Abstract
Head and neck cancers, most of which are squamous cell tumours, have an unsatisfactory prognosis despite intensive local treatment. This can be attributed, among other factors, to tumour recurrences inside or outside the treated area, and metastases at more distal locations. These tumours therefore require not only the standard surgical and radiation treatments, but also effective systemic modalities. The main option here is antineoplastic chemotherapy, which is firmly established in the palliative treatment of recurrent or metastatic stages of disease, and is used with curative intent in the form of combined simultaneous or adjuvant chemoradiotherapy in patients with inoperable or advanced tumour stages. Neoadjuvant treatment strategies for tumour reduction before surgery have yet to gain acceptance. Induction chemotherapy protocols before radiotherapy have to date been used in patients at high risk of distant metastases or as an aid for decision-making (“chemoselection”) in those with extensive laryngeal cancers, prior to definitive chemoradiotherapy or laryngectomy. Triple-combination induction therapy (taxanes, cisplatin, 5-fluorouracil) shows high remission rates with significant toxicity and, in combination with (chemo-)radiotherapy, is currently being compared with simultaneous chemoradiotherapy; the current gold standard with regards to efficacy and long-term toxicity.
A further systemic treatment strategy, called “targeted therapy”, has been developed to help increase specificity and reduce toxicity. An example of targeted therapy, EGFR-specific antibodies, can be used in palliative settings and, in combination with radiotherapy, to treat advanced head and neck cancers. A series of other novel biologicals such as signal cascade inhibitors, genetic agents, or immunotherapies, are currently being evaluated in large-scale clinical studies, and could prove useful in patients with advanced, recurring or metastatic head and neck cancers. When developing a lasting, individualised systemic tumour therapy, the critical evaluation criteria are not only efficacy and acute toxicity but also (long-term) quality-of-life and the identification of dedicated predictive biomarkers.
Keywords: head-neck carcinoma, squamous-cell carcinoma of the head and neck, medical therapy, chemotherapy, antibody, targeted therapy, biological
1 Introduction
German registry data indicate that the annual incidence of newly diagnosed malignant head and neck tumours is approximately 14,000 [88]. Over 90% of these are head and neck squamous cell carcinomas (HNSCC). For smaller, localised HNSCC (Stages I and II), surgery and/or radiation are the therapy of choice and outcomes are generally favourable. For patients with local or regionally advanced disease (Stage III or IV), the prognosis is much worse. Despite the use of intense, localised therapy, 50–60% of such patients have a local or regional recurrence after their first treatment course, and remote metastases appear in up to 20% [192], [108], [14]. For patients with uncontrolled local and regional metastases, effective systemic treatment is required (e.g. chemotherapy [CTX]).
2 Cytostatics
Since the introduction of cytostatic therapy in the 1940s, a number of effective anti-neoplastic agents have been synthesised or isolated from biological substances. Developments have focused on finding cytostatic or cytotoxic agents that are as selective as possible against malignant cells, but have minimal effects on healthy tissues. Since normal and malignant cells do not usually differ sufficiently for chemotherapeutic agents to have such tumour-specificity, dose-limiting side effects are often observed. The effect of most anti-neoplastic medications is based on the interaction of macromolecules that are required for maintaining cellular integrity and proliferation such as: nucleic acids, enzymes, and structural and surface proteins. Some chemotherapeutics limit their cytostatic or cytotoxic effects to single stages of the cell cycle (phase-specific agents). Others work across many phases of the cycle (non-phase-specific). In HNSCC, the most widely used chemotherapeutic agents are cisplatin, carboplatin, 5-fluorouracil, methotrexate, and the taxanes (e.g. paclitaxel and docetaxel). They are generally administered as a two-or three-agent combination, and can be curative or palliative.
The following sections summarise the use of cytostatics as single therapy or combination therapy in the neoadjuvant, induction, and adjuvant settings. Use of a newer class of anti-neoplastic agent, EGFR monoclonal antibodies (mAbs), will also be discussed. The first of these agents, cetuximab, is now licensed for the treatment of HNSCC patients. Other classes of systemic chemotherapeutic compounds that are yet to be licensed in HNSCC are listed in Figure 1 (Fig. 1).
Figure 1. Overview of systemic tumour therapies.
2.1 Individual cytostatic agents
2.1.1 Platinum derivatives
Extensive evidence shows that platinum derivatives are one of the most effective treatment classes in patients with HNSCC. Cisplatin is a planar heavy-metal complex that targets deoxyribonucleic acid (DNA) for its anti-tumour activity. Cisplatin forms many kinds of DNA adducts, 90% of which are 1,2-intrastrand cross-links, where platinum coordinates to two adjacent guanine residues or an adjacent adenine and guanine. The remaining are other intrastrand cross-links, interstrand cross-links, monofunctional adducts, or protein-DNA cross-links [177].
In 288 patients with recurring and metastasised head-neck carcinoma, cisplatin monotherapy achieved an average remission rate of 28% [7], corresponding approximately to the results achieved with the “reference” treatment of that time, methotrexate. A meta-analysis of palliative chemotherapy studies in HNSCC [29] showed that remission rates and overall survival with cisplatin monotherapy were comparable to that of methotrexate. Nausea and vomiting were observed in most patients between the standard dosage range of 50 to 120 mg/m2. Common dose-limiting side effects included nephrotoxicity, ototoxicity, and peripheral neuropathy. To increase tolerability, cisplatin-containing regimens requiring 100 mg per day, are divided into five separate cisplatin doses of 20 mg each when administered in Germany.
Carboplatin has a more favourable nephrotoxic, ototoxic, and emetogenic profile, but is more myelotoxic [34], [8], [9]. When compared to cisplatin, a higher concentration of carboplatin is required to achieve equivalent DNA binding. This has been attributed to the fact that it forms intrastrand DNA cross-links at a slower rate then cisplatin, and that the elimination constant (Km) of free platinum is 10-fold lower with carboplatin than with cisplatin [102], [64]. This means that when administered as monotherapy, comparable remission rates are achieved with a higher relative carboplatin dose of 400 mg/m2 [63]. However, when used as part of a combination CTX regimen, carboplatin appears to be inferior to cisplatin [71], [49].
2.1.2 Methotrexate
Methotrexate (MTX) is one of the early effective therapies for CTX of head-neck carcinomas. Starting from the observation that folic acid can block the growth of tumours [114], MTX was developed as a cytostatic folic acid analogue [72]. Methotrexate blocks the formation of tetrahydrofolic acid because of its high affinity for dihydrofolic acid reductase, a co-enzyme for C1-metabolism during the synthesis of nucleic acid. It also suppresses protein synthesis in the G1 phase.
High-dose MTX treatment became possible with the introduction of leucovorin rescue, which prevents normal cells being affected by MTX-induced folic acid deficiency. For recurring or highly advanced HNSCC, MTX monotherapy is associated with a remission rate of 31% [7]. High-dose MTX therapy combined with leucovorin rescue evokes a higher response rate compared to monotherapy alone, but does not significantly improve survival. Hepatotoxic, pulmotoxic, and nephrotoxic side effects and the appearance of dermatitis have been observed with MTX.
2.1.3 5-Fluorouracil
The anti-metabolite 5-fluorouracil (5-FU) was developed by Heidelberger et al. in 1957 [80], based on the observation that during DNA synthesis, the uracil base was used more effectively by tumour cells than normal cells.
Various mechanisms have been suggested to explain its anti-neoplastic effect. It is postulated that following intracellular nucleotide metabolism, 5-FU blocks the key enzyme, thymidilate synthetase [171], which leads to a reduction of desoxythymide triphosphate (dTTP), a preliminary product of DNA synthesis. Its effect also seems to be due to a direct block on RNA synthesis [164].
When used as monotherapy in HNSCC, 5-FU evokes only a moderate remission rate of about 15% [7], so a combination with cisplatin is of particular therapeutic importance [29]. The most common 5-FU-associated side effects are found in the gastrointestinal tract (stomatitis, ulceration, diarrhoea), ocular tissue (blepharitis, conjunctivitis, lacrimal-duct stenosis), and skin (dermatitis).
2.1.4 Mitomycin C
Mitomycin is an antibiotic that was isolated in the late 1950s from Streptomyces caespitosus. It inserts itself between two strands of DNA, and causes irreversible damage that triggers a cytotoxic signal cascade.
Under hypoxic conditions, mitomycin C works as an oxidant. It is a biologically active alkylating agent which exerts its effect via enzymatic reduction. It is used either as monotherapy or in combination with 5-FU. When this combination is administered concurrently with RTX, it can improve survival compared with RTX alone [31]. Mitomycin C is rapidly inactivated by enzymes in the liver, kidneys, spleen, and heart. It is excreted mainly through the kidneys. Typical side effects concern the skin (necrosis), kidneys, and lungs (fibrosis).
2.1.5 Taxanes
Taxanes are naturally occurring cytostatic compounds which have been used in cancer therapy since the early 1990s. Paclitaxel, originally obtained from the Pacific yew, was followed by the semi-synthesised docetaxel. Taxanes block cell division and tumour growth via inhibition of the spindle apparatus.
In HNSCC, docetaxel monotherapy is associated with a major response rate of 42% [59]. Side effects include nausea, vomiting, bone-marrow suppression, paraesthesias, and reversible hair loss. High remission rates have been achieved with taxanes, particularly when used in combination with 5-FU and a platin. However, when administered as induction therapy, relatively high toxicity rates have been reported [151], [187].
2.1.6 Bleomycin
The antibiotic, bleomycin, is a complex glycoprotein which is isolated from Streptomyces verticillus [182]. It binds specifically to guanine and cleaves single and double strands of DNA [130]. Its efficacy in squamous epithelial carcinoma, and its pulmonary and cutaneous side effects are due to the subsequent absence of bleomycin-inactivated hydrolase in the lungs and epithelium. During bleomycin monotherapy, remission rates of 6–45% (average 21%) have been achieved in patients who have exhausted conventional therapies [7]. Because of its low myelotoxicity, bleomycin is suitable in combination with myelosuppressive cytostatics. However, in recent times, its use has decreased significantly.
2.1.7 Vincristine
Vincristine sulphate is a natural alkaloid of the evergreen plant Cantharanthus roseus. By binding to tubulin, the spindle poison inhibits polymerisation to microtubuli and induces metaphasic arrest. Although its primary cytotoxic effect is generated by disturbing mitosis, there is also evidence that its lethal effects are evoked via other mechanisms. Vincristine-associated cytotoxic effects have been observed in non-proliferating cells in the G1 and S phases of the cell cycle [122].
In HNSCC, alkaloids are seldom used as monotherapy [169], [36], but have been included in combination regimens. Vincristine is rarely used today, due in part to its dose-limiting side effects, which include peripheral neuropathy. The third-generation derivative of vincristine, vinorelbine, is available in an oral formulation.
2.1.8 Other cytostatics
Other rarely-used cytostatics include ifosfamide (a nitrogen mustard alkylating agent), gemcitabine (an analogue of the nucleotide, cytidine), pemetrexed (a folic acid analogue which blocks thymidylate synthase, dihydrofolate reductase and glycinamide ribonucleotide formyltransferase) and oral etoposide (a topoisomerase inhibitor).
2.2 Cytostatic combinations
Since the 1990s, cytostatic combination therapies have been frequently used (Figure 2 (Fig. 2)). In addition to the classic chemotherapeutics: methotrexate, cisplatin, 5-FU, and bleomycin; taxanes, gemcitabine, vinorelbin, and oral etopocide have all been studied. CTX monotherapy usually evokes a response rate of 10–30%, but these rates increase significantly when agents are used in combination. In general, it should be taken into consideration that the efficacy of the CTX depends significantly on the stage of the disease. In randomised studies, the use of cisplatin in combination with 5-FU was the accepted reference therapy for quite some time. Triple-therapy combinations, which normally included a platin, achieved the highest response rates of up to 80%. This intensive CTX however, was also associated with significantly higher toxicity, and was therefore not widely administered. In addition, the increased response rates did not necessarily translate into an increase in survival time.
Figure 2. Historical development of multimodal cytostatic therapy from palliation to cure (adapted from Dietz et al. [53]).
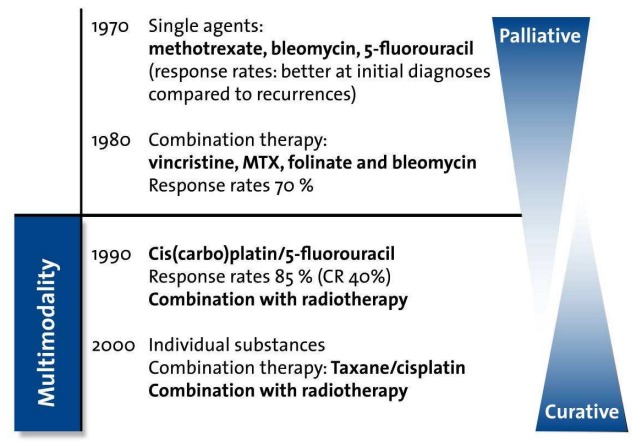
For many years, the use of a single CTX regimen (as monotherapy or as part of dual or triple therapy) was reserved for palliative care. The curative breakthrough occurred in the 1990s with the introduction of combinations comprising a platin-analogue/5-FY or mitomycin C/carboplatin with simultaneous or subsequent RTX (Figure 2 (Fig. 2)). Other combinations consist of a platin-analogue + docetaxel (neurotoxicity) ± 5-FU (mucositis, diarrhoea, gastrointestinal sepsis) ± cetuximab (see below). The evidence supporting the use of these combinations as part of a multimodal therapy approach is presented more extensively in the following chapters.
2.3 Cytostatic therapy with curative intent
The exclusive use of curative chemotherapy regimens can help to avoid the well-documented side effects of radiotherapy (e.g. mucositis and function-impairing fibrosis) and organ-ablating surgery.
Laccourreye et al. showed that non-metastatic laryngeal cancers (T1-3(4)) could be controlled solely by CTX [105], [106], with 5-year survival rates exceeding 85%. A study by Holsinger et al. involved 31 patients with T2-T4a N0-N1 tumours which were eligible for control with partial laryngeal resection [87]. For a third of these patients, the use of 3–4 cycles with paclitaxel, ifosfamide and cisplatin achieved durable disease remission with no evidence of recurrence over a median follow-up time of 5 years.
For advanced laryngeal cancers, however, a CTX-alone approach may not be appropriate. In a study of 32 patients, none of the four patients who achieved a complete histologic complete response after a single neoadjuvant cycle of CTX, were relapse-free after further CTX-only treatment [54]. Moreover, even when control of the primary tumour is achieved, cervical metastases can cause complications [54].
Overall, a curative CTX-only regimen is a potential experimental option for certain laryngeal cancers, but is far removed from the current standard. If considered at all, it should only be used in very carefully selected patients and/or for research purposes.
2.4 Chemosensitivity determination and chemoselection
The use of CTX regimens is based on empirically-collected response rates from large populations which do not differentiate tumour specificity. Tumours which appear histologically identical can respond differently to the same CTX regimen, so in this regard, the use of tumour-specific anti-oncograms, similar to those used in antibiotic therapy, could help to predict response.
As long ago as 1957, Wright et al. [209] tried to cultivate tumour cells in vitro in order to predict in vivo chemosensitivity. Various clinical studies have since been attempted, to try to dispel any general reservations about chemosensitivity predictions. The most closely aligned predictive correlation for determining in vivo chemosensitivity from in vitro results is for the clonogenic assay [200]. Von Hoff et al. [194] showed that when clonogenic assay-predicted chemosensitivity was considered, although survival times were not prolonged, the partial response rate in patients with metastatic tumours increased from 3% to 21%. To date, predictive chemosensitivity has yet to be accepted into routine clinical practice [56], [86], [51]. Various reasons exist: firstly, there are effective treatment regimens which can, if necessary, be modified within a short time; and secondly, non-chemotherapy-naïve tumours are rarely refractory to further cytostatic therapy. Therefore, the use of a predictive in vitro chemosensitivity assay will not usually convey an additional benefit.
In future, chemosensitivity testing could have an increased role as a selection criterion in patients with HNSCC (i.e. to determine whether treatment should be surgical or multimodal). This can be crucial, as although most new tumours are resectable, some surgical procedures necessitate an accompanying loss of the organ (larynx). Conversely, multimodal “organ-preserving” treatment options can belatedly cause function-impairing high toxicity levels [51]. According to current data, a satisfactory response can only be expected in about 30% of tumours, so the use of effective predictive information should help to ensure that patients receive the most suitable therapeutic intervention.
Another approach, known as “chemoselection”, consists of determining chemosensitivity in vivo. As long ago as the 1980s, HNSCC patients showed an improved response to RTX if they had previously responded to CTX induction therapy [65]. Similarly, in the early 1990s, a study that became known as the “VA Trial” (Veterans Affair Laryngeal Cancer Group), showed a clear survival advantage for patients with extensive laryngeal cancer who had responded well to 1–2 cycles of CTX induction prior to receiving radiotherapy [179].
