Abstract
The cellular attachment receptor for adenovirus (Ad), Coxsackie adenovirus receptor (CAR), required for delivery of Ad into primary cells, is not present on all cell types, thus restricting Ad-gene delivery systems. To circumvent this constrain, a transgenic mouse has been generated that expresses a truncated human CAR in all tissues analyzed. These mice allowed efficient in vitro infections at low multiplicities into lymphoid, myeloid, and endothelial cells. Furthermore, in vivo administration of Ad-vectors results in infection of macrophages, lymphocytes, and endothelial cells. In addition, tail vein injection resulted in targeting of virus into previously inaccessible areas, such as the lung and the capillaries of the brain. The CAR transgenic mice will be useful for rapid functional genomic analysis in vivo, for testing the efficacy of gene therapy procedures or as a source of easily transducible cells.
Entry of subclass C, serotypes 2 and 5 adenovirus (Ad) into cells requires two receptors; a primary receptor, Coxsackie and adenovirus receptor (CAR) for attachment, and probably secondary receptors, such as the αVβ3 and αVβ5 integrins, for internalization (1). The recent cloning of the primary attachment receptor CAR (2, 3) revealed that this transmembrane protein of 46 kDa belongs to the immunoglobulin superfamily (4). Its function has not yet been elucidated, but recent data suggest that CAR may function as an adhesion molecule (5) and have tumor-inhibitory activity (6). The expression pattern of CAR varies, not only at different developmental stages and tissues, but also between species. Although CAR is abundantly expressed in epithelial cells during embryogenesis, its expression in adult mice is restricted to fewer cell types (7, 8). This is in contrast to the homogeneous expression pattern of αV-integrins (7), suggesting that limited expression of CAR influences susceptibility to Ad-infection more than that of αV-integrins (9).
Materials and Methods
Generation of Transgenic Mice.
The transgene construct pbUbiC-hCAR(1–262) contains the human ubiquitin-C promoter (10) (position −1225 to −6) and the human CAR (hCAR) gene (amino acids 1 to 262), which lacks the cytoplasmic tail. pbUbiC-hCAR(1–262) contains a rabbit β-globin splice/polyadenylation signal from the pSCT expression vector (11). The transgene was cut with XhoI and SphI, purified, and injected into fertilized oocytes as described (12). All mice used were heterozygous.
Cell Cultures.
B cell, T cell, and bone marrow cell cultures were derived from splenocytes and femurs of wild-type and transgenic mice. The red blood cells were lysed by treatment with Gey's solution. T cells were maintained for three days in α-MEM supplemented with 5% FCS (Life Technologies), 10 mm Hepes, and 1000 units/ml recombinant IL-2. B lymphocytes were grown in RPMI medium 1640 supplemented with 15% FCS (Life Technologies), 50 μM 2-mercaptoethanol, and 30 nM phorbol 12-myrisate 13-acetate (PMA) (both Sigma). Dendritic cells (DCs) were generated by culture of bone marrow cells in presence of GM-CSF and IL-4 as described (13). Seven days after culture initiation, adherent DCs were harvested and 3.0 × 106 cells were replated in 6-cm Petri dishes with supplemented DMEM containing granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-4. In vitro-maintained DCs were infected on days 8–9. Heart aorta was isolated and cultured in Ham's F-12 medium supplemented with 10% FBS. After 7–10 days, endothelial cells were trypsinized and expanded. The presence of endothelial cells in the cell cultures was checked by receptor-mediated uptake of fluorochrome-labeled acetylated low-density lipoprotein (14) (DIl-Ac-LDL, Biomedical Technologies, Stoughton, MA) and by indirect immunostaining with an anti-mouse CD31 (PECAM-1) monoclonal antibody (PharMingen). All media contained 100 units/ml penicillin/streptomycin and 2 mM glutamine (Life Technologies).
Virus Infections.
