Abstract
Head and Neck Squamous Cell Carcinomas (HNSCC) are the 6th most common cancers worldwide. While incidence rates for cancer of the hypopharynx and larynx are decreasing, a significant increase in cancer of the oropharynx (OSCC) is observed. Classical risk factors for HNSCC are smoking and alcohol. It has been shown for 25 to 60% of OSCC to be associated with an infection by oncogenic human papilloma viruses (HPV). The development of “common” cancer of the head and neck is substantially enhanced by an accumulation of genetic changes, which lead to an inactivation of tumor suppressor genes or activation of proto-oncogenes. A more or less uniform sequence of different DNA-damages leads to genetic instability. In this context, an early and frequent event is deletion on the short arm of chromosome 9, which results in inactivation of the p16-gene. In contrast, for HPV-induced carcinogenesis, expression of the viral proteins E6 and E7 is most important, since they lead to inactivation of the cellular tumor-suppressor-proteins p53 and Rb. The natural route of transoral infection is a matter of debate; peroral HPV-infections might be frequent and disappear uneventfully in most cases. Smoking seems to increase the probability for developing an HPV-associated OSCC. The association of HNSCC with HPV can be proven with established methods in clinical diagnostics. In addition to classical prognostic factors, diagnosis of HPV-association may become important for selection of future therapies. Prognostic relevance of HPV probably surmounts many known risk-factors, for example regional metastasis. Until now, no other molecular markers are established in clinical routine. Future therapy concepts may vary for the two subgroups of patients, particularly patients with HPV-associated OSCC may take advantage of less aggressive treatments. Finally, an outlook will be given on possible targeted therapies.
Keywords: head and neck cancer, human papillomavirus, carcinogenesis, prognosis, molecular markers
1 Epidemiology of Head and Neck Squamous Cell Carcinoma
Worldwide, Head and Neck Squamous Cell Carcinoma (HNSCC) is the sixth most malignant tumor. Yearly, 650.000 new cases are estimated; nearly 50% will later die due to the disease [124]. In the USA, 45.660 new cases were diagnosed in 2007, corresponding 3.2% of all new cancer cases [72]. The incidence for Germany and other western European states as well as for the USA is estimated to be 15/100.000. A long-term observation of the incidence statistics revealed that incidence and morbidity rates are changing. In this context it is noteworthy that the dynamics of statistics differ within different localizations. On principle, high incidence rates would be expected in regions with high tobacco and alcohol consumption. Yearly incidence rates around 30/100.000 male inhabitants were reported for Spain, Hong Kong, India, Europe, Brazil and for Afro-American US-citizens.
Also, high incidence rates (>10/100.000) for females have been reported for the Indian subcontinent, for Hong Kong and the Philippines. The highest value for male persons was found in the Alsace (64/100.000) and with 16/100.000 for females in Madras/India [148]. General trends of incidence rates reflect in- or decrease of risk factors as well as incidence rates of various subgroups. The 5-year survival rate varies between 20% and more than 90%, depending on tumor stadium and localization of the primary tumor.
1.1 Trends at various subsites
As mentioned before, in the western world the proportion of Head and Neck Squamous Cell Carcinoma is less than 5% of new tumor diseases [72]. Thereby age-corrected incidence rates are reported from various countries to decrease for all carcinoma of the head-neck region. Mainly in USA separate age-corrected incidence rates for carcinoma of the larynx, oral region and hypopharynx, referred to a standard population of the year 2000 were found to decrease [172]. According to an estimation of the Robert-Koch-Institut, age-corrected morbidity decreased by about 40% for males with laryngeal carcinoma since 1980. While for carcinoma of the oral cavity and the pharynx, after a significant rise until 1990, the age-corrected morbidity and mortality rates decreased in males, these rates remained constant for females. This decrease is mainly due to a decrease in smokers during the last 40 years. As is well known for the same period of observation, the 5-year survival rates have hardly changed. Simultaneously observed decreasing mortality rates may be consequently caused by a decrease in new diseases.