This type of “chemoselection” was investigated by the working group from Michigan [183]. A single 5-FU/platinum cycle was used to pre-select patients with advanced laryngeal cancers for further treatment. Non-responders underwent surgery, which also helped them to avoid the complications of subsequent “salvage laryngectomy” [154], whereas patients with tumour regression underwent organ-preserving CRT. The organ preservation and survival data for the responders were so promising that further studies were performed in patients with oropharyngeal cancers. From these studies, it appears that CTX induction + CRT is most suited to patients with HPV16 (human papillomavirus)-positive tumours [104], [207], [208]. Large-scale prospective studies are now being performed to investigate the suitability of HPV, or of surrogate markers such as p16, as predictors of a response to CTX or RTX.
2.5 Induction chemotherapy
Based on the observation that tumours without prior surgical or radiotherapy treatment respond better to cytostatic therapy, induction CTX is usually administered prior to local or regional standard therapy [205], [199], [198], [74], [100], Figure 3 (Fig. 3)). This can devitalise both local and distal tumour manifestations that may not be ameliorated by localised surgery or radiation therapy, but that could potentially lead to locoregional recurrences [39]. A further advantage of induction CTX is that the initial response can help clinicians decide whether organ preservation or surgery is the most appropriate option (see 2.4 Chemosensitivity determination and chemoselection).
Figure 3. Overview of systemic cytostatic therapy.
Induction CTX is usually followed by irradiation, as contrary to lymphoproliferative diseases, the response is only temporary and must be consolidated by local measures. Induction CTX is used mainly in the course of organ-preserving therapy, particularly with laryngeal/hypopharyngeal cancers (avoidance of laryngectomy), and recently increasingly also with (HPV-positive) oropharyngeal neoplasia (preservation of the base of the tongue).
The concept of induction CTX is based mainly on the classically ground-breaking laryngeal organ-preserving studies: the VA trial [179] and the study by the European Organisation for Research and Treatment of Cancer (EORTC [112], Table 1 (Tab. 1)).
Table 1. Selected clinical studies of induction CTX with treatment arms, response rates and overall survival. The p values were given only where there were borderline significant differences.
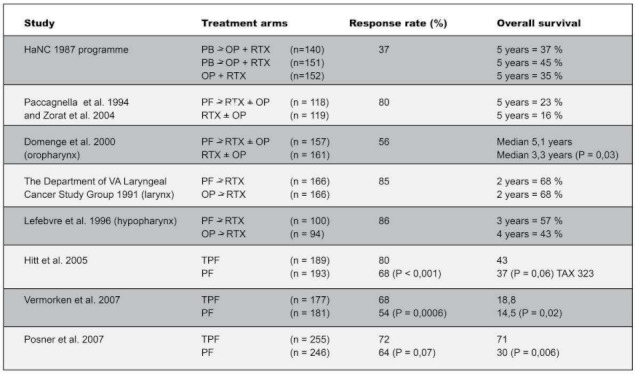
These studies showed no significant difference in survival between the two study arms a) “organ-preserving” induction CTX with platinum analogues/5-FU and RTX compared with b) “organ-ablating” surgery and adjuvant RTX. A detailed and critical description of these studies, along with analyses of quality-of-life and distant metastases, can be found in the paper by F. Wenz. Consequently, a large number of studies were performed using the dual-therapy induction regimen of cisplatin/5-FU, irrespective of tumour stage and tumour location, and predominantly in combination with conventionally fractionated RTX. This clear heterogeneity is why no clear proof could be found of any improvement in locoregional tumour control or any clear survival advantage [147].
Nevertheless, well-structured individual Phase III studies [141], [213], [57] have suggested a survival advantage of induction CTX when compared with local therapy alone (RTX ± surgery). This advantage was found to apply mainly in patients with inoperable tumours (RTX only). However, the studies were not performed using the current standard treatment for advanced unresectable tumours, which consists of simultaneous CRT. Indeed, the studies included in the meta-analysis were generally those with dual-therapy induction CTX followed by RTX. However, the MACH-NC meta-analysis [148], described extensively in the paper by F. Wenz, showed significantly improved overall survival when CRT was administered simultaneously, compared with patients treated with staged induction CTX + RTX.
In the TAX323 and TAX324 studies, the integration of a triple combination consisting of taxanes, platinum derivatives and 5-FU (TPF) followed by RTX (TAX323) or carboplatin-containing CRT (TAX324) led to renewed efforts being devoted to comparative studies [186], [151], [119]. Posner et al. [151] (TAX324) showed, in 501 patients with locally advanced HNSCC, that induction therapy with a TPF triple combination (docetaxel, cisplatin and 5-FU), compared with the “tried and tested” treatment regimen consisting of a combination of cisplatin and 5-FU (PF), produced a more than two-fold increase in median survival time (70.6 vs. 30.1 months) in a relatively cost-effective manner [143]. This was equivalent to a 30% relative reduction in the risk of death. In addition, when compared with the PF treatment regimen, TPF was associated with both a significant reduction in local tumour progression and a reduction in distant metastases [151]. However, these results must be treated with caution, since the effect was observed mainly in both elderly patients and those with oropharyngeal cancers, and the study group included about 60% primarily operable patients.
In the TAX323 study it was shown that, when compared with PF, the TPF regimen significantly prolonged mean survival, albeit to a small degree (18.6 vs. 14.6 months, p≤0.005) [186]. The additional administration of docetaxel in the induction CTX did not lead to any increase in grade 3/4 toxicity. From this, it can be deduced that the TPF regimen is the new “induction standard” in unresectable and locally advanced tumours, but that it still has to prove its worth in randomised studies versus the previous “standard treatment” of simultaneous CRT.
For this reason, comparative studies have been designed to assess whether the “new” TPF induction CTX (in combination with CRT) can improve locoregional tumour control or overall survival (e.g. SWOG Phase III trial in oropharyngeal cancer (SO427), Michigan, USA; Paradigm Phase III trial, Boston; TREMPLIN on larynx preservation, Lefebvre, Lille, France; ICRAT Phase II, Budach V, Berlin; Phase II, Padua [140]). Other clinical benefits such as reduction in distant metastases and preservation of function (speech, swallowing), e.g. through less late tissue fibrosis (the “wildfire” of simultaneous CRT), have also been assessed [101], [52]. The results of the German induction CTX initiative suggested moderate late toxicity using this approach [52]; so in order to avoid severe surgical complications after salvage laryngectomy [154], early surgical intervention shortly after the chemoselecting induction phase is recommended.
Extended induction CTX is not without its problems. Poor patient compliance directly affects survival rates, and drug-related toxicity can lead to death [149], [17]. The recent study by the German laryngeal organ preservation group (DeLOS-II, Dietz A, Leipzig) reported four treatment-related deaths with TPF (+/-cetuximab), resulting in the study being discontinued and re-designed. This study was re-initiated in Autumn 2009 after 5-FU had been eliminated from both arms because of TPF-induced neutropenia had resulted in an increased risk of sepsis. Similarly, in another German study which reported acceptable toxicity, induction therapy (TP) was given prior to CRT [167].
This led to TPF therapy being recommended in a sub-population of patients, selected on the basis of either comorbidity (Charlson scale) or ECOG performance status. Moreover, in patients with malnutrition/cancerous cachexia, a high-calorie diet was recommended; in those with febrile neutropenia, the administration of G-CSF and prophylactic antibiotic therapy (e.g. with quinolones) was recommended; and in patients with diarrhoea, loperamide administration and adequate fluid replacement were recommended. The severely vein-irritant cytostatics are generally administered via a central venous port (Figure 4 (Fig. 4)). It is hoped that new studies which are directly comparing TPF+CRT with simultaneous CRT can answer further questions about efficacy (i.e. long-term survival, organ preservation, locoregional control, and distant metastases), toxicity (i.e. compliance and treatment-related deaths) and preservation of function (i.e. swallowing and speech).
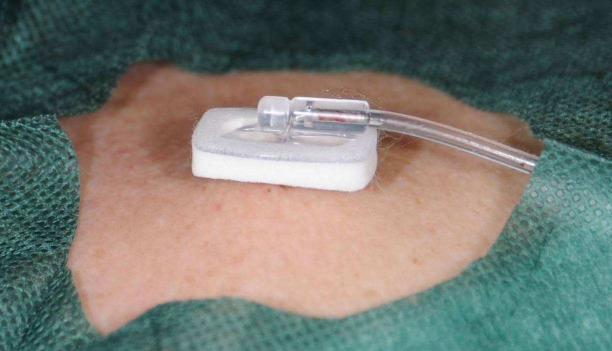
Figure 4. Port catheter in situ. The needle in the port chamber can also be used for the central venous administration of strongly vein-irritant chemotherapeutic agents.
Further recent developments have led to a reduction in the dose of the induction CTX, particularly in patients with HPV-positive oropharyngeal cancers (see below), which have been used in the study protocols of the Eastern Cooperative Oncology Group (ECOG 1308) and other study groups [145]. This seems sensible, particularly considering the high toxicity of the TPF regimen, and would benefit patients with a high comorbidity level. As an alternative to dose reduction, drugs in the induction protocol can be replaced with alternative agents (including anti-EGFR antibodies and signal cascade inhibitors), some of which are already being investigated (see below).
On the basis of the current data, there are clear-cut clinical situations in which the use of induction CTX seems useful, such as in patients with a high risk of distant metastases (e.g. those with extensive lymph node metastases or hypopharyngeal cancers). Induction CTX in chemoselected patients (see above, [183]) could also be of benefit to patients with T4 laryngeal/hypopharyngeal cancers in whom the role of laryngeal preservation is unclear.
In summary, induction CTX is not currently regarded as standard therapy for patients with advanced HNSCC. Current studies investigating the use of this approach (and particularly that of the relatively toxic TPF triple therapy), at various disease stages, will help to further establish its value versus simultaneous CRT. The crucial question about how to reduce the function-impairing late toxicity observed after simultaneous CRT also needs to be answered.
2.6 Neoadjuvant chemotherapy
The term induction CTX is generally used in the context of organ preservation (e.g. of the larynx). In contrast, with neoadjuvant CTX, the emphasis is on reducing (“downstaging” and “downsizing”) the primary tumour followed by resection (Figure 3 (Fig. 3)). It was previously thought that the subsequent resection had to be performed to the same specifications that were used before the advent of CTX. However, downstaging of the kind practised with oesophageal cancers, which was thought to be more or less obsolete, is now the focus of renewed interest [151].
The main neoadjuvant chemotherapeutic agents are dual or triple combinations comprising platinum analogues, taxanes and 5-FU. However, with one exception [57] (GETTEC), the few available studies show that neoadjuvant CTX followed by resection evokes no clear improvement in survival [162], [141], [213].
The integration of CTX in a neoadjuvant RCT setting, prior to the radical resection of oral cancers, (e.g. in accordance with the DÖSAG [“German-Austrian-Swiss Association for Tumours in the Maxillofacial Region”] protocol) is associated with moderate toxicity and seems promising. However, whether there is a survival advantage relative to other treatment concepts is yet to be established in large-scale randomised studies [128], [95], [62], [60].
It is also not completely clear whether downstaging can be reliably achieved through neoadjuvant CTX (before surgery). The possible advantages of effective downstaging lie in the less radical resection of the primary tumour with improved postoperative quality-of-life. Large-scale, prospective, randomised studies with consistent histological evaluation would be a suitable means of determining the efficacy of neoadjuvant CTX.
2.7 Simultaneous chemoradiotherapy
Simultaneous chemoradiotherapy (CRT, Figure 3 (Fig. 3)), a detailed account of which is given in the paper by F. Wenz, places the emphasis more on the locally intensified effect of the radiotherapy, rather than the systemic effect of the CTX. Indeed, CTX is intended to supplement local tumour control with irradiation, destroy micrometastases (additively), and contribute to an intensified radiotherapeutic effect (synergistically), for example by inhibiting the repair of sublethal lesions [192]. The main agents that can be administered simultaneously with RTX are cisplatin, carboplatin, 5-FU and mitomycin C. The positive effect of cisplatin has repeatedly been demonstrated, whilst its well-documented nephrotoxicity can be reduced by using lower doses at shorter intervals, and by ensuring hydration measures are effective. Carboplatin has a comparable radiotherapy-intensifying effect to cisplatin [93], and results with mitomycin look promising since it complements the action of the RTX, particularly on hypoxic cells. However, the main disadvantage with this agent is the risk of more severe cytopenia, which must not be ignored in nutritionally compromised HNSCC patients.
To optimise the desired effect, the different treatment modalities are administered shortly after one another or simultaneously. A meta-analysis published by Munro [132] revealed that in patients with advanced HNSCC, survival time was improved by 12% in patients receiving simultaneous CRT, when compared with those treated with RTX alone. This superiority has since been confirmed in several randomised studies, and is essentially attributable to improved locoregional control [28], [33], [55], [92], [174].
Similarly, the two Pignon meta-analyses which include 63 and 93 studies respectively [147], [148], showed a significant survival advantage for HNSCC patients following simultaneous CRT, when compared with those receiving induction CTX prior to RTX. This superiority was confirmed in the three-armed RTOG study 91–11 which investigated organ-preserving therapy in patients with advanced but operable laryngeal cancers [69]. In this randomised Phase III study, the simultaneous CRT arm (cisplatin and standard fractionated RTX) proved superior to the induction arm and the RTX-only arm, in terms of both larynx preservation and disease-free survival. However, there was no significant difference in overall survival between study arms, and even the initially observed difference in larynx preservation levelled out during long-term follow-up [70]. The increased acute toxicity of simultaneous CRT is adequately documented in the Forastiere study, and although other studies documented only sporadic late toxicities [117], [50], [158], they were particularly concerning in the first German laryngeal organ preservation study (Phase II) [160]: after three years, 25% of the surviving patients had to be tracheotomised because of pronounced, refractory late oedema which occurred as a result of the simultaneous CRT. It is clear from this study that the use of simultaneous CRT as an organ-preserving approach is not the ideal solution in patients with resectable laryngeal or hypopharyngeal cancers. This may be contrary to the ASCO recommendation [146], but organ preservation does not simply mean that the organ should remain anatomically intact; it should also retain its functionality [181]. When compared with the organ-preserving protocols associated with induction, simultaneous platinum-based CRT produces the lowest “laryngo-oesophagus dysfunction-free survival” rates, an endpoint recently defined by Lefebvre and Ang [110], [111]. However, the most recent MACH-NC meta-analysis by Pignon [148] showed significantly improved overall survival in the simultaneous CRT arm, when compared with patients treated with a (dual combination) induction approach of CTX + RT. Large-scale clinical studies are currently being performed to see whether a triple combination in the induction phase followed by (C)RT confirms this finding.
In summary, simultaneous CRT can be used in primarily inoperable HNSCC and is an organ-preserving strategy for advanced-stage tumours (in the larynx, hypo-and oropharynx). However, the associated late toxicities which can result in a dysfunctional organ have not yet been adequately detected and evaluated [196].
2.8 Adjuvant chemotherapy
Adjuvant CTX (Figure 3 (Fig. 3)) is used in an attempt to catch microscopic tumour residues after a surgical and/or radiotherapeutic intervention has been carried out. Despite impressive remission rates, mainly with inductive CTX, no reliable improvement in survival time has so far been demonstrated using adjuvant CTX alone [163], [91], [68], [109], [15]. Adjuvant CTX is most effectively used in combination with irradiation therapy in the form of adjuvant CRT (see the paper by F. Wenz). The two most important recent studies which investigated adjuvant CTX in combination with RTX are the EORTC 22931 study of Bernier et al. [22] and the RTOG 9501 study of Cooper et al. [47]. Without any significant increases in early or late toxicities, both studies showed an improvement in tumour-free survival (overall survival only in the EORTC study) and locoregional tumour control rate, but no reduction in distant metastases in the adjuvant CRT arm.
In a meta-analysis of both studies [21], extracapsular growth and small (less than 0.5 cm) resection margins were the demographic parameters that led to the most marked benefit with combined CRT. This level I evidence showed that conventionally fractionated, platinum-containing postoperative CRT in patients with the specified risk factors, improved local control and survival. In this situation, postoperative combined CRT can be regarded as the standard.
2.9 Palliative chemotherapy
At some stage, in about half of all patients with HNSCC, the malignancy will no longer be able to be treated appropriately by means of surgery or irradiation (e.g. no further radiation treatment will be possible because of local recurrences or distant metastases). In these patients, drug therapy must be given with palliative intent and on a strictly individual basis, so that there is a balance between the expected efficacy and the expected toxicity of the treatment. Often, sequentially administered monotherapy is preferred to combination treatment. The main agents used are MTX, platinum derivatives, 5-FU, taxanes and cetuximab (see below). These agents can be used “first-line” or “second-line”. “First-line” refers to a regimen that is used first; if there is no response, treatment is expanded to include additional agents (“second line”).