The recombinant Ads vmAdCG (AdGFP) and pTG-Z (AdLacZ) have been described (15, 16). Ad vectors were prepared by infection of HER911 cells and purified on CsCl gradients. Viral titers were determined with the cytopathic effect assay (TCID50) on HER911 cells and calculations were done according to the method of Reed and Munch (15). Nonadherent cells were resuspended in 200 μl of serum-free medium and transferred to a 5-ml polystyrene round-bottom tube, in which the designated amount of AdGFP was added. The tube was gently agitated for 25 min at room temperature followed by 25 min at 37°C with occasional shaking. Finally, the infected cells were cultured in the supplemented medium for an additional 48 h before evaluation of green fluorescent protein (GFP) expression by FACS analysis. Adherent cells were washed with PBS, covered with serum-free medium, and mixed with the designated amount of AdGFP. After 25 min of gentle agitation at room temperature and 25 min at 37°C, the virus was removed and cells were cultured with the appropriate medium for an additional 48 h before FACS analysis.
In Vivo Administration of Ad.
Mice were killed 3 days after administration of 5.0 × 1010 plaque-forming units (pfu) of AdLacZ in the tail vein. Tissues samples were stained for β-galactosidase activity and subsequently embedded, counterstained with hematoxylin and eosin, and cut into 10-μm sections for histological analysis. Mice were killed ≈30 h after intraperitoneal injection of 3.3 × 107 pfu or 108 pfu of AdGFP and cells were harvested. Adherent cells (macrophages) were separated from floating cells by incubation on plastic for 2 h.
Flow Cytometry Analyses.
The anti-mouse antibodies, B220, CD3, and CD11c [phycoerythrin (PE)-labeled] and mac-1, Gr-1, CD4, CD8, CD18, CD34, and c-Kit (FITC-labeled) were all obtained from PharMingen. The mouse monoclonal RmcB has been described (17), and the secondary FITC or PE-conjugated rabbit anti-mouse immunoglobulin antibodies were purchased from Dako (Denmark). Cells were analyzed on a FACScaliber flow cytometer and CELLQUEST software version 3.1f (both from Becton Dickinson).
Western Blotting.
Tissues were homogenized in 1% deoxycholate 1% Triton X-100 together with PMSF and protease inhibitors (complete, Boehringer Mannheim) for 1 h at 4°C and centrifuged at 20,000 × g for 15 min. The supernatant was analyzed by Western blotting as described (3, 8).
Indirect Immunostaining.
Endothelial cells were incubated with DiI-Ac-LDL diluted to 1 μg/ml in culture medium for at least 4 h at 37°C. The cells were fixed with 4% paraformaldehyde in PBS and incubated with RmcB antibody (8.3 μg/ml in PBS with 0.5% BSA) overnight at 4°C. The cells were then incubated with 1:200 dilution of FITC-conjugated anti-mouse Ig (Amersham Pharmacia) for 1 h, and examined with a confocal laser-scanning microscope (Zeiss).
Results
Broad Transgenic Expression of Truncated hCAR.
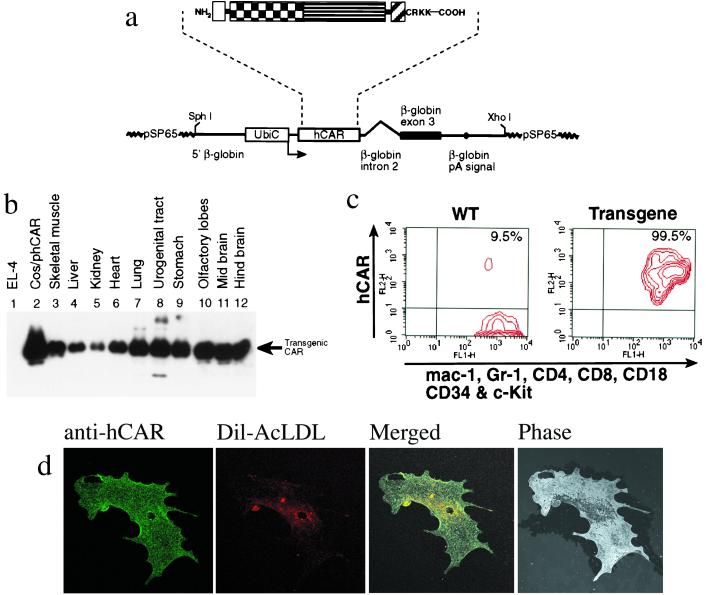
A transgenic mouse strain expressing hCAR driven by the ubiquitin-C promoter (Fig. 1a) was generated. The intracellular domain was removed because (i) it is not required for virus infection (18, 19) and (ii) it contains potential phosphorylation sites (3) that may elicit an intracellular signaling pathway. Mice were screened for the presence of the transgene by PCR and integration was confirmed by Southern blot analysis. Fifteen independent founders were identified. Male founders were used to derive several independent transgenic lines, and progenies were screened by FACS analysis and Western blotting for hCAR expression. One line expressing high levels of the transgenic hCAR was chosen for all further experiments. The animals appear healthy and have no obvious defects. The expression of the hCAR protein in different organs was monitored by Western blot. As shown in Fig. 1b, the transgenic protein was present in many organs and in addition was also detected in the thymus, intestine, esophagus, trachea, salivary glands, spleen, and bone marrow (data not shown). Cell surface expression of hCAR on some representative cell types was confirmed by FACS analysis and by indirect immunofluorescence. We found that the hCAR transgene is expressed on all cells present in bone marrow (Fig. 1c). Finally, in vitro cultured endothelial cells from the aorta showed a homogeneous staining over the entire plasma membrane (Fig. 1d).