In spite of the observation that the number of new cases decreases, these effects are not consistent with age and subgroups. If sublocations are considered, the decreasing incidence rate of head-neck-tumors is compensated by a significant increase of the incidence values for OSCC [59], [156], [172]. Shiboski et al. reported in 2007 about an increase of oral and oropharynx carcinoma in young adults [156]. Especially for tonsillar and sublingual carcinoma increased incidence rates were published several times. In a recent publication from Denmark, a sixfold increase of incidence rates for tonsillar carcinoma in male patients up to the age of 60 years was reported for the time between 1978 and 2007 [9]. Recently several Spanish cancer registers were evaluated and published. Likewise, the result was a significant increase of new OSCC diseases between 1991 and 2001 in the male population [27]. In the US-population the yearly increase of new OSCC was estimated to be 5% [171]. Similarly in Norway between 1991 and 2005 increasing incidence rates of 5% for males and 4.2% for females were published for OSCC. A twofold increase of new diseases was predicted for 2020 [110].
Apparently the number of new OSCC seems to increase, whereby a great portion of these new OSCC is represented by men in their fourth to sixth decade [19]. Within the last two decades it was also shown that oncogenic HPV act as an own risk factor for the development of OSCC [2], [33], [83]. For the same period it was observed that the portion of OSCC characterized by an HPV-association increased.
In a recent publication from Finland, the portion of p16-protein-positiv biopsies (i.e. HPV-positive tumors) of head and neck carcinoma from the years 1990 to 2000 and from 2000 to 2007 was determined, resulting in a doubling of positive tumors from 22 to 41%. With decreasing numbers of new cases of head and neck carcinoma on the one hand and the coincident identification of oncogenic HPV as a new risk factor for OSCC and increasing incidence rates, it is obvious that a virus epidemic is discussed, which might cause cancer. The reason for the increased spread of oncogenic HPV in the head and neck region is seen as a consequence of a changed sexual behavior with special relevance to oral sexual practice [32].
1.2 HPV-associated carcinoma
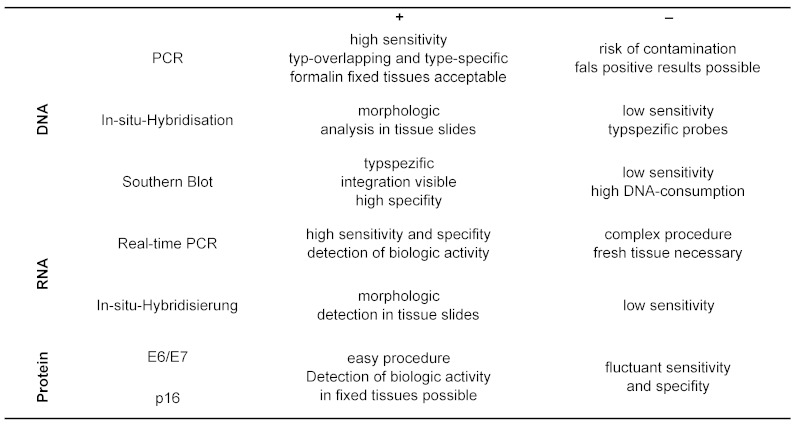
In 1985 the DNA of HPV16 was found in a carcinoma of the oral cavity by means of Southern blot-technique [98]. Meanwhile a connection between HPV and carcinoma has been proven by several epidemiologic and molecular biological investigations [33]. Subsequently, several case series with different prevalence of HPV-DNA in OSCC were published. On an international basis, the portion of OSCC with an association to HPV varies significantly within one country and also with time. In publications from the USA the prevalence for HPV-positive OSCC varies between 40 and 80%. In Germany values between 30 and 40% became known and from Scandinavia very recently values of more than 90% were published [59], [104], [114]. During the last years with improved techniques, the prevalence rate for an association of OSCC with oncogenic HPV could be determined for Germany to be between 30 and 50% [80], [82].