In palliative CTX (Figure 3 (Fig. 3)), the aim is to prolong life, and/or improve a patient’s quality-of-life by reducing tumour-related symptoms. Various treatment protocols have been investigated in palliative patient populations. Although the number of complete and partial remissions in cisplatin-containing treatment regimens was sometimes much higher than in protocols containing no cisplatin [191], [29], comparison of the survival times revealed minimal differences. The same observation was made in a direct comparison of mono-and combination therapies [16], [45]. The combination of cisplatin and 5-FU produced a higher response rate (~30% vs. 15%), when compared with the respective monotherapy, but although side effects were more frequent, survival time was only marginally improved [71], [29], [40], [75], (Table 2 (Tab. 2)).
Table 2. Palliative first-line therapy with various cytostatic combinations.
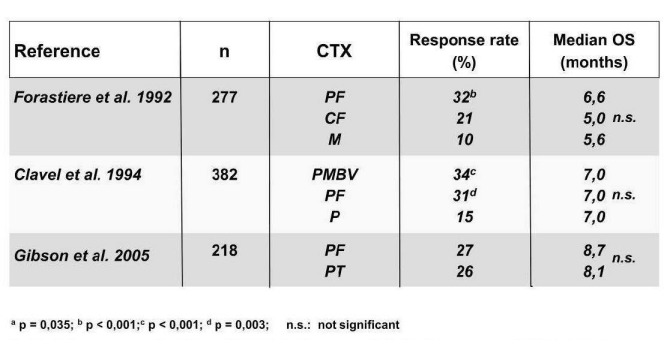
The combination of taxanes with platinum derivatives, and potentially 5-FU, does not appear to be significantly superior to the combination of platinum and 5-FU [75], [20]. In the metastatic stage, median survival is approximately 6 months, irrespective of the CTX protocol used.
A significant improvement in survival time from 7.4 to 10.1 months was achieved through the integration of an anti-EGFR antibody (see below), to supplement conventional CTX with platinum and 5-FU in a first-line setting [185]. Despite disadvantages, such as the need to discontinue treatment in one in five patients, and the development of ten CTX-related deaths from a total of 442 treated patients, this form of treatment is currently regarded as the “new standard” for the palliative treatment of patients with HNSCC. It should be noted, however, that a recent Health Technology Assessment (HTA) analysis of this combination did not confer an appropriate cost-benefit ratio [77].
There is currently no standard “second-line” treatment with palliative intent, and at present, such an approach is associated with an extremely poor response rate [113]. However, the use of novel “biologicals” or modified chemotherapeutic agents, such as capecitabine, an oral prodrug of 5-FU which is currently being investigated [123], may help to improve outcomes.
3 Targeted therapy/biologicals
The term “targeted therapy” or “biological” refers to a substance (e.g. a small molecule) that has a specific effect on a particular molecular target (e.g. growth factors that stimulate intracellular signalling pathways in tumour cells). Other examples of targeted therapies include monoclonal antibodies (mAbs), substances which are synthesised by identical immune cells that are all clones of a unique parent cell. Compared to classical antineoplastic CTX, the hope for this type of therapy is that their highly targeted tumour-specific activity will evoke fewer side effects. This is particularly relevant for patients with HNSCC, whom experience high levels of comorbidity which can restrict their ability to tolerate conventional CTX.
Various biologicals are being investigated in Phase III studies. Some of the most extensively researched molecules are the homologous EGF Receptor Kinase (HER) family, which comprises various sister molecules (e.g. HER1 and HER2), the former of which is better known as epidermal growth factor receptor (EGFR).
3.1 Monoclonal antibodies against EGFR
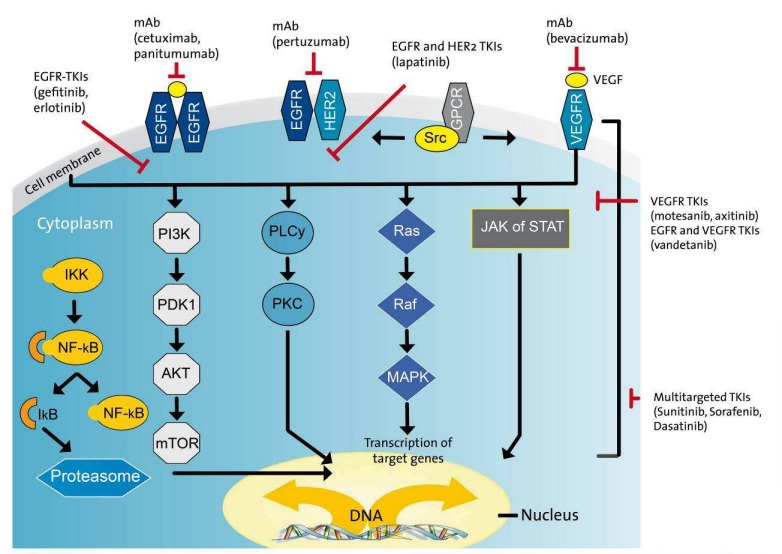
Growth factors are polypeptides that are synthesised and secreted by various cell types. They bind to specific membrane-based glycoprotein receptors and, depending on the target cell, can cause different phenotypic changes. After binding to the receptor, a signalling cascade, comprising a virtually continuous chain of signalling proteins, is activated (Figure 5 (Fig. 5)). Many of these signalling pathways culminate in the activation of transcription factors and a change in the cell’s gene expression. As a result, growth factors commonly stimulate proliferation, prevent differentiation, or protect the cell from apoptosis. Since the process of carcinogenesis is associated with the uncontrolled expression of growth factors, growth factor receptors and components of the intracellular signalling cascade, these molecules are regarded as key elements of the formation of a degenerated cell. Selective interference of the underlying signalling mechanisms could therefore open up new avenues of cancer treatment. For example, ligand binding suppression using targeted mAbs could evoke an antiproliferative effect.
Figure 5. Pleiotropic effects of the intracellular and nuclear EGFR signalling cascade after ligand binding. The binding of different ligands triggers a conformation change and autophosphorylation of EGFR. This is followed by consecutive activation of the intracellular tyrosine kinase and various intracellular signalling pathways, and culminates in tumour-cell proliferation, survival, and metastasis. Inhibition of this ligand binding or receptor phosphorylation is a new therapeutic approach in HNSCC.
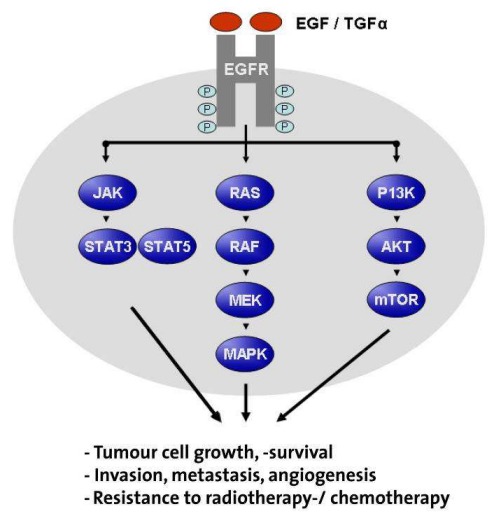
EGFR is one of the most extensively researched growth factor receptors, and consists of four members of the proto-oncogene family, (i.e. c-erbB-1, -2, -3 and -4). EGFR (from c-erbB-1) is a 170 kDa transmembrane phosphoglycoprotein which, with the exception of haematopoietic cells, is found within all adult tissue. The cysteine-rich extracellular domain is responsible for binding ligands, including EGF and TGF alpha. This leads to intracellular tyrosine kinase activation and induction of the signalling cascade (Ras/Raf/MAPK, Figure 5 (Fig. 5)). Further protein phosphorylation then triggers transcription factors which ultimately lead to a change in the cell’s gene expression. In head and neck squamous carcinoma cells, EGFR is often extensively overexpressed [159].
Because of the frequency and intensity of EGFR expression, its importance in the development and maintenance of the malignant phenotype, and the accessible position of the EGF/TGFα receptor on the cell surface, anti-EGFR mAbs have been studied extensively in HNSCC.
Several monoclonal antibodies target EGFR. These include: the “chimeric” IgG1 anti-EGFR antibody, cetuximab (Erbitux®), which comprises human and murine fractions; the humanised EMD72000, matuzumab, which is a version of IgG1 with an elongated half-life [23]; or the completely humanised antibodies, panitumumab (IgG2a), and zalutumumab (IgG1). In principle, these entities bind to EGFR with a higher affinity than the endogenous ligands, thus preventing dimerisation, internalisation, and autophosphorylation. Preclinical studies have demonstrated an inhibition of proliferation and induction of apoptosis in the tumour; an antibody-dependent cell-mediated cytotoxicity (ADCC), particularly with chimeric antibodies, and evidence of additive/synergistic interactions with CTX and RTX [82], [85], [24]. Cetuximab has demonstrated its efficacy in HNSCC in several clinical papers. In one pioneering study in patients with locally advanced HNSCC without a primary surgical treatment option, cetuximab, in combination with RTX, was compared to RTX alone. Relative to the RTX-only arm, cetuximab + RTX increased median survival time by 19.7 months (49.0 vs. 29.3 months), and median time to locoregional failure by 9.5 months (24.4 vs. 14.9 months) [26], [27]. However, patients at the T4 or N0 stage and those who had a poor Karnofsky index and were over 65 years of age did not benefit.
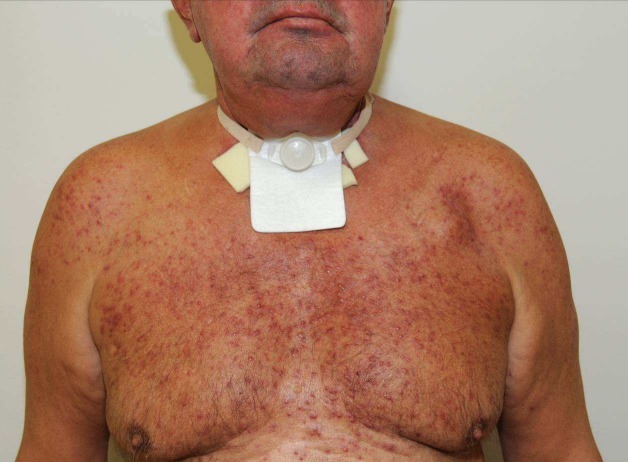
Two Phase III studies have examined the efficacy of anti-EGFR therapy in a first-line setting. In a study by Burtness et al. [32], the combination of cisplatin and cetuximab was compared with cetuximab and placebo. The response rate in the cisplatin-containing arm was significantly higher than that of the placebo-containing arm (26% vs. 10%; p=0.03). However, there were no significant differences in median progression-free survival and median overall survival, potentially because the study was inadequately powered. As noted by other investigators [27], there was a correlation between treatment efficacy and antibody-associated skin toxicity (skin rash, Figure 6 (Fig. 6)).
Figure 6. The typical picture of cetuximab/Erbitux®-associated skin rash, consisting of acneiform, pustular or maculopapillary hyperkeratotic follicular exanthem, preferentially affects seborrhoeic areas of skin. Concomitant dermatological treatment is indicated.
In the second study [185], 442 patients were randomised to receive either CTX alone or CTX + cetuximab. In addition to the known transient skin toxicities, infusion reactions, sepsis and hypomagnesaemia were observed during antibody therapy. Compared to the CTX-only arm, median survival in the antibody-containing arm increased significantly by 2.7 months (10.1 vs. 7.4 months) and was described by the authors as a “major breakthrough” in the palliative systemic treatment of patients with head and neck cancer. This combination was mostly effective in patients under 65 years of age with a Karnofsky index of over 80 who received cisplatin and not carboplatin, and those with tumours of the oral cavity but not of the hypopharynx or larynx [185]. For both groups, the estimated two-year survival was less than 20%, but addition of the antibody did not cause any reduction in quality-of-life [127]. Interestingly, in this study the EGFR gene expression of the treated tumours was not a predictive marker for an antibody response [115].
On the basis of these results, in 2006, the cetuximab/Erbitux® + RTX combination was registered for the treatment of patients with advanced HNSCC. In 2008, it was also approved in combination with first-line CTX for patients with recurrent/metastatic HNSCC. Cetuximab monotherapy also appears to be efficacious in the second-and third-line treatment of cisplatin-refractory patients [187]. A further registration (FDA fast-track status) for the palliative treatment of HNSCC patients for whom treatment options have been exhausted was granted for the humanised antibody zalutumumab. This followed a demonstration of prolonged progression-free survival, although overall survival was unaffected [121].
Ongoing studies are attempting to determine whether the combination of RTX + cetuximab is equivalent or even superior to the previous gold-standard in the primary treatment of HNSCC: simultaneous CRT (i.e. whether it also reduces late toxicity). The results of the RTOG 0522 Phase III study presented at ASCO 2011 were sobering however, as they failed to demonstrate either progression-free or overall survival for the CRT + cetuximab combination, when compared to CRT alone. CRT + cetuximab was also associated with a higher side effect incidence (i.e. mucositis and skin reactions) [11]. Following these disappointing results, a planned Phase III study of adjuvant CRT ± the humanised antibody, panitumumab (EORTC 24071, Budach W, Düsseldorf), was discontinued. The adoption of the RTX + cetuximab regimen is now very much likely to depend on the findings of comparative trials versus primary CRT, and the resultant subgroup analysis. Thus, several Phase III studies are underway to investigate the interaction of cetuximab with radiotherapy (see paper by F. Welz) or induction CTX: RTOG-0920 (Machtay M, Cleveland, OH, USA) which is investigating whether the additional of cetuximab to RTX improves overall survival in postoperative patients with intermediate risk; the four-arm study by Paccagnella (GSTTC Italian Collaborative Group) which is comparing CRT, RTX + cetuximab, TPF + CRT, and TPF + RTX + cetuximab; the INTERCEPTOR study (Merlano, Italy) which is comparing TPF induction followed by CRT with RTX + cetuximab; the GORTEC2007-01 study which is comparing RTX + cetuximab with CRT + cetuximab; and the GORTEC2007-02 study which is comparing CRT with induction TPF followed by RTX + cetuximab.
Further studies are investigating the efficacy of anti-EGFR antibodies in combination with taxanes (e.g. CeFCID, Keilholz, Berlin; DeLOS II, Dietz, Leipzig; ICRAT, Budach, Berlin; [12]). Irrespective of the results, there is sufficient evidence to date to suggest that anti-EGFR antibodies are efficacious in HNSCC. Thus, targeted therapy is now firmly established as a fourth pillar of treatment alongside surgery, irradiation, and classic CTX.
3.2 Immunotherapy
There are four main principles of an immunotherapy intervention: active or passive, and specific or nonspecific. Active immunotherapy involves the induction of an immune response in the tumour host, whereas passive immunisation is based on the transfer of suitable, e.g. ex vivo reproduced and/or conditioned immune cells or the infusion of immunoglobulins (antibodies). The general features of all four immunotherapy types are shown below (Figure 7 (Fig. 7)).
Figure 7. The four principles of an immunotherapy intervention: active or passive, and specific or nonspecific.
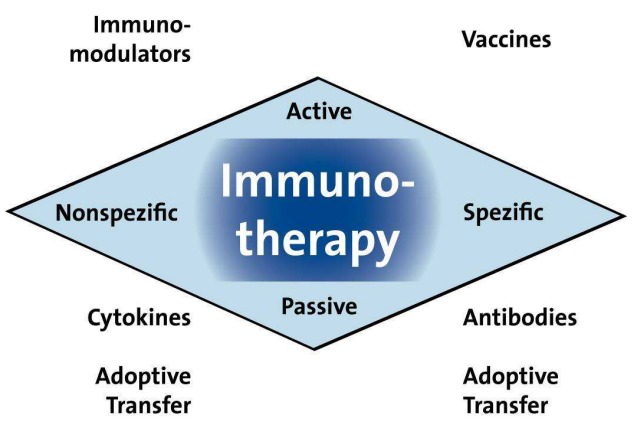
The concept behind immunotherapy is to build up or support an effective immune response directed against tumour-associated antigens (TAA). This is done in an attempt to strengthen the antigenicity and/or immunogenicity of the TAAs and to correct the inhibitory mechanisms that act at various levels of the immunological defences.
Essentially, immunotherapy uses the body’s own specific and systemic defences, as part of the “main treatment” of tumour cells that would not otherwise be targeted [84], [201]. Current antitumour immunotherapy techniques comprise, amongst others, nonspecific immunostimulation, genetic modifications of tumour or immune cells and the use of monoclonal antibodies, adoptive immunotherapy, and vaccination/immunisation.