Figure 1.
Structure of the transgene construct and hCAR protein expression pattern in different organs. (a) Outlined is the truncated hCAR (SP, signal peptide; IG1 and IG2, Ig-like domain 1 and 2, respectively; TM, transmembrane region). Note that the cytoplasmic domain has only the first four amino acids (CRKK) C-terminal to TM. Below is a schematic map of the pβUbiC-hCAR(1–262) plasmid. The human ubiquitin-C promoter and the truncated hCAR are shown as open boxes. The black box and the thick line denote the rabbit β-globin sequences, as indicated. The polyadenylation signal is indicated by a black dot. (b) The expression patterns of the hCAR transgene in different tissues was analyzed by Western blot using a mouse monoclonal antibody (RmcB) specific for the transgenic hCAR. An arrow indicates the signal corresponding to the transgenic hCAR. Lane 1, negative control of the CAR-deficient EL-4 mouse thymoma cell line. Lane 2, positive control of COS cells transfected with the hCAR expression plasmid pβUbiC-hCAR(1–262). (c) Detection of hCAR expression in transgenic bone marrow cells by flow cytometry. Bone marrow (BM) cells were depleted of the B lymphocytes by B220 immunomagnetic beads (Miltenyli Biotech, Germany). The mouse monoclonal anti-hCAR antibody RmcB and a PE-conjugated secondary rabbit anti-mouse immunoglobulin antibody were used to detect the transgene. The cells were also stained with FITC-labeled antibodies for the markers indicated at the bottom of the diagram. These markers stain all hematopoietic lineages in BM. The hCAR+ population (9.5%) detected with wild-type BM cells are B lymphocyte contaminants that escaped the immunomagnetic depletion step. (d) Indirect immunostaining of transgenic aorta endothelial cells. (Left) hCAR staining. (Left Center) Specific staining of aorta endothelial cells after receptor mediated uptake of fluorochrome-labeled acetylated low-density lipoprotein (DIl-Ac-LDL). (Right Center) Merged picture (hCAR and DIl-Ac-LDL staining). The control staining, with only FITC-conjugated anti-mouse Ig (Amersham Pharmacia) secondary antibody, was negative, as were the stainings with wild-type endothelial cells (data not shown). (Right) Same field, phase-contrast.
Efficient in Vitro Adenovirus Infection of Cells of the Hematopoietic System.
