Cintron A, Wesley RA, Danner RL. Signaling by eNOS through a superoxide-dependent p42/44 mitogen-activated protein kinase pathway. Am J Physiol Cell Physiol. 2001;281:C544–554. doi: 10.1152/ajpcell.2001.281.2.C544. [DOI] [PubMed] [Google Scholar]
- 116.Werner E. GTPases and reactive oxygen species: switches for killing and signaling. J Cell Sci. 2004;117:143–153. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 117.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 118.Woo NH, Abel T, Nguyen PV. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur J Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- 119.Wu J, Huang KP, Huang FL. Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+− and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J Neurochem. 2003;86:1524–1533. doi: 10.1046/j.1471-4159.2003.01963.x. [DOI] [PubMed] [Google Scholar]
- 120.Wu J, Li J, Huang KP, Huang FL. Attenuation of protein kinase C and cAMP-dependent protein kinase signal transduction in the neurogranin knockout mouse. J Biol Chem. 2002;277:19498–19505. doi: 10.1074/jbc.M109082200. [DOI] [PubMed] [Google Scholar]
- 121.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 122.Yeh SH, Lin CH, Lee CF, Gean PW. A requirement of nuclear factor-kappaB activation in fear-potentiated startle. J Biol Chem. 2002;277:46720–46729. doi: 10.1074/jbc.M206258200. [DOI] [PubMed] [Google Scholar]
- 123.Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc Natl Acad Sci USA. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zekry D, Epperson TK, Krause KH. A role for NOX NADPH oxidases in Alzheimer’s disease and other types of dementia? IUBMB Life. 2003;55:307–313. doi: 10.1080/1521654031000153049. [DOI] [PubMed] [Google Scholar]
- 125.Zorumski CF, Izumi Y. Nitric oxide and hippocampal synaptic plasticity. Biochem Pharmacol. 1993;46:777–785. doi: 10.1016/0006-2952(93)90484-e. [DOI] [PubMed] [Google Scholar]