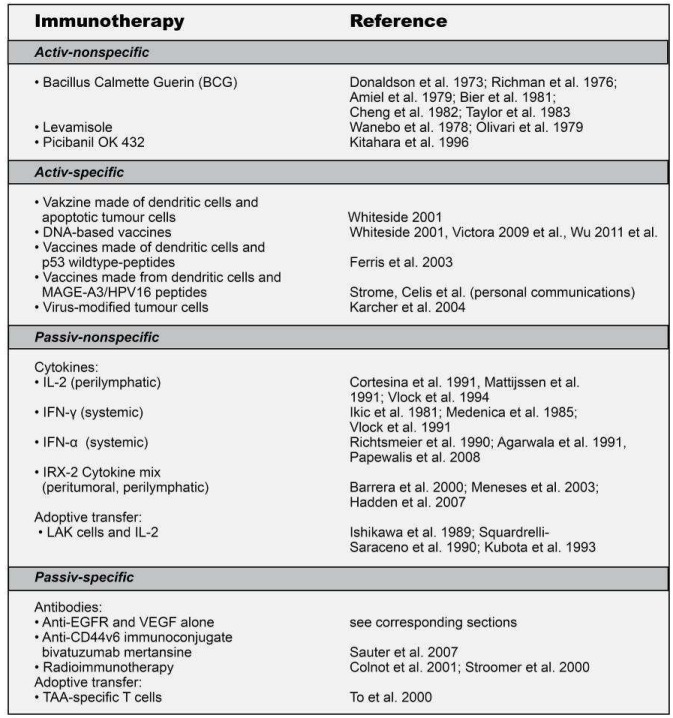
The use of (chimeric) anti-EGFR antibodies – although often classed as biological or targeted therapy – can also be regarded as a passive immunotherapy technique. Apart from the anti-EGFR antibodies however, no other classes of immunotherapy agents are supported by consistent Phase III study results, and are therefore unlikely to be registered for use in HNSCC in the near future (Table 3 (Tab. 3)). Researchers are optimistic about viral target structures such as the Epstein-Barr virus (mainly in China) and HPV, which are already being targeted for immunotherapy with other tumour entities [82], [10], [145], [197], [204].
Table 3. Selected clinical studies of the immunotherapy of patients with HNSCC.
HPV is a double-stranded DNA virus which, via its E6/E7 region, inactivates the tumour suppressor genes p53 and pRb, and causes uncontrolled cell proliferation and tumour development [131]. It is the most common sexually transmitted virus in the world, and infection with HPV-16 carries a 15-fold increased risk of developing oropharyngeal cancer. HPV-positive HNSCCs often develop in the oropharynx, but can also culminate in cystic metastases. Despite showing relatively little differentiation compared to HPV-negative HNSCCs, HPV-positive HNSCCs do not appear to be directly correlated with tobacco and alcohol consumption [3]. In recent years, an increased incidence of HPV-positive HNSCC and oropharyngeal cancers has been observed. Current data suggest that a change in the classification of HNSCC (e.g. whether patients are HPV-positive or HPV-negative) is warranted, since their prognosis differs considerably – HPV-positive HNSCCs are generally more sensitive to RTX and CTX, and have a more favourable postoperative long-term prognosis [116], [99], [10].
HPV vaccines (Gardasil®, Cervarix®) are available for the prevention of cervical cancer and are registered for use in girls and young women. However, since it is mostly young men who develop HPV-positive HNSCC, studies have been initiated to investigate the activity and efficacy of HPV vaccination in men. Whether these vaccinations will lead to a reduction in HPV-associated HNSCC is yet to be determined. It must also be noted that Gardasil® and Cervarix® are prophylactic vaccines, whereas therapeutic vaccines are required in HNSCC patients with an existing tumour load. Since T-cell responses to HPV constituents have been observed in HNSCC patients [5], [83], HPV may become an attractive target molecule for immunotherapy, e.g. for adoptive transfer [6].
Although immunotherapy has yet to become established as the standard for the adjuvant treatment of HNSCC, basic immunological research has contributed to our understanding of the complex immunobiology of HNSCC (Figure 8 (Fig. 8)). To date, this has not transferred to an evidence-based clinical benefit (Table 3 (Tab. 3)), but there is reason to believe that future studies will incorporate these insights for the benefit of HNSCC patients.
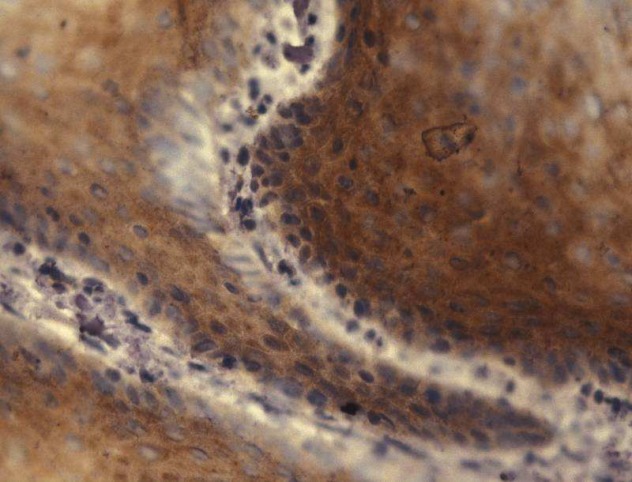
Figure 8. Immunohistochemical determination of HPV16 E7 in the nucleus and cytoplasm of a representative HNSCC. HPV16 E7 is said to be a potential target molecule for immunotherapy.
3.3 Anti-angiogenesis
Preclinical and clinical studies suggest that vascular endothelial growth factor (VEGF) plays a major role in the regulation of neoplastic angiogenesis in solid tumours.
In HNSCC, it has been shown that a correlation exists between tumoral VEGF expression and tumour stage, vascular invasion and survival. It is therefore logical to use either a monoclonal, humanised anti-VEGF antibody, (bevacizumab, Avastin®), or a tyrosine kinase inhibitor (TKI) that attacks intracellularly at the receptor domain (e.g. sunitinib/sorafenib) (Figure 9 (Fig. 9)). Initial studies with bevacizumab alone produced unfavourable response rates, so combinations with CTX in prognostically unfavourable HNSCC patients were investigated [166]. In a further Phase I study in patients with advanced HNSCC, a combination of bevacizumab with sirolimus was found to have an acceptable side effect profile [44].
Figure 9. The complex signalling cascade at cellular level in HNSCC with potential points of therapeutic systemic action by biologicals.
On the assumption that VEGF-mediated angiogenesis could be involved in resistance to an anti-EGFR targeted therapy, erlotinib was combined with bevacizumab in a Phase I/II study in patients with recurrent or metastatic HNSCC. Side effects included diarrhoea and rash, and also severe bleeding from the tumour (3/46 patients). A response was seen mainly in patients with a high ratio of phosphorylated VEGFR2 or EGFR to total protein [42].
In a Phase II study, bevacizumab was used with the antifolate, pemetrexed, in the first-line treatment of patients with recurrent or metastatic HNSCC. Tumours were well controlled, and the overall response rate was 30%, but severe haemorrhagic complications were seen in 6/40 patients [13].
It is not currently possible to provide a final assessment of the efficacy of bevacizumab in HNSCC, but an ECOG Phase III study (E1305) investigating its use when combined with CTX (cisplatin + docetaxel or 5-FU) in patients with recurrent or metastatic HNSCC is expected to provide clarity. In general, the decision to use bevacizumab must assess the possibility of haemorrhagic complications, and take precautions accordingly.
Cilengitide, a cyclic pentapeptide, has antiangiogenic properties which stem from “integrin” inhibition. In addition to several individual case reports [153], cilengitide was combined with cetuximab and platinum-containing CTX in a Phase I study in patients with HNSCC. A 2000 mg dosage was selected on the basis of its favourable side effect profile in the randomised Phase II (ADVANTAGE) study that is currently being evaluated [184].
Sunitinib inhibits various receptor tyrosine kinases such as those for VEGF, PDGF, c-Kit, FLT, CSF and RET (Figure 9 (Fig. 9)). One study [38] was discontinued early on account of haemorrhagic complications. In a study [120] involving orally administered sunitinib, partial remission was observed in only one of 38 patients, but 16% of patients reported haemorrhagic complications.
3.4 Hypoxic cells as target structures
Tirapazamine is a bioreductive substance with selective cytotoxicity for hypoxic (tumour) cells. It also potentiates the action of cisplatin. Phase I/II studies in advanced HNSCC have shown that tirapazamine has an acceptable toxicity profile in cisplatin-based CRT protocols. The additive effect of TPZ in CRT was investigated in a Phase III study which included 853 patients with untreated advanced HNSCC (TROG CE, “Tirapazamine Radiation and Cisplatin Evaluation”, [157]). No improvement in survival time or any other evaluated parameter was achieved by adding tirapazamine.
3.5 Signalling cascade inhibition
3.5.1 Tyrosine kinases (TKIs)
In HNSCC, receptor variants such as the EGFRvIII mutation appear to be responsible for the constitutive activation of the downstream signalling cascade and any resistance to EGFR inhibition by the corresponding mAbs [170]. To overcome resistance, the inhibition of signalling cascades downstream from the receptor, such as EGFR-associated tyrosine kinase, could be an interesting treatment approach (Figure 9 (Fig. 9)).
An EGFR-specific TKI was presented as long ago as 1994 by Fry et al. [73]. These small molecules selectively suppress EGFR autophosphorylation, thus preventing receptor-mediated intracellular signal transmission, and show antitumoral activity in vitro and in vivo [30]. More recent developments, including their current clinical use, are presented below and in Table 4 (Tab. 4).
Table 4. Selected clinical studies with TKI for HNSCC treatment.

Erlotinib (Tarceva®) is a selective inhibitor of the tyrosine kinase domain of the EGF receptor (HER1). It has been registered in Germany for the treatment of non-small cell lung cancer since 2005, and in pancreatic cancer since early 2007.
Gefitinib (Iressa®) is a selective EGFR inhibitor which is registered for the treatment of non-small cell lung cancer with activating EGFR mutations.
The main problem with using single inhibitors is the rapid development of mutated kinases which become resistant to treatment. A more logical approach is to combine several inhibitors and/or develop ones with several sites of attack. Such approaches are described below.
Lapatinib (Tyverb®) is a dual TKI (HER1 and 2), which is licensed for combination treatment with capecitabine in women with advanced or metastatic HER2-positive breast cancer who have already been treated with chemotherapy. The registration has now been extended to include combination therapy with an aromatase inhibitor for the treatment of postmenopausal women with hormone receptor-and HER2-positive metastatic breast cancer.
Like lapatinib, BIBW-2992 (afatinib) shows dual (irreversible) tyrosine kinase inhibition and could play a role in cetuximab resistance [211]. Afatinib is currently being evaluated in a Phase II study in HNSCC patients [167]; Phase III studies are in preparation.
Sorafenib (Nexavar®) is a multi-kinase inhibitor that has several points of attack: the inhibition of Raf kinase to reduce cell division and proliferation; and the inhibition of other tyrosine kinases, including those involved in the VEGF signalling pathway, to reduce tumour angiogenesis. Sorafenib has received approval for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma. Other multi-kinase inhibitors are sunitinib (see above), BIBF 1120 (VEGFR, PDGFR, FGFR), vandetanib (VEGFR, EGFR) and dasatinib (among others Src kinase, Figure 9 (Fig. 9)).
All of the inhibitors listed above are oral formulations, although this is not necessarily advantageous in patients with neoplasia of the mouth and upper airways (dysphagia, PEG). The most common side effects are acne-like rash and diarrhoea.
Various studies involving signalling cascade inhibitors in HNSCC are listed in Table 4 (Tab. 4). It is worth noting that in the Phase III study by Stewart et al. [175], there was no improvement in response or survival time in the gefitinib arms (250 and 500 mg/day), when compared with MTX therapy. The role of these agents in combination with CTX has not yet been established [79] and is the subject of ongoing studies (e.g. ECOG 1302: Phase III randomised, placebo-controlled trial of docetaxel versus docetaxel plus ZD1839 (Iressa, gefitinib) in performance status 2 or previously treated patients with recurrent or metastatic head and neck cancer).
3.5.2 mTOR and other signalling molecules
Sirolimus/rapamycin (Rapamune®) is an immunosuppressant with a macrolide structure which is isolated from streptomycetes. Sirolimus inhibits a number of cytokine-mediated signal transduction pathways via complexation of the protein mTOR (mammalian target of rapamycin), a 282 kDa phosphoinositide 3-kinase which is often activated in HNSCC. The deactivation of mTOR prevents mTOR-dependent cell metabolism which disrupts the cell cycle and inhibits cell growth (Figure 9 (Fig. 9)). Even though sirolimus is a novel treatment approach, studies in HNSCC are currently limited to Phase I trials [44]. Rapamycin derivatives, such as everolimus, temsirolimus and deforolimus, are potent mTOR inhibitors that are more stable and soluble than rapamycin. Early clinical studies of these agents as monotherapy (e.g. TEMHEAD study at the Hannover Medical School) or in combination with (induction) CTX/CRT have now been initiated.
Other therapeutic points of attack that target the tumour’s own signalling cascade include the inhibition of protein kinase C [35] or the proteasome NF-kappaB by bortezomib [61].
4 Gene and stem-cell therapy
4.1 Gene therapy
Gene therapy involves the insertion of genetic material either directly into tumour cells (thus producing cytotoxicity), or indirectly via the introduction of DNA into healthy cells (thus activating the body’s immune system to act specifically against the cancer). The aim of gene therapy is to eliminate tumour cells as selectively as possible, without creating any accompanying toxicity in adjacent, non-malignant cells. HNSCCs are particularly suited to this form of treatment, as in most cases, there is good accessibility for the intratumoral injection of vectors and a treatment-monitoring biopsy. Although the efficacy of gene therapy is restricted to locoregional control, its use in HNSCC seems worthwhile, since locoregional recurrences often occur during the course of the disease and can affect overall survival. The following conditions must be met before gene therapy can be used “routinely”: a) optimisation of the type and route of access so that the genetic material can be selectively introduced into the target tissue in a sufficiently high concentration; b) selection of the most effective and safest gene sequences; c) creation of a means of regulating expression of the therapeutic gene, and if necessary, stopping it completely.
Various treatment strategies have been developed, such as the replacement of mutated tumour suppressor genes (e.g. p53), the expression of alloantigens, or the inhibition of oncogenes [76], [107]. If necessary, these approaches can also be used in combination, to help potentiate the therapeutic effect.
Many different vehicles for gene administration have been tested, but viral vectors, particularly those derived from adeno-and retroviruses, are still considered the most efficient. The most extensively researched gene therapy approaches in HNSCC focus on the “repair” of the tumour suppressor gene p53, which is present in mutated form in well over half of HNSCC cases, and is associated with an unfavourable prognosis [66], [18]. p53 is a central protein in cell cycle control and protects the cell from genotoxic stress by causing G1/S cell cycle arrest in genetically altered cells. p53 mutations can therefore cause genetically damaged cells to reproduce uncontrollably. In preclinical studies, gene therapy involving the replacement of mutated p53 led to reduced growth of HNSCC and increased radiochemical sensitivity [118].
Initial clinical studies involving Advexin (Ad5CMV-p53), a modified p53-coding adenovirus in which the E1 region is replaced by the cDNA of the p53 gene, showed a clinical response in some advanced HNSCC patients [41], [212]. Side effects mainly comprised flu-like symptoms and localised pain. Two Phase III studies (T301, T302) have been designed to compare the safety, efficacy and survival of Advexin, as monotherapy or in combination with CTX, in patients with HNSCC. In the T301 study, 123 patients with tumour recurrence following RTX and CTX with cisplatin/taxanes, received either intratumoral Advexin or MTX [133]. Although no significant differences in overall survival were observed in the two study arms (Advexin 6.1 vs. MTX 4.4 months), Advexin improved survival (7.2 vs. 2.7 months) in patients with a “favourable” profile (normal p53 gene sequences and low p53 protein expression), when compared with those with an “unfavourable” p53 profile (high expression of mutated p53, [134]). The second Phase III study (T302) will involve 288 patients with recurrent HNSCC, who will be treated with cisplatin, 5-FU ± Advexin. Advexin is not yet licensed for use in HNSCC.
Onyx-015 is another adenovirus, but one from which the E1B region has been removed. The E1B region is responsible for the binding and inactivation of the p53 tumour suppressor protein, and is required for virus replication in normal tissue. For this reason, onyx-015 is only suitable in cells that have disturbed p53 function. Although various in vitro studies have questioned its selectivity, clinical trials have been performed to investigate onyx-015 as monotherapy or in combination with CTX [96], [136], [137], [135]. Remission was achieved in some patients, but there appeared to be no correlation with p53 status. This led researchers to again question the agent’s selectivity, and as a consequence, all studies were discontinued.
Another gene therapy that has been clinical tested is Gendicine®, a replication-deficient adenoviral vector with an RSV promoter which codes for the human wild-type p53 and is manufactured in human embryogenic renal cells (bioreactor). In China, Gendicine has been commercially available commercially for the treatment of HNSCC since 2004 (SiBiono, Shenzhen). Gendicine is therefore the first gene therapy to receive marketing authorisation following clinical testing. In the western world however, this registration was viewed with scepticism, as it was solely based on a Chinese Phase II/III study that involved only 135 patients (85% nasopharyngeal carcinoma) – a study population which was regarded by many experts to be inadequate. In this study, combination therapy comprising irradiation and Gendicine evoked complete tumour regression approximately three times more frequently than RTX alone [144]. No correlation with p53 status was established. A multicentre randomised Phase IV study involving over 300 patients has now been initiated in China. Criticism of the drug’s evidence base remains however, as virtually all relevant scientific and clinical studies have been published in Chinese-language journals. This makes it much more difficult for western scientists to scrutinise the data.