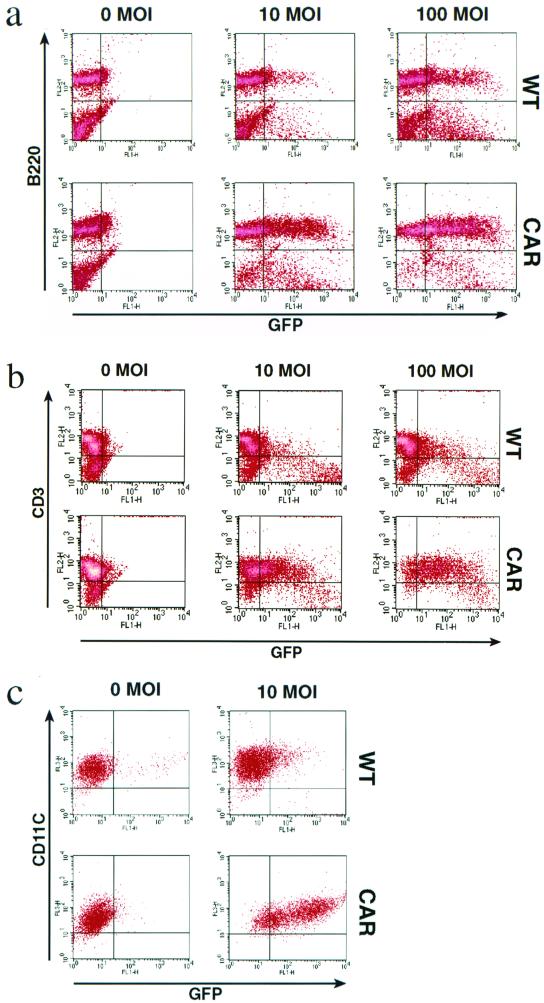
Primary lymphocytes are generally resistant to most currently available gene transfer techniques, including Ad vectors (18). The low expression levels of the CAR have been proposed to be the main reason why Ad infection of lymphocytes is inefficient (7, 18). Splenocytes stimulated with PMA or IL-2 were infected with recombinant Ad expressing GFP. As seen in Fig. 2 a and b, transduction of the transgenic cells was much more efficient. In particular, at a multiplicity of infection (MOI) of 100, 73% of the B220+ and 85% of the CD3+ transgenic cells were GFP positive, compared with 29 and 21% of the control cells, respectively. Moreover, density plot analysis shows a rightward shift of the transgenic B and T cell populations, indicating that the majority of, if not all, cells were transduced.
Figure 2.
The expression of the transgenic hCAR in B lymphocytes, T lymphocytes, and dendritic cells confers enhanced susceptibility to Ad transduction. (a) PMA-stimulated splenocytes were infected with the indicated MOI (0, 10, or 100) of AdGFP. After 48 h, the live cells were analyzed by flow cytometry for the expression of GFP and for the presence of the B lymphocyte marker B220. (Upper) wild type (WT). (Lower) hCAR transgene (CAR). Percentage of GFP-positive cells: WT; 10 MOI, 20%; 100 MOI, 38%. CAR; 10 MOI, 55%; 100 MOI, 75%. (b) IL-2-stimulated splenic T cells were infected with the indicated MOI (0, 10, or 100) of AdGFP. The live cells were analyzed by flow cytometry for the expression of GFP and for the presence of the T lymphocyte marker CD3. Percentage of GFP positive cells: WT; 10 MOI, 24%; 100 MOI, 28%. CAR; 10 MOI, 52%; 100 MOI, 85%. (c) Mature in vitro generated dendritic cells were infected with the indicated MOI (0 or10) of AdGFP. After 48 h, the live cells were analyzed by flow cytometry for the expression of GFP and for the presence of the CD11c as a marker for mature DCs. Percentage of GFP-positive cells: WT; 10 MOI, 16%. CAR; 10 MOI, 81%.
Dendritic cells are a prime target for immunization protocols such as vaccination, tolerance, and antitumor immunity (20). However, mature DCs are relatively resistant to Ad-mediated gene delivery and high viral titers (MOI >100) are required to achieve significant gene transfer because mature DCs do not express CAR (20). Approximately 80% of the CAR-transgenic DCs were infected, compared with 15% of the control cells (Fig. 2c). Again a rightward shift of GFP expression could be observed. According to published data, ≈80% of normal mature DCs can be infected but with MOIs of 100 or higher (20). Thus, the experiments with CAR-transgenic DCs show that infection can be obtained with at least 10-fold less virus particles per cell.
Ad Targeting in Vivo After Local or Systemic Application.
Ad-mediated gene transfer has limitations in vivo (7, 21, 22). However, the transgenic expression of hCAR may allow uptake of Ad vectors in tissues and organs that are not normally targeted in adult mice.
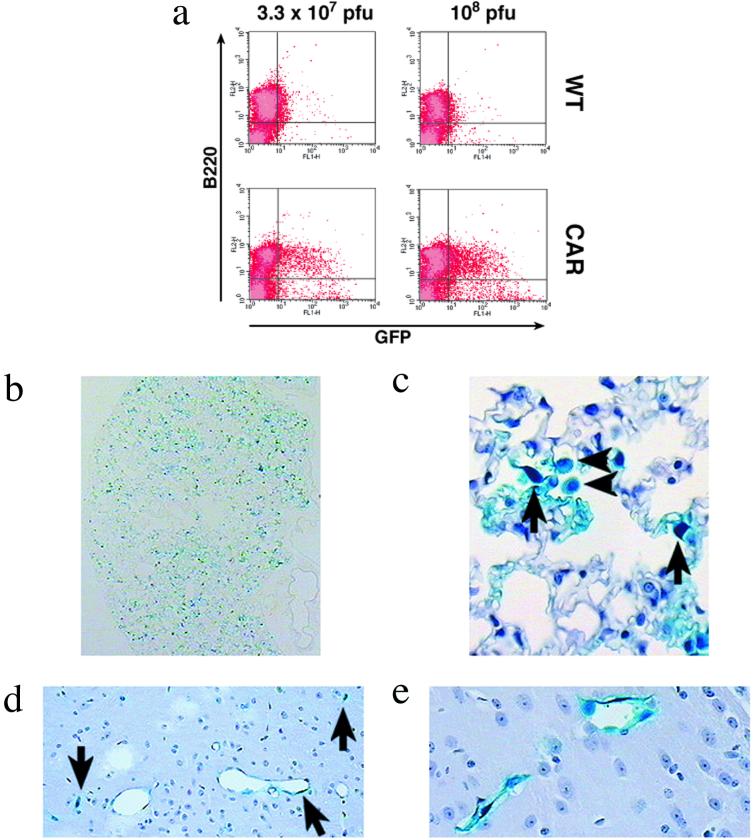
In an initial experiment, AdGFP was administrated into the peritoneal cavity of transgenic and control mice. Infection of the hCAR B1a lymphocytes was much more efficient over the two MOIs tested (Fig. 3a). As reported by others (15, 23), we found that non-transgenic macrophages are relatively susceptible to Ad infection and a significant number of cells were GFP positive. However, the number of transgenic GFP-positive macrophages was at least twice the number of GFP-positive control cells (data not shown).
Figure 3.
In vivo administration of Ad vectors. (a) Infection of B1a lymphocytes after injection of AdGFP into the peritoneal cavity. The mice were injected with the indicated amount of AdGFP. Nonadherent cells harvested from the injected mice were analyzed by flow cytometry for the expression of GFP and for the presence of the B lymphocyte marker B220. Percentage of GFP positive: 7% and 15% (Left); 5% and 28% (Right). (b–e) Histological sections of lung (b and c) and brain (d and e) after tail-vein injection of AdLacZ in the transgenic hCAR mice. (b) An overview of the distribution of β-galactosidase-expressing cells in the lung is shown. Positive, infected cells can be seen throughout the section, indicating widespread Ad uptake. (c) Higher-resolution image demonstrating cell types infected. Arrows indicate endothelial cells of septal capillaries and arrowheads indicate alveolar macrophages. (d) The blue staining for β-galactosidase expression in the endothelium of many of the cerebral capillaries (arrows) is shown. (e) Magnification of two capillaries in the plexus choroideus showing a β-galactosidase-positive signal lining the inside of the vessels. (Section b is unstained, sections c to e are counterstained with hematoxylin and eosin.)
Systemic i.v. injection in the tail vein is one of the most convenient routes of vector application, resulting in almost selective hepatic expression of the marker gene (7, 22). In an initial experiment, AdGFP was injected into the tail vein of a transgenic mouse and control mice, and GFP expression was monitored 48 h after injection. Although GFP expression was confined to the liver in wild-type mice as reported in refs. 7 and 22, the hCAR mouse displayed strong expression in several other organs, most notably in the lung. This experiment was repeated with an Ad vector expressing the β-galactosidase gene (AdLacZ) to confirm the expression patterns of the marker gene. The main differences noticed in the staining for β-galactosidase between the hCAR and the control mice were in the lung and the capillary bed of the brain. The lungs of transgenic mice displayed an irregular but intense staining of fusiform cells, the endothelial cells lining the septal capillaries (Fig. 3 b and c). Interestingly, alveolar macrophages were also found to express β-galactosidase (Fig. 3c). In contrast to the transgenic animals, pulmonary tissues of nontransgenic animals were β-galactosidase-negative. Moreover, the hCAR endothelium of cerebral capillaries was β-galactosidase-positive, but not in brain capillaries from control animals (Fig. 3 d and e). Taken together, these in vivo experiments show that the transgenic expression of hCAR leads to a change in tissue distribution of Ad vectors. The hCAR mouse allows efficient gene delivery to the lung, lung alveolar macrophages, and the capillaries of the brain.