Numerous other gene therapy approaches are under investigation in HNSCC (e.g. REOLYSIN®, a wild-type oncolytic (RNA) reovirus with selective toxicity for tumour cells). Initial results are encouraging, and suggest that gene therapy can be used clinically, particularly as part of a combination regimen. However, disadvantages, such as high costs and the current inability to assess its safety, must firstly be overcome before its use becomes standardised.
4.2 Stem-cell therapy
Stem cells can be differentiated into various cell types or tissues – depending on the type of stem cells and the influences upon them, they can become generic tissue (embryonic stem cells) or a specified tissue type (adult stem cells). Stem cells can be regarded as a reservoir of new cells that replaces defective or dead cells. In oncology, the tumour stem cell is characterised by specific markers with properties of self-renewal, potentially uncontrolled proliferation, and the fact that it remains in the resting phase of the cell cycle and is consequently resistant to chemo-and radiotherapy. Treatments that specifically target HNSCC-specific tumour stem cells could, in principle, improve the chance of finding a cure. For this hope to be realised however, dedicated molecular characterisation of the corresponding cell is required, meaning that such an approach is far from routine clinical use (review in [206] and [129]).
5 General conclusions
HNSCC is mainly treated using surgery and/or irradiation. Systemic cytostatic therapy has an established place in combination with primary or adjuvant irradiation, and in the palliative setting with previously treated recurrences or distant metastases. The value of induction CTX, which currently comprises triple combination with a taxane, platinum derivative, and 5-FU, must be investigated in randomised studies, using the current standard therapy (simultaneous CRT) as a comparator.
Following the registration of the anti-EGFR antibody, cetuximab, a fourth “pillar” in the form of targeted therapy was established. However, before it can be integrated into existing protocols and/or replace established methods, further investigations are required. Innovative molecular substances (e.g. multi-kinase inhibitors) and immunotherapy or gene therapy approaches – which to date have not received EU marketing authorisation in HNSCC – should improve future treatment options and have favourable side effect profiles. These must now be tested in clinical studies with sufficiently large patient populations, preferably in a first-line setting.
In order to identify the treatment that is most suitable for any particular patient, clinical and/or molecular predictive markers must also be identified. These will help to further advance the individualisation of tumour therapy. To this end, a clear and renewed impetus in HNSCC translational research is long overdue.
The right individual therapy, along with effective multidisciplinary communication, can not only help to ensure that the tumour is controlled and survival prolonged, but also that the patient’s quality-of-life is maintained or restored.
Abbreviations
B – Bleomycin
CR – Complete Response
CTX – Chemotherapy
CRT – Chemoradiotherapy
ECOG – Eastern Cooperative Oncology Group
EGFR – Epidermal Growth Factor Receptor
EORTC – European Organisation for Research and Treatment of Cancer
5-FU/F – 5-Fluorouracil
HNSCC – Head and Neck Squamous Cell Carcinoma
HPV – Human Papillomavirus
MACH-NC – Metaanalysis of chemotherapy in head and neck cancer
MTX/M – Methotrexate
OP – Operation
OR – Overall Response
OS – Overall Survival
P – Platinum
PF – Platinum and 5-FU
PR – Partial Response
RTOG – Radiation Therapy Oncology Group
RTX – Radiotherapy
SD – Stable Disease
TAA – Tumour-associated antigens
TKI – Tyrosine kinase inhibitor
TPF – Taxane, Platinum, 5-FU
TTP – Time to Progression
Notes
Competing interests
The author declares that he has no competing interests.
References
- 1.Abidoye OO, Cohen EE, Wong SJ, Kozloff MF, Nattam SR, Stenson KM, Blair EA, Day S, Dancey DE, Vokes EEl. A phase II study of lapatinib (GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2006;24(18S;June 20 suppl):abstr 5568. [Google Scholar]
- 2.Agarwala S, Vlock D, Johnson JT, et al. Phase II trial of interferon-alpha in locally recurrent or metastatic head and neck cancer: results of ECOG trial P-Z386. Proc Am Soc Clin Oncol. 1991;10:205. [Google Scholar]
- 3.Agrawal Y, Koch WM, Xiao W, Westra WH, Trivett AL, Symer DE, Gillison ML. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:7143–7150. doi: 10.1158/1078-0432.CCR-08-0498. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aisner J, Sinibaldi V, Eisenberger M. Carboplatin in the treatment of squamous cell head and neck cancers. Semin Oncol. 1992;19:60–65. [PubMed] [Google Scholar]
- 5.Albers A, Abe K, Hunt J, Wang J, Lopez-Albaitero A, Schaefer C, Gooding W, Whiteside TL, Ferrone S, DeLeo A, Ferris RL. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–11155. doi: 10.1158/0008-5472.CAN-05-0772. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 6.Albers AE, Hoffmann TK, Klussmann JP, Kaufmann AM. Prophylactic and therapeutic vaccines against human papilloma virus. HNO. 2010;58:778–790. doi: 10.1007/s00106-010-2118-6. Available from: http://dx.doi.org/10.1007/s00106-010-2118-6. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sarraf M. Chemotherapeutic management of head and neck cancer. Cancer Metast Rev. 1987;6:191–198. doi: 10.1007/BF00144263. Available from: http://dx.doi.org/10.1007/BF00144263. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sarraf M. Management strategies in head and neck cancer: The role of carboplatin. In: Brunns PA, Canetta R, Ozols RF, editors. Carboplatin: Current perspectives and future directions. Philadelphia: W.B. Saunders; 1990. [Google Scholar]
- 9.Amiel JL, Sancho-Garnier H, Vandenbrouck C, Eschwege F, Droz JP, Schwaab G, Wibault P, Stromboni M, Rey A. First results of a randomized trial on immunotherapy of head and neck tumors. Recent Results Cancer Res. 1979;68:318–323. doi: 10.1007/978-3-642-81332-0_48. [DOI] [PubMed] [Google Scholar]
- 10.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. Available from: http://dx.doi.org/10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan P, Sherman EJ, Weber RS, Galvin JM, Schwartz DL, El-Naggar AK, Gillison ML, Jordan R, List MA, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):abstr 5500. [Google Scholar]
- 12.Argiris A, Heron DE, Smith RP, Kim S, Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala RR, Dacic S, Gooding W, Grandis JR, Johnson JT, Ferris RL. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–5300. doi: 10.1200/JCO.2010.30.6423. Available from: http://dx.doi.org/10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argiris A, Karamouzis MV, Gooding WE, Branstetter BF, Zhong S, Raez LE, Savvides P, Romkes M. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2011;29:1140–1145. doi: 10.1200/JCO.2010.33.3591. Available from: http://dx.doi.org/10.1200/JCO.2010.33.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. Available from: http://dx.doi.org/10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armand JP, Couteau C. Chemotherapy in head and neck cancer. Eur J Cancer. 1995;31:819–822. doi: 10.1016/0959-8049(95)00124-2. Available from: http://dx.doi.org/10.1016/0959-8049(95)00124-2. [DOI] [PubMed] [Google Scholar]
- 16.Armand JP, Cvitkovik E, Recondo G, Wibault P, Schwaab G, Domenge C, Tellez-Bernal E, Gandia D, Luboinski B, Eschwege F, et al. Salvage chemotherapy in recurrent head and neck cancer: The Institut Gustave Roussy Experience. Am J Oncol. 1993;14:301–306. doi: 10.1016/0196-0709(93)90087-n. [DOI] [PubMed] [Google Scholar]
- 17.Atassi B, Ozgursoy O, Yoo GH, Jacobs JR, Bhatti NS, Mal M, Kim H, Lin H, Sukari A. Influence of induction chemotherapy on patients’ compliance to radiotherapy in patients with locally advanced head and neck squamous cell carcinoma. J Clin Oncol. 2011;29(suppl):abstr 5558. [Google Scholar]
- 18.Balz V, Scheckenbach K, Götte K, Bockmühl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63(6):1188–1191. [PubMed] [Google Scholar]
- 19.Barrera JL, Verastegui E, Meneses A, Zinser J, delaGarza J, Hadden JW. Combination immunotherapy of squamous cell carcinoma of the head and neck: a phase 2 trial. Arch Otolaryngol Head Neck Surg. 2000;126:345–351. doi: 10.1001/archotol.126.3.345. [DOI] [PubMed] [Google Scholar]
- 20.Benasso M, Ponzanelli A, Merlano M, Numico G, Ricci I, Vigo V, Grossi F, Amadori D, Cavallo G, Capaccetti B, Taveggia P, Boni L, Rosso R. Paclitaxel, cisplatin and 5-fluorouracil in recurrent squamous cell carcinoma of the head and neck: a phase II trial from an Italian cooperative group. Acta Oncol. 2006;45:168–174. doi: 10.1080/02841860500468919. Available from: http://dx.doi.org/10.1080/02841860500468919. [DOI] [PubMed] [Google Scholar]
- 21.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. Available from: http://dx.doi.org/10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 22.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. Available from: http://dx.doi.org/10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 23.Bier H, Hoffmann T, Hauser U, Wink M, Ochler M, Kovar A, Müser M, Knecht R. Clinical trial with escalating doses of the antiepidermal growth factor receptor humanized monoclonal antibody EMD 72 000 in patients with advanced squamous cell carcinoma of the larynx and hypopharynx. Cancer Chemother Pharmacol. 2001;47:519–524. doi: 10.1007/s002800000270. Available from: http://dx.doi.org/10.1007/s002800000270. [DOI] [PubMed] [Google Scholar]
- 24.Bier H, Hoffmann T, van Lierop A. Anti-(epidermal growth factor) receptor monoclonal antibodies for the induction of antibody-dependent cell-mediated cytotoxicity against squamous cell carcinoma lines of the head and neck. Cancer Immunol Immunother. 1998;46:167–173. doi: 10.1007/s002620050475. Available from: http://dx.doi.org/10.1007/s002620050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bier J, Rapp HJ, Borsos T. Randomized study on intratumoral BCG-cell wall preparation (CWP) therapy in patients with squamous cell carcinoma in the head and neck. Immunotherapy. 1981;12:71–75. [Google Scholar]
- 26.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. Available from: http://dx.doi.org/10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 27.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. Available from: http://dx.doi.org/10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 28.Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, George SL, Huang AT, Prosnitz LR. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. Available from: http://dx.doi.org/10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 29.Browman GP, Cronin L. Standard chemotherapy in squamous cell head and neck cancer: what we learned from randomized trials. Semin Oncol. 1994;21:311–319. [PubMed] [Google Scholar]
- 30.Buchdunger E, Mett H, Trinks U, Regenass U, Müller M, Meyer T, Beilstein P, Wirz B, Schneider P, Traxler P, Lyodon NB. 4,5-bis(4-fluoroanilino) phtalimide: a selective inhibitor of the epidermal growth factor receptor signal transduction pathway with potent in vivo antitumor activity. Clin Cancer Res. 1995;1:813–821. [PubMed] [Google Scholar]
- 31.Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, Lammert I, Jahnke K, Stueben G, Herrmann T, Bamberg M, Wust P, Hinkelbein W, Wernecke KD. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J Clin Oncol. 2005;23:1125–1135. doi: 10.1200/JCO.2005.07.010. Available from: http://dx.doi.org/10.1200/JCO.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. Available from: http://dx.doi.org/10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 33.Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Bertrand P. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharyngeal carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. Available from: http://dx.doi.org/10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 34.Canetta R, Rozencweig M, Carter SK. Carboplatin: the clinical spectrum to date. Cancer Treat Rev. 1985;12:125–136. doi: 10.1016/0305-7372(85)90027-1. Available from: http://dx.doi.org/10.1016/0305-7372(85)90027-1. [DOI] [PubMed] [Google Scholar]
- 35.Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, Cole P, Sullivan R, Riddle J, Schmidt J, Enas N, Sinha V, Thornton DE, Herbst RS. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–4099. doi: 10.1200/JCO.2005.05.3447. Available from: http://dx.doi.org/10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 36.Cheng E, Young CW, Wittes RE. Phase II trial of vindesine in advanced head and neck cancer. Cancer Treat Rep. 1980;64:1141–1142. [PubMed] [Google Scholar]
- 37.Cheng VS, Suit HD, Wang CC, Raker J, Kaufman S, Rothman K, Walker A, Mcnulty P. Clinical trial of Corynebacterium parvum (intra-lymph-node and intravenous) and radiation therapy in the treatment of head and neck carcinoma. Cancer. 1982;49:239–244. doi: 10.1002/1097-0142(19820115)49:2<239::aid-cncr2820490208>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Choong NW, Kozloff M, Taber D, Hu HS, Wade J, 3rd, Ivy P, Karrison TG, Dekker A, Vokes EE, Cohen EE. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs. 2010;28:677–683. doi: 10.1007/s10637-009-9296-7. Available from: http://dx.doi.org/10.1007/s10637-009-9296-7. [DOI] [PubMed] [Google Scholar]
- 39.Clark JR, Frei E. Chemotherapy for head and neck cancer: progress and controversy in management of patients with Mo disease. Sem Oncol. 1989;16:44–57. [PubMed] [Google Scholar]
- 40.Clavel M, Vermorken JB, Cognetti F, Cappelaere P, de Mulder PH, Schornagel JH, Tueni EA, Verweij J, Wildiers J, Clerico M, et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1994;5:521–526. doi: 10.1093/oxfordjournals.annonc.a058906. [DOI] [PubMed] [Google Scholar]
- 41.Clayman GL, el-Naggar AK, Lippman SM, Henderson YC, Frederick M, Merritt JA, Zumstein LA, Timmons TM, Liu TJ, Ginsberg L, Roth JA, Hong WK, Bruso P, Goepfert H. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16:2221–2232. doi: 10.1200/JCO.1998.16.6.2221. [DOI] [PubMed] [Google Scholar]
- 42.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, Kozloff MF, Clark JI, Yan DH, Liu W, Pierce C, Dancey JE, Stenson K, Blair E, Dekker A, Vokes EE. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. Available from: http://dx.doi.org/10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen EE, Kane MA, List MA, Brockstein BE, Mehrotra B, Huo D, Mauer AM, Pierce C, Dekker A, Vokes EE. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 44.Cohen EE, Sharma MR, Janisch L, Llobrera M, House L, Wu K, Ramirez J, Fleming GF, Stadler WM, Ratain MJ. A phase I study of sirolimus and bevacizumab in patients with advanced malignancies. Eur J Cancer. 2011;47:1484–1489. doi: 10.1016/j.ejca.2011.02.017. Available from: http://dx.doi.org/10.1016/j.ejca.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–2652. doi: 10.1200/JCO.2005.05.3348. Available from: http://dx.doi.org/10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 46.Colnot DR, Quak JJ, Roos JC, de Bree R, Wilhelm AJ, Snow GB, van Dongen GA. Radioimmunotherapy in patients with head and neck squamous cell carcinoma: initial experience. Head Neck. 2001;23:559–565. doi: 10.1002/hed.1078. Available from: http://dx.doi.org/10.1002/hed.1078. [DOI] [PubMed] [Google Scholar]
- 47.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. Available from: http://dx.doi.org/10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 48.Cortesina G, De Stefani A, Galeazzi E, Cavallo GP, Jemma C, Giovarelli M, Vai S, Forni G. Interleukin-2 injected around tumor draining lymph nodes in head and neck cancer. Head Neck. 1991;13:125–131. doi: 10.1002/hed.2880130208. Available from: http://dx.doi.org/10.1002/hed.2880130208. [DOI] [PubMed] [Google Scholar]
- 49.De Andrés L, Brunet J, López-Pousa A, Burgués J, Vega M, Tabernero JM, Mesía R, López JJ. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin Oncol. 1995;13:1493–1500. doi: 10.1200/JCO.1995.13.6.1493. [DOI] [PubMed] [Google Scholar]