Discussion
Possible Applications of the hCAR Mouse.
Here we describe a mouse model that facilitates efficient delivery of genes with Ad vectors. Transgenic B and T lymphocytes are now efficiently transduced with Ad. The stimulation with PMA of these lymphocytes was required to drive reporter gene expression from the cytomegalovirus (CMV) promoter of our Ad vectors. This is in line with previous reports, which have shown that stimulation of lymphocytes and other cell types is a prerequisite for successful expression from the CMV promoter/enhancer (24–26). Thus, the results are dependent not only on the susceptibility of the target cells to Ad infection, but also on the activity of the promoter that drives the marker gene. Therefore, it is possible that other promoters may allow detection of marker gene expression in other cells and organs of the hCAR mouse. Interestingly, we observed that B1a cells, when infected in vivo by AdGFP, efficiently express the marker gene without the need for stimulation.
DCs and macrophages represent an interesting target for genetic modification because of their role in immune responses and in inflammatory disorders. However, these cells are relatively resistant to Ad infection and high viral titers (MOI >100) are required to achieve efficient gene transfer (20). We found that the transgenic DCs and macrophages were more susceptible to Ad-mediated gene delivery, allowing infection at low MOIs. This low MOI could be important in preserving the normal physiology of these cells. Moreover, we were able to infect the transgenic alveolar macrophages after i.v. injection of AdLacZ. Alveolar macrophages, as reported recently (27), are difficult targets for Ad infection, even in vitro.
The peritoneal and tail vein injection experiments revealed that the transgenic expression of hCAR results in targeting of cells that are normally resistant to Ad-mediated gene delivery. To our knowledge, this is the first time that Ads after i.v. injection in the tail vein have efficiently infected the lung. Furthermore, many different cell types, such as alveolar macrophages, peritoneal macrophages, and B1a cells, have been infected in vivo. In particular, endothelial cells in various organs of the transgenic mouse seem to be susceptible to Ad transduction. This accessibility will allow for interesting gain or loss of function experiments in areas such as cardiovascular research and in the search for the underlying molecular mechanisms in the development of atherosclerosis (28). In the hCAR transgenic mice, the liver and the lung harbor the majority of tail vein-injected Ad particles. However, the broad expression of the transgene in these animals should allow for the development of other organ-specific injection protocols.
One major goal in gene therapy is the development of Ad vectors that target a gene to a specific cell type or organ (29). This requires both the introduction of tissue-specific ligands and the abrogation of binding of Ad to its cognate receptor. The hCAR transgenic mice will provide a test system to assess whether the retargeted Ad vector avoids interaction with CAR and homes to its newly generated receptor, thereby selectively localizing gene expression to the tissue of interest.
The possibilities offered by the hCAR mice to deliver Ad vectors to many different primary cells and tissues will be useful for many applications related to functional analysis of specific genes. Alternatively, crossing the hCAR mice with genetically modified mice will make it possible to test for gain of function experiments or to evaluate the introduction of an alleviating factor and its effect on a particular phenotype. Such readouts may help to dissect several cellular and signaling pathways and could serve as a screen for gene therapy candidates and for the identification of new drug targets.
Acknowledgments
We thank Dr. S. Rusconi and Dr. S. Brenz Verca for their help with the adenovirus work and for their generous gift of the vmAdCG (AdGFP) and pTG-Z (AdLacZ). We also thank Dr. P. Angel for the Ubi-JunB plasmid. We are indebted to Dr. R. Tomko, Dr. K. Sollerbrant, J. Elmén, M. L. Spångberg, A. Berg, Dr. P. Percipalle, and Dr. N. French for helpful suggestions and technical assistance. Special thanks are given to Dr. R. Feinstein, Dr. P. Höglund, C. Johansson, N. Veitonmäki, and R. Wallin. This work was supported by Pharmacia Corporation, the Swedish Foundation for Strategic Research, and the Medical Research Council to S.P. and from the Swedish CancerFonden to L.P. T.T. is supported by a fellowship from the Swiss National Science Foundation.
Abbreviations
- Ad
adenovirus
- CAR
Coxsackie and adenovirus receptor
- PMA
phorbol 12-myristate 13-acetate
- DCs
dendritic cells
- AdGFP
recombinant Ad vmAdCG
- AdLacZ
recombinant Ad pTG-Z
- PE
phycoerythrin
- hCAR
human CAR
- GFP
green fluorescent protein
- MOI
multiplicity of infection
References
- 1.Nemerow G R. Virology. 2000;274:1–4. doi: 10.1006/viro.2000.0468. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Tomko R P, Xu R, Philipson L. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrétien I, Marcuz A, Courtet M, Katevuo K, Vainio O, Heath J K, White S J, Du Pasquier L. Eur J Immunol. 1998;28:4094–4104. doi: 10.1002/(SICI)1521-4141(199812)28:12<4094::AID-IMMU4094>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, Kobayashi K, Kumanishi T, Watanabe Y G, Odani S, Kuwano R. Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 6.Okegawa T, Li Y, Pong R-C, Bergelson J M, Zhou J, Hsieh J-T. Cancer Res. 2000;60:5031–5036. [PubMed] [Google Scholar]
- 7.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R G, Van Veghel R, Schultheiss H-P, Lamers J M J. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 8.Tomko R P, Johansson C B, Totrov M, Abagyan R, Frisén J, Philipson L. Exp Cell Res. 2000;255:47–55. doi: 10.1006/excr.1999.4761. [DOI] [PubMed] [Google Scholar]
- 9.Hidaka C, Milano E, Leopold P L, Bergelson J M, Hackett N R, Finberg R W, Wickham T J, Kovesdi I, Roelvink P, Crystal R G. J Clin Invest. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schorpp M, Jäger R, Schellander K, Schenkel J, Wagner E F, Weiher H, Angel P. Nucleic Acids Res. 1996;24:1787–1788. doi: 10.1093/nar/24.9.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Severne Y, Wieland S, Schaffner W, Rusconi S. EMBO J. 1988;7:2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan B, Costantini E, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 13.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Maramatsu S, Steinman R M. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voyta J C, Via D P, Butterfield C E, Zetter B R. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heider H, Brenz Verca S, Rusconi S, Asmis R. BioTechniques. 1999;28:260–268. doi: 10.2144/00282st02. [DOI] [PubMed] [Google Scholar]
- 16.Brenz Verca S. Ph.D. thesis. Fribourg, Switzerland: Univ. of Fribourg; 1997. [Google Scholar]
- 17.Hsu K-H, Lonberg-Holm K, Alstein B, Crowell R L. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, De Gregori J. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Bergelson J M. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirk C J, Mulé J J. Hum Gene Ther. 2000;11:797–806. doi: 10.1089/10430340050015419. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Singh R, Moore M A S, Song W-R, Crystal R G. Hum Gene Ther. 1998;9:1223–1231. doi: 10.1089/hum.1998.9.8-1223. [DOI] [PubMed] [Google Scholar]
- 22.Mittal S K, McDermott M R, Johnson D C, Prevec L, Graham F L. Virus Res. 1993;28:67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- 23.Schneider S D, Rusconi S, Seger R A, Hossle J P. Gene Ther. 1997;4:524–532. doi: 10.1038/sj.gt.3300432. [DOI] [PubMed] [Google Scholar]
- 24.Löser P, Jennings G S, Strauss M, Sandig V. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M R, Piekos B, Cabatingan M S, Woodland R T. J Immunol. 2000;165:4112–4119. doi: 10.4049/jimmunol.165.7.4112. [DOI] [PubMed] [Google Scholar]
- 26.Wan Y Y, Leon R P, Marks R, Cham C M, Schaack J, Gajewski T F, DeGregori J. Proc Natl Acad Sci USA. 2000;97:13784–13789. doi: 10.1073/pnas.250356297. (First Published November 28, 2000; 10.1073/pnas.250356297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worgall S, Singh R, Leopold P L, Kaner R J, Hackett N R, Topf N, Moore M A S, Crystal R G. Blood. 1999;93:655–666. [PubMed] [Google Scholar]
- 28.Gerard R D, Collen D. Cardiovasc Res. 1997;35:451–458. doi: 10.1016/s0008-6363(97)00134-x. [DOI] [PubMed] [Google Scholar]
- 29.Wickham T J. Gene Ther. 2000;7:110–114. doi: 10.1038/sj.gt.3301115. [DOI] [PubMed] [Google Scholar]