- 50.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Calais G. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharyngeal carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys. 2003;55:93–98. doi: 10.1016/S0360-3016(02)03819-1. Available from: http://dx.doi.org/10.1016/S0360-3016(02)03819-1. [DOI] [PubMed] [Google Scholar]
- 51.Dietz A, Boehm A, Horn IS, Kruber P, Bechmann I, Golusinski W, Niederwieser D, Dollner R, Remmerbach TW, Wittekind C, Dietzsch S, Hildebrandt G, Wichmann G. Assay-based response evaluation in head and neck oncology: requirements for better decision making. Eur Arch Otorhinolaryngol. 2010;267:483–494. doi: 10.1007/s00405-009-1191-5. Available from: http://dx.doi.org/10.1007/s00405-009-1191-5. [DOI] [PubMed] [Google Scholar]
- 52.Dietz A, Rudat V, Dreyhaupt J, Pritsch M, Hoppe F, Hagen R, Pfreundner L, Schroder U, Eckel H, Hess M, Schroder M, Schneider P, Jens B, Zenner HP, Werner JA, Engenhardt-Cabillic R, Vanselow B, Plinkert P, Niewald M, Kuhnt T, Budach W, Flentje M. Induction chemotherapy with paclitaxel and cisplatin followed by radiotherapy for larynx organ preservation in advanced laryngeal and hypopharyngeal cancer offers moderate late toxicity outcome (DeLOSI-trial) Eur Arch Otorhinolaryngol. 2009;266:1291–1300. doi: 10.1007/s00405-008-0846-y. Available from: http://dx.doi.org/10.1007/s00405-008-0846-y. [DOI] [PubMed] [Google Scholar]
- 53.Dietz A, Weber A. Dollner R, Hildebrandt. Nicht resektable Kopf-Hals-Tumoren und deren Therapie. Laryngo-Rhino-Otol. 2005;84:200–206. doi: 10.1055/s-2005-861132. Available from: http://dx.doi.org/10.1055/s-2005-861132. [DOI] [PubMed] [Google Scholar]
- 54.Divi V, Worden FP, Prince ME, Eisbruch A, Lee JS, Bradford CR, Chepeha DB, Teknos TN, Hogikyan ND, Moyer JS, Tsien CI, Urba SG, Wolf GT. Chemotherapy alone for organ preservation in advanced laryngeal cancer. Head Neck. 2010;32:1040–1047. doi: 10.1002/hed.21285. Available from: http://dx.doi.org/10.1002/hed.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobrowsky W, Naudé J. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiother Oncol. 2000;57:119. doi: 10.1016/S0167-8140(00)00233-4. Available from: http://dx.doi.org/10.1016/S0167-8140(00)00233-4. [DOI] [PubMed] [Google Scholar]
- 56.Dollner R, Granzow C, Neudert M, Dietz A. Ex vivo chemosensitivity of head and neck carcinoma to cytostaticic drug combinations. Anticancer Res. 2006;26:1651–1655. [PubMed] [Google Scholar]
- 57.Domenge C, Hill C, Lefebvre JL, De Raucourt D, Rhein B, Wibault P, Marandas P, Coche-Dequeant B, Stromboni-Luboinski M, Sancho-Garnier H, Luboinski B. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tete et du Cou (GETTEC) Br J Cancer. 2000;83:1594–1598. doi: 10.1054/bjoc.2000.1512. Available from: http://dx.doi.org/10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donaldson RC. Chemoimmunotherapy for cancer of the head and neck. Am J Surg. 1973;126:507–512. doi: 10.1016/S0002-9610(73)80040-6. Available from: http://dx.doi.org/10.1016/S0002-9610(73)80040-6. [DOI] [PubMed] [Google Scholar]
- 59.Dreyfuss AI, Clark JR, Norris CM, Rossi RM, Lucarini JW, Busse PM, Poulin MD, Thornhill L, Costello R, Posner MR. Docetaxel: an active Drug for squamous cell carcinoma of the head and neck. J Clin Oncol. 1996;14:1672–1678. doi: 10.1200/JCO.1996.14.5.1672. [DOI] [PubMed] [Google Scholar]
- 60.Driemel O, Ettl T, Kölbl O, Reichert TE, Dresp BV, Reuther J, Pistner H. Outcome and histopathologic regression in oral squamous cell carcinoma after preoperative radiochemotherapy. Strahlenther Onkol. 2009;185:296–302. doi: 10.1007/s00066-009-1914-y. Available from: http://dx.doi.org/10.1007/s00066-009-1914-y. [DOI] [PubMed] [Google Scholar]
- 61.Dudek AZ, Lesniewski-Kmak K, Shehadeh NJ, Pandey ON, Franklin M, Kratzke RA, Greeno EW, Kumar P. Phase I study of bortezomib and cetuximab in patients with solid tumours expressing epidermal growth factor receptor. Br J Cancer. 2009;100:1379–1384. doi: 10.1038/sj.bjc.6605043. Available from: http://dx.doi.org/10.1038/sj.bjc.6605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eich HT, Löschcke M, Scheer M, Kocher M, Bongartz R, Wacker S, Zöller JE, Müller RP. Neoadjuvant radiochemotherapy and radical resection for advanced squamous cell carcinoma of the oral cavity. Outcome of 134 patients. Strahlenther Onkol. 2008;184:23–29. doi: 10.1007/s00066-008-1725-6. Available from: http://dx.doi.org/10.1007/s00066-008-1725-6. [DOI] [PubMed] [Google Scholar]
- 63.Eisenberger M, Van Echo D, Aisner J. Carboplatin: the experience in head and neck cancer. Semin Oncol. 1989;16:34–41. [PubMed] [Google Scholar]
- 64.Elferink F, van der Vijgh WJF, Klein I, Vermorken JB, Gall HE, Pinedo HM. Pharmacokinetics of carboplatin after i.v. administration. Cancer Treat Rep. 1987;71:1231–1237. [PubMed] [Google Scholar]
- 65.Ensley JF, Jacobs JR, Weaver A, Kinzie J, Crissman J, Kish JA, Cummings G, Al-Sarraf M. Correlation between response to cisplatinum-combination therapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54:811–814. doi: 10.1002/1097-0142(19840901)54:5<811::AID-CNCR2820540508>3.0.CO;2-E. Available from: http://dx.doi.org/10.1002/1097-0142(19840901)54:5<811::AID-CNCR2820540508>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 66.Erber R, Conradt C, Homann N, Enders C, Finckh M, Dietz A, Weidauer H, Bosch FX. TP53 DNA contact mutations are selectively associated with allelic loss and have a strong clinical impact in head and neck cancer. Oncogene. 1998;16:1671–1679. doi: 10.1038/sj.onc.1201690. Available from: http://dx.doi.org/10.1038/sj.onc.1201690. [DOI] [PubMed] [Google Scholar]
- 67.Ferris RL, DeLeo AB, Whiteside TL. Adjuvant p53 peptide loaded DC-based therapy of subjects with squamous cell cancer of the head and neck (A phase I safety and immunogenicity trial). UPCI Protocol. Pittsburgh, PA: 2003. Available from: http://de.healthetreatment.com/clinical-trial/nct00404339/ [Google Scholar]
- 68.Forastiere AA. Randomized trials of induction chemotherapy: a critical review. Hematol Oncol Clin North Am. 1991;5:725–736. [PubMed] [Google Scholar]
- 69.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. Available from: http://dx.doi.org/10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 70.Forastiere AA, Maor M, Weber RS, Pajak T, Glisson B, Trotti A, Ridge J, Ensley J, Chao C, Cooper J. Long-term results of intergroup RTOG 91-11: a phase III trial to preserve the larynx-induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. Proc Am Soc Clin Oncol. 2006;24(18S;June 20 suppl):abstr 5517. [Google Scholar]
- 71.Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10:1245–1251. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 72.Franklin AL, Belt M, Stockstad ELR, Jukes TH. Biological studies with 4-amino-10-methylpteroyl-glutamic acid. J Biol Chem. 1949;177:621–629. [PubMed] [Google Scholar]
- 73.Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Conners RW, Bridges AJ. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. Available from: http://dx.doi.org/10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 74.Ganzer U, Bier H, Bachert C. Kritische Anmerkungen zur Chemotherapie bösartiger Kopf- und Halsgeschwülste. Laryng Rhinol Otol. 1987;66:200–204. doi: 10.1055/s-2007-998638. Available from: http://dx.doi.org/10.1055/s-2007-998638. [DOI] [PubMed] [Google Scholar]
- 75.Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, Forastiere AA Eastern Cooperative Oncology Group. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. Available from: http://dx.doi.org/10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 76.Gleich LL, Gluckman JL, Armstrong S, Biddinger PW, Miller MA, Balakrishnan K, Wilson KM, Saavedra HI, Stambrook PJ. Alloantigen gene therapy for squamous cell carcinoma of the head and neck: results of a phase-1 trial. Arch Otolaryngol Head Neck Surg. 1998;124:1097–1104. doi: 10.1001/archotol.124.10.1097. [DOI] [PubMed] [Google Scholar]
- 77.Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, Proudlove C, Shaw R. Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck. Health Technol Assess. 2009;13:49–54. doi: 10.3310/hta13suppl3/08. [DOI] [PubMed] [Google Scholar]
- 78.Hadden JW, Verastegui E, Hadden E. IRX-2 and thymosin alpha1 (Zadaxin) increase T lymphocytes in T lymphocytopenic mice and humans. Ann N Y Acad Sci. 2007;1112:245–255. doi: 10.1196/annals.1415.032. Available from: http://dx.doi.org/10.1196/annals.1415.032. [DOI] [PubMed] [Google Scholar]
- 79.Hainsworth JD, Spigel DR, Burris HA, 3rd, Markus TM, Shipley D, Kuzur M, Lunin S, Greco FA. Neoadjuvant chemotherapy/gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients with locally advanced squamous carcinoma of the head and neck. Cancer. 2009;115:2138–2146. doi: 10.1002/cncr.24265. Available from: http://dx.doi.org/10.1002/cncr.24265. [DOI] [PubMed] [Google Scholar]
- 80.Heidelberger C, Chandhari NK, Dannenberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines; a new class of tumor inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. Available from: http://dx.doi.org/10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 81.Hitt R, López-Pousa A, Martínez-Trufero J, Escrig V, Carles J, Rizo A, Isla D, Vega ME, Martí JL, Lobo F, Pastor P, Valentí V, Belón J, Sánchez MA, Chaib C, Pallarés C, Antón A, Cervantes A, Paz-Ares L, Cortés-Funes H. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. Available from: http://dx.doi.org/10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann T, Hafner D, Balló H, Haas I, Bier H. Antitumor activity of anti-epidermal growth factor receptor (eGFR) monoclonal antibodies and cisplatin in ten human head and neck squamous cell carcinoma (HNSCC) lines. Anticancer Res. 1997;17:4419–4426. [PubMed] [Google Scholar]
- 83.Hoffmann TK, Arsov C, Schirlau K, Bas M, Friebe-Hoffmann U, Klussmann JP, Scheckenbach K, Balz V, Bier H, Whiteside TL. T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int J Cancer. 2006;118:1984–1991. doi: 10.1002/ijc.21565. Available from: http://dx.doi.org/10.1002/ijc.21565. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann TK, Bier H, Whiteside TL. Targeting the immune system: novel therapeutic approaches in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2004;53:1055–1067. doi: 10.1007/s00262-004-0530-z. Available from: http://dx.doi.org/10.1007/s00262-004-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffmann TK, Schirlau K, Sonkoly E, Brandau S, Lang S, Pivarcsi A, Balz V, Muller A, Homey B, Boelke E, Reichert T, Friebe-Hoffmann U, Greve J, Schuler P, Scheckenbach K, Schipper J, Bas M, Whiteside TL, Bier H. A novel mechanism for anti-eGFR antibody action involves chemokine-mediated leukocyte infiltration. Int J Cancer. 2009;124:2589–2596. doi: 10.1002/ijc.24269. Available from: http://dx.doi.org/10.1002/ijc.24269. [DOI] [PubMed] [Google Scholar]
- 86.Hoffmann TK, Sonkoly E, Hauser U, van Lierop A, Whiteside TL, Klussmann JP, Hafner D, Schuler P, Friebe-Hoffmann U, Scheckenbach K, Erjala K, Grénman R, Schipper J, Bier H, Balz V. Alterations in the p53 pathway and their association with radio-and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:1100–1109. doi: 10.1016/j.oraloncology.2008.02.006. Available from: http://dx.doi.org/10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 87.Holsinger FC, Kies MS, Diaz EM, JR, Gillenwater AM, Lewin JS, Ginsberg LE, Glisson BS, Garden AS, Ark N, Lin HY, Lee JJ, El-Naggar AK, Ki Hong W, Shin DM, Khuri FR. Durable long-term remission with chemothreapy alone for stage II to IV laryngeal cancer. J Clin Oncol. 2009;27:1976. doi: 10.1200/JCO.2008.17.6396. Available from: http://dx.doi.org/10.1200/JCO.2008.17.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Husmann G, Kaatsch P, Katalinic A, Bertz J, Haberland J, Kraywinkel K, Wolf U. Cancer Incidence and Trends. A joint publication of the Robert Koch Institute and the Society of Cancer Registries in Germany v5. Updated in 2006. [Google Scholar]
- 89.Ikic D, Padovan I, Brodarec I, Knezevic M, Soos E. Application of human leucocyte interferon in patients with tumours of the head and neck. Lancet. 1081;1:1025–1027. doi: 10.1016/s0140-6736(81)92189-9. [DOI] [PubMed] [Google Scholar]
- 90.Ishikawa T, Ikawa T, Eura M, Fukiage T, Masuyama K. Adoptive immunotherapy for head and neck cancer with killer cells induced by stimulation with autologous or allogeneic tumor cells and recombinant interleukin-2. Acta Otolaryngol. 1989;107:346–351. doi: 10.3109/00016488909127519. Available from: http://dx.doi.org/10.3109/00016488909127519. [DOI] [PubMed] [Google Scholar]
- 91.Jacobs C, Makuch R. Efficacy of adjuvant chemotherapy for patients with resectable head and neck cancer: a subset analysis of the head and neck contracts program. J Clin Oncol. 1990;8:838–847. doi: 10.1200/JCO.1990.8.5.838. [DOI] [PubMed] [Google Scholar]
- 92.Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, Vaskovic Z, Tadic L. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol. 2000;18:1458–1464. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 93.Jeremic B, Shibamoto Y, Stanisavljevic B, Milojevic L, Milicic B, Nikolic N. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol. 1997;43:29–37. doi: 10.1016/S0167-8140(97)00048-0. Available from: http://dx.doi.org/10.1016/S0167-8140(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 94.Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, Ziouta Y, Helmke BH, Weidauer H, Schirrmacher V, Herold-Mende C. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004;64:8057–8061. doi: 10.1158/0008-5472.CAN-04-1545. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-04-1545. [DOI] [PubMed] [Google Scholar]
- 95.Kessler P, Grabenbauer G, Leher A, Bloch-Birkholz A, Vairaktaris E, Neukam FW, Sauer R. 5-Jahres-Überlebenswahrscheinlichkeit von Patienten mit primären Plattenepithelkarzinomen der Mundhöhle. Vergleich von zwei Behandlungsstrategien in einer prospektiven Studie. Strahlenther Onkol. 2007;4:184–189. doi: 10.1007/s00066-007-1469-8. Available from: http://dx.doi.org/10.1007/s00066-007-1469-8. [DOI] [PubMed] [Google Scholar]
- 96.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. Available from: http://dx.doi.org/10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 97.Kirby AM, A'Hern RP, D'Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, Box C, Eccles SA, Nutting CM, Harrington KJ. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94:631–636. doi: 10.1038/sj.bjc.6602999. Available from: http://dx.doi.org/10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitahara S, Ikeda M, Inouye T, Matsunaga T, Yamaguchi K, Takayama E, Healy GB, Tsukuda M. Inhibition of head and neck metastasis and/or recurrent cancer by local administration of multi-cytokine inducer OK-432. J Laryngol Otol. 1996;110:449–453. doi: 10.1017/S0022215100133948. Available from: http://dx.doi.org/10.1017/S0022215100133948. [DOI] [PubMed] [Google Scholar]
- 99.Klussmann JP, Preuss SF, Speel EJ. Human papillomavirus and cancer of the oropharynx. Molecular interaction and clinical implications. HNO. 2009;57:113–122. doi: 10.1007/s00106-008-1867-y. Available from: http://dx.doi.org/10.1007/s00106-008-1867-y. [DOI] [PubMed] [Google Scholar]
- 100.Knecht R. Strahlen-, Chemo- und Targettherapie von Kopf-Hals-Karzinomen. HNO. 2009;57:436–445. doi: 10.1007/s00106-009-1909-0. Available from: http://dx.doi.org/10.1007/s00106-009-1909-0. [DOI] [PubMed] [Google Scholar]
- 101.Knecht R, Baghi M, Hambek M, Zamboglou N, Tesch H. TPF induction chemotherapy followed by concurrent radiochemotherapy in the first line therapy of advanced carcinomas of the larynx and pharynx (phase IIb trial) J Clin Oncol. 2006;24(18S;June 20 suppl):abstr 5562. [Google Scholar]
- 102.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer drugs: evidence that cis-diammin-dichloroplatinum (II) and cis-diammine (1,1-cyclobutanedicarboxylato) platinum (II) differ only in their interaction with DNA. Cancer Res. 1986;46:1972–1979. [PubMed] [Google Scholar]
- 103.Kubota E, Katano M, Kurokawa H, Imamura H, Katsuki T, Yamamoto H, Hisatsugu T, Nagumo F, Tadano J. Tumoricidal effect of human PBMC following stimulation with OK-432 and its application for locoregional immunotherapy in head and neck cancer patients. J Craniomaxillofac Surg. 1993;21:30–37. doi: 10.1016/S1010-5182(05)80529-3. Available from: http://dx.doi.org/10.1016/S1010-5182(05)80529-3. [DOI] [PubMed] [Google Scholar]
- 104.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D'Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE. eGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. Available from: http://dx.doi.org/10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laccourreye O, Brasnu D, Bassot V, Ménard M, Khayat D, Laccourreye H. Cisplatin-fluorouracil exclusive chemotherapy for T1–T3N0 glottic squamous cell carcinoma complete clinical responders: five-year results. J Clin Oncol. 1996;14:2331–2336. doi: 10.1200/JCO.1996.14.8.2331. [DOI] [PubMed] [Google Scholar]
- 106.Laccourreye O, Veivers D, Hans S, Ménard M, Brasnu D, Laccourreye H. Chemotherapy alone with curative intent in patients with invasive squamous cell carcinoma of the pharyngolarynx classified as T1–T4N0M0 complete clinical responders. Cancer. 2001;92:1504–1511. doi: 10.1002/1097-0142(20010915)92:6<1504::AID-CNCR1475>3.0.CO;2-V. Available from: http://dx.doi.org/10.1002/1097-0142(20010915)92:6<1504::AID-CNCR1475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 107.Lai SY, Koppikar P, Thomas SM, Childs EE, Egloff AM, Seethala RR, Branstetter BF, Gooding WE, Muthukrishnan A, Mountz JM, Lui VW, Shin DM, Agarwala SS, Johnson R, Couture LA, Myers EN, Johnson JT, Mills G, Argiris A, Grandis JR. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanisms. J Clin Oncol. 2009;27:1235–1242. doi: 10.1200/JCO.2008.17.8251. Available from: http://dx.doi.org/10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lang S, Wollenberg B, Dellian M, Steuer-Vogt MK, Schwenzer K, Sautier W, Chucholowski M, Eckel R, Faas I, Wilmes E, Ehrenfeld M, Arnold W, Kastenbauer E, Hölzel D. Clinical and epidemiological data of patients with malignomas of the head and neck. Laryngorhinootologie. 2002;81:499–508. doi: 10.1055/s-2002-33285. Available from: http://dx.doi.org/10.1055/s-2002-33285. [DOI] [PubMed] [Google Scholar]
- 109.Laramore GE, Scott CB, al Sarraf M, Haselow RE, Ervin TJ, Wheeler R, Jacobs JR, Schuller DE, Gahbauer RA, Schwade JG. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on intergroup study 0034. Int J Radiat Oncol Biol Phys. 1992;23:705–713. doi: 10.1016/0360-3016(92)90642-U. Available from: http://dx.doi.org/10.1016/0360-3016(92)90642-U. [DOI] [PubMed] [Google Scholar]
- 110.Lefebvre JL, Ang KK, Ang K, Bardet E, Barry B, Bernier J, Bourhis J, Budach V, Calais G, Conley B, Forastiere A, Knecht R, Langendijk J, Lefebvre JL, Leemans R, Licitra L, Posner M, de Raucourt D, Rolland F, Temam S, Vermorken JB, Vokes E, Weber RS, Wolf G. Larynx preservation clinical trial design: key issues and recommendations – a consensus panel summary. Head Neck. 2009;31:429–441. doi: 10.1002/hed.21081. Available from: http://dx.doi.org/10.1002/hed.21081. [DOI] [PubMed] [Google Scholar]
- 111.Lefebvre JL, Ang KK Larynx Preservation Consensus Panel. Larynx preservation clinical trial design: key issues and recommendations-a consensus panel summary. Int J Radiat Oncol Biol Phys. 2009;73:1293–1303. doi: 10.1016/j.ijrobp.2008.10.047. Available from: http://dx.doi.org/10.1016/j.ijrobp.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 112.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. J Natl Cancer Inst. 1996;88:890–899. doi: 10.1093/jnci/88.13.890. Available from: http://dx.doi.org/10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 113.León X, Hitt R, Constenla M, Rocca A, Stupp R, Kovács AF, Amellal N, Bessa EH, Bourhis J. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol) 2005;17:418–424. doi: 10.1016/j.clon.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 114.Leuchtenberger C, Lewisohn R, Laslo D, Leuchtenberger R. Folic acid – a tumor growth inhibitor. Exp Biol Med. 1944;55:204–205. [Google Scholar]
- 115.Licitra L, Mesia R, Rivera F, Remenár E, Hitt R, Erfán J, Rottey S, Kawecki A, Zabolotnyy D, Benasso M, Störkel S, Senger S, Stroh C, Vermorken JB. Evaluation of eGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol. 2011;22:1078–1087. doi: 10.1093/annonc/mdq588. Available from: http://dx.doi.org/10.1093/annonc/mdq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantù G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. Available from: http://dx.doi.org/10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 117.List MA, Siston A, Haraf D, Schumm P, Kies M, Stenson K, Vokes EE. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol. 1999;17:1020–1028. doi: 10.1200/JCO.1999.17.3.1020. [DOI] [PubMed] [Google Scholar]
- 118.Liu TJ, Zhang WW, Taylor DL, Roth JA, Goepfert H, Clayman GL. Growth suppression of human head and neck cancer cells by the introduction of a wild-type p53 gene via a recombinant adenovirus. Cancer Res. 1994;54:3662–3667. [PubMed] [Google Scholar]
- 119.Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE, Posner MR TAX 324 Study Group. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–159. doi: 10.1016/S1470-2045(10)70279-5. Available from: http://dx.doi.org/10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S, Moxhon A, Duprez T, Guigay J. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28:21–28. doi: 10.1200/JCO.2009.23.8584. Available from: http://dx.doi.org/10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 121.Machiels JP, Subramanian S, Ruzsa A, Repassy G, Lifirenko I, Flygare A, Sørensen P, Nielsen T, Lisby S, Clement PM. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12:333–343. doi: 10.1016/S1470-2045(11)70034-1. Available from: http://dx.doi.org/10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 122.Madoc-Jones H, Mauro F. Interphase action of vinblastine and vincristine: differences in their lethal action through the mitotic cycle of cultured mamalian cells. J Cell Physiol. 1968;72:185–196. doi: 10.1002/jcp.1040720306. Available from: http://dx.doi.org/10.1002/jcp.1040720306. [DOI] [PubMed] [Google Scholar]
- 123.Martinez-Trufero J, Isla D, Adansa JC, Irigoyen A, Hitt R, Gil-Arnaiz I, Lambea J, Lecumberri MJ, Cruz JJ. Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. Br J Cancer. 2010;102:1687–1691. doi: 10.1038/sj.bjc.6605697. Available from: http://dx.doi.org/10.1038/sj.bjc.6605697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattijssen V, De Mulder PH, Schornagel JH, Verweij J, van den Broek P, Galazka A, Roy S, Ruiter DJ. Clinical and immunopathological results of a phase II study of perilymphatically injected recombinant interleukin-2 in locally far advanced, nonpretreated head and neck squamous cell carcinoma. J Immunother. 1991;10:63–68. doi: 10.1097/00002371-199102000-00009. Available from: http://dx.doi.org/10.1097/00002371-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 125.Medenica R, Slack N. Clinical results of leukocyte interferon-induced tumor regression in resistant human metastatic cancer resistant to chemotherapy and/or radiotherapy-pulse therapy schedule. Cancer Drug Deliv. 1985;2:53–76. doi: 10.1089/cdd.1985.2.53. Available from: http://dx.doi.org/10.1089/cdd.1985.2.53. [DOI] [PubMed] [Google Scholar]
- 126.Meneses A, Verastegui E, Barrera JL, de la Garza J, Hadden JW. Lymph node histology in head and neck cancer: impact of immunotherapy with IRX-2. Int Immunopharmacol. 2003;3:1083–1091. doi: 10.1016/S1567-5769(03)00017-1. Available from: http://dx.doi.org/10.1016/S1567-5769(03)00017-1. [DOI] [PubMed] [Google Scholar]
- 127.Mesía R, Rivera F, Kawecki A, Rottey S, Hitt R, Kienzer H, Cupissol D, De Raucourt D, Benasso M, Koralewski P, Delord JP, Bokemeyer C, Curran D, Gross A, Vermorken JB. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21:1967–1973. doi: 10.1093/annonc/mdq077. Available from: http://dx.doi.org/10.1093/annonc/mdq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohr C, Bohndorf W, Carstens J, Härle F, Hausamen JE, Hirche H, Kimmig H, Kutzner J, Mühling J, Reither J, Sack H, Schettler D, Stellmach R, Wagner W, Wannenmacher MF. Preoperative radiochemotherapy and radical surgery in comparison with radical surgery alone. A prospective, multicentric randomized DÖSAK study of advanced squamous cell carcinoma of the oral cavity and the oropharynx (a 3-year follow-up) Int J Oral Maxillofac Surg. 1994;23:140–148. doi: 10.1016/S0901-5027(05)80288-7. Available from: http://dx.doi.org/10.1016/S0901-5027(05)80288-7. [DOI] [PubMed] [Google Scholar]
- 129.Mozet C, Wichmann G, Dietz A. Translational approaches in cancer stem cell research. HNO. 2011;59:859–865. doi: 10.1007/s00106-011-2363-3. Available from: http://dx.doi.org/10.1007/s00106-011-2363-3. [DOI] [PubMed] [Google Scholar]
- 130.Müller WEG, Zahn RK. Bleomycin: mode of action on DNA. Gann Monogr. 1976;19:51–62. [Google Scholar]
- 131.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/S0168-1702(02)00190-9. Available from: http://dx.doi.org/10.1016/S0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 132.Munro AJ. An overview of randomised controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer. 1995;71:83–91. doi: 10.1038/bjc.1995.17. Available from: http://dx.doi.org/10.1038/bjc.1995.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nemunaitis J. A phase 3, MULTI-center, open-LABEL, randomized study of Adenoviral p53 Gene Therapy (ADVEXIN(R)) versus methotrexate in patients with recurrent, refractory squamous cell carcinoma of the head and neck (SCCHN) investigated the clinical benefit of ADVEXIN vs methotrexate to address an unmet medical need. 12th Annual Meeting of the American Society of Gene Therapy; 2009 May 27-30; San Diego, CA, USA. 2009. [Google Scholar]
- 134.Nemunaitis J, Clayman G, Agarwala SS, Hrushesky W, Wells JR, Moore C, Hamm J, Yoo G, Baselga J, Murphy BA, Menander KA, Licato LL, Chada S, Gibbons RD, Olivier M, Hainaut P, Roth JA, Sobol RE, Goodwin WJ. Biomarkers Predict p53 Gene Therapy Efficacy in Recurrent Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2009;15:7719–7725. doi: 10.1158/1078-0432.CCR-09-1044. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-09-1044. [DOI] [PubMed] [Google Scholar]
- 135.Nemunaitis J, Cunningham C, Tong AW, Post L, Netto G, Paulson AS, Rich D, Blackburn A, Sands B, Gibson B, Randlev B, Freeman S. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10:341–352. doi: 10.1038/sj.cgt.7700585. Available from: http://dx.doi.org/10.1038/sj.cgt.7700585. [DOI] [PubMed] [Google Scholar]
- 136.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 137.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, Romel L, Randlev B, Kaye S, Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 138.Nemunaitis JJ. Efficacy of adenoviral p53 gene therapy (advexin) in pivotal clinical trials for recurrent squamous cell carcinoma of the head and neck. 11th Annual Meeting of the American Society of Gene Therapy; 2008 May 28-June 1; Boston, MA, USA. 2008. [Google Scholar]
- 139.Olivari AJ, Pradier R, Califano L, Guardo A, Glait HM. Levamisole in squamous cell carcinoma of the head and neck. Cancer Treatment Rep. 1979;63:983–990. [PubMed] [Google Scholar]
- 140.Paccagnella A, Ghi MG, Loreggian L, Buffoli A, Koussis H, Mione CA, Bonetti A, Campostrini F, Gardani G, Ardizzoia A, Dondi D, Guaraldi M, Cavallo R, Tomio L, Gava A Gruppo di Studio Tumori della Testa e del Collo XRP 6976 F/2501 Study. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol. 2010;21:1515–1522. doi: 10.1093/annonc/mdp573. Available from: http://dx.doi.org/10.1093/annonc/mdp573. [DOI] [PubMed] [Google Scholar]
- 141.Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, Jirillo A, Tomio L, Fila G, Fede A, Endrizzi L, Bari M, Sampognaro E, Balli M, Gava A, Pappagallo GL, Fiorention MV. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86:265–272. doi: 10.1093/jnci/86.4.265. Available from: http://dx.doi.org/10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 142.Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, Willenberg HS, Schinner S, Cohnen M, Seissler J, Zacharowski K, Scherbaum WA, Schott M. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol. 2008;180:1462–1470. doi: 10.4049/jimmunol.180.3.1462. [DOI] [PubMed] [Google Scholar]
- 143.Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2009;31:1255–1262. doi: 10.1002/hed.21096. Available from: http://dx.doi.org/10.1002/hed.21096. [DOI] [PubMed] [Google Scholar]
- 144.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. Available from: http://dx.doi.org/10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 145.Peres J. HPV-positive oropharyngeal cancer: data may justify new approach. J Natl Cancer Inst. 2010;102:1456–1459. doi: 10.1093/jnci/djq403. Available from: http://dx.doi.org/10.1093/jnci/djq403. [DOI] [PubMed] [Google Scholar]
- 146.Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ, Ang KK, Clayman GL, Fisher SG, Forastiere AA, Harrison LB, Lefebvre JL, Leupold N, List MA, O'Malley BO, Patel S, Posner MR, Schwartz MA, Wolf GT. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24:3693–3704. doi: 10.1200/JCO.2006.07.4559. Available from: http://dx.doi.org/10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 147.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 148.Pignon JP, le Maître A, Maillard E, Bourhis J MACH-NC Collaborative Group. Metaanalysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. Available from: http://dx.doi.org/10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 149.Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M, Calais G. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498–506. doi: 10.1093/jnci/djp007. Available from: http://dx.doi.org/10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 150.Posner MR. Paradigm shift in the tratment of head and neck cancer: the role of neoadjuvant chemotherapy. Oncologist. 2005;10:11–19. doi: 10.1634/theoncologist.10-90003-11. Available from: http://dx.doi.org/10.1634/theoncologist.10-90003-11. [DOI] [PubMed] [Google Scholar]
- 151.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM, Jr, Haddad RI TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. Available from: http://dx.doi.org/10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 152.Program HaNC: Adjuvant chemotherapy for advanced head and neck squamous cell carcinoma: Final report of Head and Neck Contracts Program. Cancer. 1987;60:301. doi: 10.1002/1097-0142(19870801)60:3<301::aid-cncr2820600306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 153.Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Cilengitide (EMD 121974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral Oncol. 2004;40:228–230. doi: 10.1016/j.oraloncology.2003.08.003. Available from: http://dx.doi.org/10.1016/j.oraloncology.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 154.Relic A, Scheich M, Stapf J, Voelter C, Hoppe F, Hagen R, Pfreundner L. Salvage surgery after induction chemotherapy with paclitaxel/cisplatin and primary radiotherapy for advanced laryngeal and hypopharyngeal carcinomas. Eur Arch Otorhinolaryngol. 2009;266:1799–1805. doi: 10.1007/s00405-009-0946-3. Available from: http://dx.doi.org/10.1007/s00405-009-0946-3. [DOI] [PubMed] [Google Scholar]
- 155.Richman SP, Livingston RB, Gutterman JU, Suen JY, Hersh EM. Chemotherapy versus chemo-immunotherapy of head and neck cancer: report of a randomized study. Cancer Treatment Rep. 1976;60:535–539. [PubMed] [Google Scholar]
- 156.Richtsmeier WJ, Koch WM, McGuire WP, Poole ME, Chang EH. Phase I-II study of advanced head and neck squamous cell carcinoma patients treated with recombinant human interferon gamma. Arch Otolaryngol Head Neck Surg. 1990;116:1271–1277. doi: 10.1001/archotol.1990.01870110043004. Available from: http://dx.doi.org/10.1001/archotol.1990.01870110043004. [DOI] [PubMed] [Google Scholar]
- 157.Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, Trotti A, Bernier J, Bourhis J, Ringash J, Henke M, Kenny L. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. Available from: http://dx.doi.org/10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 158.Rosenthal DI, Lewin JS, Eisbruch A. Dysphagia after radiation therapy or chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. Available from: http://dx.doi.org/10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 159.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and eGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. Available from: http://dx.doi.org/10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 160.Rudat V, Eckel H, Volling P, Schröder M, Staar S, Wallner F, Wannenmacher M, Dietz A. Long-term results of a prospective multicenter phase II study to preserve the larynx function using concomitant boost radiochemotherapy with carboplatin. Radiother Oncol. 2008;89:33–37. doi: 10.1016/j.radonc.2008.06.005. Available from: http://dx.doi.org/10.1016/j.radonc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 161.Sauter A, Kloft C, Gronau S, Bogeschdorfer F, Erhardt T, Golze W, Schroen C, Staab A, Riechelmann H, Hoermann K. Pharmacokinetics, immunogenicity and safety of bivatuzumab mertansine, a novel CD44v6-targeting immunoconjugate, in patients with squamous cell carcinoma of the head and neck. Int J Oncol. 2007;30:927–935. [PubMed] [Google Scholar]
- 162.Schneider I, Holzgraefe M, Schröder M. Carboplatin and paclitaxel induction chemotherapy for patients with oropharyngeal carcinoma. Laryngo-Rhino-Otol. 2008;87:719–722. doi: 10.1055/s-2008-1077307. Available from: http://dx.doi.org/10.1055/s-2008-1077307. [DOI] [PubMed] [Google Scholar]
- 163.Schuller DE, Metch B, Stem DW, Mattox D, McCracken JD. Preoperative chemotherapy in advanced resectable head and neck cancer: final report of the southwest oncology group. Laryngoscope. 1988;98:1205–1211. doi: 10.1288/00005537-198811000-00011. Available from: http://dx.doi.org/10.1288/00005537-198811000-00011. [DOI] [PubMed] [Google Scholar]
- 164.Schütz JD, Collins JM, Wallace HJ, Diasio RB. Alteration of the secondary structure of newly synthesized DNA from murine bone marrow cells by 5-fluorouracil. Cancer Res. 1986;46:119–123. [PubMed] [Google Scholar]
- 165.Seiwert TY, Clement PM, Cupissol D, Del Campo J, de Mont-Serrat H, Thurm HC, Blackman HS, Cohen EE. BIBW 2992 versus cetuximab in patients with metastatic or recurrent head and neck cancer (SCCHN) after failure of platinum-containing therapy with a cross-over period for progressing patients: Preliminary results of a randomized, open-label phase II study. J Clin Oncol. 2010;28(15S;suppl):abstr 5501. [Google Scholar]
- 166.Seiwert TY, Haraf DJ, Cohen EE, Stenson K, Witt ME, Dekker A, Kocherginsky M, Weichselbaum RR, Chen HX, Vokes EE. Phase I study of bevacizumab added to fluorouracil-and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26:1732–1741. doi: 10.1200/JCO.2007.13.1706. Available from: http://dx.doi.org/10.1200/JCO.2007.13.1706. [DOI] [PubMed] [Google Scholar]
- 167.Semrau S, Waldfahrer F, Lell M, Linke R, Klautke G, Kuwert T, Uder M, Iro H, Fietkau R. Feasibility, toxicity, and efficacy of short induction chemotherapy of docetaxel plus cisplatin or carboplatin (TP) followed by concurrent chemoradio-therapy for organ preservation in advanced cancer of the hypopharynx, larynx, and base of tongue. Early results. Strahlenther Onkol. 2011;187:15–22. doi: 10.1007/s00066-010-2178-2. Available from: http://dx.doi.org/10.1007/s00066-010-2178-2. [DOI] [PubMed] [Google Scholar]
- 168.Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, Francis P, Harvey L, Klein M, Zhang W, Dancey J, Eisenhauer EA, Winquist E. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol. 2007;25:2178–2183. doi: 10.1200/JCO.2006.07.6547. Available from: http://dx.doi.org/10.1200/JCO.2006.07.6547. [DOI] [PubMed] [Google Scholar]
- 169.Smart C, Rochlin D, Nahum A, Silva A, Wagner D. Clinical experience with vinblastine sulphate in squamous cell and other malignancies. Cancer Chemoth Rep. 1964;34:31–45. [PubMed] [Google Scholar]
- 170.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, Grandis JR. Mutant epidermal growth factor receptor (eGFRvIII) contributes to head and neck cancer growth and resistance to eGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 171.Sommer A, Santi DV. Purification and amino acid analysis of an active site peptide from thymidylate synthetase containing covalently bound 5´-fluoro-2´-deoxyuridylate and methylene tetrachloride. Biochem Biophys Res Commun. 1974;57:689–696. doi: 10.1016/0006-291X(74)90601-9. Available from: http://dx.doi.org/10.1016/0006-291X(74)90601-9. [DOI] [PubMed] [Google Scholar]
- 172.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. Available from: http://dx.doi.org/10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 173.Squadrelli-Saraceno M, Rivoltini L, Cantu G, Ravagnani F, Parmiani G, Molinari R. Local adoptive immunotherapy of advanced head and neck tumors with LAK cells and interleukin-2. Tumori. 1990;76:566–571. doi: 10.1177/030089169007600611. [DOI] [PubMed] [Google Scholar]
- 174.Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, Flentje M, Eckel HE, Mueller RP. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy – results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–1171. doi: 10.1016/S0360-3016(01)01544-9. Available from: http://dx.doi.org/10.1016/S0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 175.Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Hirsch FR, Varella-Garcia M, Ghiorghiu S, Hargreaves L, Armour A, Speake G, Swaisland A, Vokes EE. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. Available from: http://dx.doi.org/10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 176.Stroomer JW, Roos JC, Sproll M, Quak JJ, Heider KH, Wilhelm BJ, Castelijns JA, Meyer R, Kwakkelstein MO, Snow GB, Adolf GR, van Dongen GA. Safety and biodistribution of 99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in head and neck cancer patients. Clin Cancer Res. 2000;6:3046–3055. [PubMed] [Google Scholar]
- 177.Takahara PM, Frederick CA, Lippard SJ. Crystal Structure of the Anticancer Drug Cisplatin Bound to Duplex DNA. J Am Chem Soc. 1996;118:12309–12321. doi: 10.1021/ja9625079. Available from: http://dx.doi.org/10.1021/ja9625079. [DOI] [Google Scholar]
- 178.Taylor SG, Raynor WJ, Jr, Bytell DE, Sisson GA. A randomized trial of adjuvant BCG immunotherapy in head and neck cancer. Arch Otolaryngol. 1983;109:544–549. doi: 10.1001/archotol.1983.00800220050013. Available from: http://dx.doi.org/10.1001/archotol.1983.00800220050013. [DOI] [PubMed] [Google Scholar]
- 179.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The VA Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. Available from: http://dx.doi.org/10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 180.To WC, Wood BG, Krauss JC, Strome M, Esclamado RM, Lavertu P, Dasko D, Kim JA, Plautz GE, Leff BE, Smith V, Sandstrom-Wakeling K, Shu S. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: a phase 1 study. Arch Otolaryngol Head Neck Surg. 2000;126:1225–1231. doi: 10.1001/archotol.126.10.1225. [DOI] [PubMed] [Google Scholar]
- 181.Trotti A, Bentzen SM. The need for adverse effects reporting standards in oncology clinical trials. J Clin Oncol. 2004;22:19–22. doi: 10.1200/JCO.2004.10.911. Available from: http://dx.doi.org/10.1200/JCO.2004.10.911. [DOI] [PubMed] [Google Scholar]
- 182.Umezawa, H Bleomycin and other antitumor antibiotics of high molecular weight. Antimicrob Agents Chemother (Bethesda) 1965;5:1079–1085. [PubMed] [Google Scholar]
- 183.Urba SG, Wolf GT, Eisbruch A, Worden F, Lee J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N, Taylor J. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24:593–598. doi: 10.1200/JCO.2005.01.2047. Available from: http://dx.doi.org/10.1200/JCO.2005.01.2047. [DOI] [PubMed] [Google Scholar]
- 184.Vermorken JB, Guigay J, Mesia R, Trigo JM, Keilholz U, Kerber A, Bethe U, Picard M, Brummendorf TH. Phase I/II trial of cilengitide with cetuximab, cisplatin and 5-fluorouracil in recurrent and/or metastatic squamous cell cancer of the head and neck: findings of the phase I part. Br J Cancer. 2011;104:1691–1696. doi: 10.1038/bjc.2011.152. Available from: http://dx.doi.org/10.1038/bjc.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. Available from: http://dx.doi.org/10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 186.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL EORTC 24971/TAX 323 Study Group. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. Available from: http://dx.doi.org/10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 187.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler M, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. Available from: http://dx.doi.org/10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 188.Victora GD, Socorro-Silva A, Volsi EC, Abdallah K, Lima FD, Smith RB, Moyses RA, Zárate-Bladés CR, Michaluart P, Silva CL, Kalil J, Coelho V. Immune response to vaccination with DNA-Hsp65 in a phase I clinical trial with head and neck cancer patients. Cancer Gene Ther. 2009;16:598–608. doi: 10.1038/cgt.2009.9. Available from: http://dx.doi.org/10.1038/cgt.2009.9. [DOI] [PubMed] [Google Scholar]
- 189.Vlock DR, Johnson J, Myers E, Day R, Gooding WE, Whiteside T, Pelch K, Sigler B, Wagner R, Colao D, Rust D. Preliminary trial of non-recombinant interferon-alpha in recurrent squamous cell carcinoma of the head and neck. Head Neck. 1991;13:15–21. doi: 10.1002/hed.2880130103. Available from: http://dx.doi.org/10.1002/hed.2880130103. [DOI] [PubMed] [Google Scholar]
- 190.Vlock DR, Snyderman CH, Johnson JT, Myers EN, Eibling D, Rubin JS, Kirkwood JM, Dutcher JP, Adams GL. Phase Ib trial of the effect of peritumoral and intronodal injections of interleukin-2 in patients with advanced squamous cell carcinoma of the head and neck: an Eastern Cooperative Oncology Group trial. J Immunother. 1994;15:134–139. doi: 10.1097/00002371-199402000-00007. Available from: http://dx.doi.org/10.1097/00002371-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 191.Vokes EE. Head and neck cancer. In: Perry MC, editor. Chemotherapy Source Book. Baltimore: Williams & Wilkins; 1992. pp. 918–931. [Google Scholar]
- 192.Vokes EE, Weichselbaum RR. Concomitant chemotherapy: rationale and clinical experience in patients with solid tumors. J Clin Oncol. 1990;8:911–934. doi: 10.1200/JCO.1990.8.5.911. [DOI] [PubMed] [Google Scholar]
- 193.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. Available from: http://dx.doi.org/10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 194.von Hoff DD, Sanbach JF, Clark GM, Turner JN, Forseth BF, Piccart MJ, Colombo N, Muggia FM. Selection of cancer chemotherapy for a patient by an in vitro assay versus a clinician. J Natl Cancer Inst. 1990;82:110. doi: 10.1093/jnci/82.2.110. [DOI] [PubMed] [Google Scholar]
- 195.Wanebo HJ, Oettgen HF, Mike V, Pinsky CM, Strong EW, Hilal EY. Adjuvant trial of levamisole in patients with squamous cell cancer of the head and neck: a preliminary report. Recent Results Cancer Res. 1978;68:324–333. doi: 10.1007/978-3-642-81332-0_49. [DOI] [PubMed] [Google Scholar]
- 196.Wang CJ, Knecht R. Current concepts of organ preservation in head and neck cancer. Eur Arch Otorhinolaryngol. 2011;268:481–487. doi: 10.1007/s00405-010-1407-8. Available from: http://dx.doi.org/10.1007/s00405-010-1407-8. [DOI] [PubMed] [Google Scholar]
- 197.Wansom D, Light E, Worden F, Prince M, Urba S, Chepeha DB, Cordell K, Eisbruch A, Taylor J, D'Silva N, Moyer J, Bradford CR, Kurnit D, Kumar B, Carey TE, Wolf GT. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136:1267–1273. doi: 10.1001/archoto.2010.211. Available from: http://dx.doi.org/10.1001/archoto.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weaver A, Fleming S, Ensley J, Kish JA, Jacobs J, Kinzie J, Crissman J, Al-Sarraf M. Superior clinical response and survival rates with initial bolus of cisplatin and 120 hour infusion of 5-fluorouracil before definitive therapy for locally advanced head and neck cancer. Am J Surg. 1984;148:525–529. doi: 10.1016/0002-9610(84)90381-7. Available from: http://dx.doi.org/10.1016/0002-9610(84)90381-7. [DOI] [PubMed] [Google Scholar]
- 199.Weaver A, Flemming S, Kish J, Vandenberg H, Jacob J, Crissman J, Al-Sarraf M. Cis-platinum and 5-fluorouracil as induction therapy for advanced head and neck cancer. Am J Surg. 1982;144:445–448. doi: 10.1016/0002-9610(82)90419-6. Available from: http://dx.doi.org/10.1016/0002-9610(82)90419-6. [DOI] [PubMed] [Google Scholar]
- 200.Weisenthal LM. Predictive assays for drug and radiation resistance. In: Masters J, editor. Human cancer in primary culture. Dordrecht, NL: Kluver Verlag; 1991. pp. 103–146. [Google Scholar]
- 201.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–S283. doi: 10.1016/j.jaci.2009.09.045. Available from: http://dx.doi.org/10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Whiteside TL. Immunobiology and immunotherapy of head and neck cancer. Curr Oncol Rep. 2001;3:46–55. doi: 10.1007/s11912-001-0042-3. Available from: http://dx.doi.org/10.1007/s11912-001-0042-3. [DOI] [PubMed] [Google Scholar]
- 203.Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, Urba SG. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28:3330–3335. doi: 10.1200/JCO.2009.25.6834. Available from: http://dx.doi.org/10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wittekindt C, Wagner S, Klußmann JP. HPV-associated head and neck cancer: The basics of molecular and translational research. HNO. 2011;59:885–892. doi: 10.1007/s00106-011-2357-1. Available from: http://dx.doi.org/10.1007/s00106-011-2357-1. [DOI] [PubMed] [Google Scholar]
- 205.Wittes R, Heller K, Randolph V, Howard J, Vallejo A, Farr H, Harrold C, Gerold F, Shah J, Spiro R, Strong E. Cis-Dichlorodiammineplatinum(II)-based chemotherapy as initial treatment of advanced head and neck cancer. Cancer Treat Rep. 1979;63:1533–1538. [PubMed] [Google Scholar]
- 206.Wollenberg B. Implication of stem cells in the biology and therapy of head and neck cancer. Laryngo-Rhino-Otol. 2011;90:S110–S119. doi: 10.1055/s-0030-1270442. Available from: http://dx.doi.org/10.1055/s-0030-1270442. [DOI] [PubMed] [Google Scholar]
- 207.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, Urba SG, Eisbruch A, Teknos TN, Chepeha DB, Prince ME, Tsien CI, D'Silva NJ, Yang K, Kurnit DM, Mason HL, Miller TH, Wallace NE, Bradford CR, Carey TE. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138. doi: 10.1200/JCO.2007.12.7597. Available from: http://dx.doi.org/10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Worden FP, Moyer J, Lee JS, Taylor JM, Urba SG, Eisbruch A, Teknos TN, Chepeha DB, Prince ME, Hogikyan N, Lassig AA, Emerick K, Mukherji S, Hadjiski L, Tsien CI, Miller TH, Wallace NE, Mason HL, Bradford CR, Wolf GT. Chemoselection as a strategy for organ preservation in patients with T4 laryngeal squamous cell carcinoma with cartilage invasion. Laryngoscope. 2009;119:1510–1517. doi: 10.1002/lary.20294. Available from: http://dx.doi.org/10.1002/lary.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wright JC, Cobb JB, Gumport SL, Safadi D. Investigation of the relationship between clinical and tissue-culture response to chemotherapeutic agents on human cancer. N Engl J Med. 1957;257:1207. doi: 10.1056/NEJM195712192572502. Available from: http://dx.doi.org/10.1056/NEJM195712192572502. [DOI] [PubMed] [Google Scholar]
- 210.Wu A, Zeng Q, Kang TH, Peng S, Roosinovich E, Pai SI, Hung CF. Innovative DNA vaccine for human papillomavirus (HPV)-associated head and neck cancer. Gene Ther. 2011;18:304–312. doi: 10.1038/gt.2010.151. Available from: http://dx.doi.org/10.1038/gt.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, Ang J, Temple G, Bell S, Shahidi M, Uttenreuther-Fischer M, Stopfer P, Futreal A, Calvert H, de Bono JS, Plummer R. Phase I trial of the irreversible eGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. Available from: http://dx.doi.org/10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 212.Yoo GH, Moon J, Leblanc M, Lonardo F, Urba S, Kim H, Hanna E, Tsue T, Valentino J, Ensley J, Wolf G. A phase 2 trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene therapy followed by chemoradiotherapy for advanced, resectable squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: report of the Southwest Oncology Group. Arch Otolaryngol Head Neck Surg. 2009;135:869–874. doi: 10.1001/archoto.2009.122. Available from: http://dx.doi.org/10.1001/archoto.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zorat PL, Paccagnella A, Cavaniglia G, Loreggian L, Gava A, Mione CA, Boldrin F, Marchiori C, Lunghi F, Fede A, Bordin A, Da Mosto MC, Sileni VC, Orlando A, Jirillo A, Tomio L, Pappagallo GL, Ghi MG. Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst. 2004;96:1714–1717. doi: 10.1093/jnci/djh306. Available from: http://dx.doi.org/10.1093/jnci/djh306. [DOI] [PubMed] [Google Scholar]