In the various subgroups of head and neck carcinoma oncogenic HPV were taken responsible for the varying portions of OSCC. According to a pooled analysis of several experimental investigations to the incidence of HPV-infections in head and neck carcinoma, the probability of an HPV-association can be expected in decreasing order for the various subgroups as follows: oropharynx, → larynx/hypopharynx, → oral cavity. If all subgroups are pooled, the total portion of head and neck tumors caused by infection with oncogenic HPV-types like HPV16 and HPV18 is estimated to be 15–25% [87]. In the same review from 2005 with pooled worldwide data, the portion of OSCC with an HPV-association was expected to be 35.6%. However, in this publication analyses were included, in which carcinomas of the base of tongue were classified as oral carcinoma. So the true incidence rate of OSCC might be higher than estimated.
Contemporary with decreasing tobacco consumption, the portion of HPV-associated OSCC seems to rise. This reflects, mainly in the US the worldwide decrease in tobacco consumption of the last decades in western countries. While rates of smokers in industrialized countries stagnates or decreases, developing countries report about increasing numbers of smokers. Among US-citizens cigarette smoking was reduced by more than half from 42 to 20% between 1965 and 2006. More than 20 years later, this effect seems to be measurable as reduction newly diagnosed diseases. With patients from countries in Latin America and Europe, in which smoking is still very common, an actual paper reports a portion of HPV-induced OSCC of less than 5% [141]. The worldwide variance of HPV-associated OSCC can, at least in part, be explained by various and diverse spread of further risk factors. In summary, due to the increasing incidence rate of OSCC and the contemporary reduced cigarette consumption, the portion as well as the absolute number of HPV-caused OSCC seems to rise.
1.3 Risk factors
Exposition to exogenic carcinogens like nutrition, poor oral hygiene, infections, family background and other anamnestic tumor diseases are in general alone or in combination commonly accepted risk factors for the development of head and neck tumors. Cigarette smoking and consumption of chewing tobacco, snuff and alcohol are the most known and dominant risk factors, in particular for carcinoma of the oral cavity, the oro- and hypopharynx and larynx. Probably 85% of all new cases are caused by tobacco consumption.
Alcohol and nicotine have a synergistic effect by inducing carcinogenesis. In Southeast Asia betel chewing is very popular and common since centuries. According to recent estimates for East Africa and Asia, more than 450 million people practice betel chewing. In India chewing of betel nuts has made carcinoma of the oral cavity to one of the most frequent cancerous diseases. The active agent of the betel nut is an alkaloid (such as Arecolin). More exogenic risk factors are listed in Table 1 (Tab. 1). An example for an endogenous risk factor for head and neck cancer is Fanconi’s anemia, a recessive autosomal hereditary disease with genomic instability [90].
Table 1. Risk factors in HNSCC, besides HPV, alcohol and smoking.
1.3.1 Tobacco and alcohol
After World War II smoking of cigarettes was identified as risk factor for cancer of the oral cavity and the pharynx [190]. In the subsequent years nicotine and alcohol were confirmed as the dominant risk factors [41]. A dose-effect was established for both substances while their interactions seem to be more synergistic than additive [176]. The tumor inducing action of tobacco is mainly due to the genotoxic effects of carcinogens (nitrosamine, polycyclic hydrocarbons) in tobacco smoke. A high consumption of alcohol is considered as risk factor especially for carcinoma of the hypopharynx. Alcohol metabolites like acetaldehyde interfere with synthesis and repair of DNA. The carcinogenic efficacy of alcohol seems to lie in its ability to act as solvent for the constituents of tobacco smoke and by thereby potentiating their carcinogenic effects. Alcohol per se has no carcinogenic potency [119]. It is important to know that up to 25% of new diseases were patients who never had smoked or drunken alcohol. This was especially true with patients younger than 45 at the time of diagnosis [97]. According to a publication by Koch et al. tumors in nonsmokers were preferably found in the oral cavity (tongue), which is clinically known as juvenile carcinoma of the tongue [85].
The comparableness of various publications however is commonly limited by differences in definitions like non-smoker/ex-smokers, amount of alcohol consumption etc. In a case-control study published in 2004 the data were adjusted for amount of alcohol and tobacco consumption. Both, alcohol and tobacco were identified equivocal as separate risk factors. Both substances acting in combination had a more than additive effect (non-smoker/non-alcoholic: OR=1.0; non-smoker/alcoholic: OR=1.7; smoker/non-alcoholic: OR=1.6; smoker/alcoholic: OR=12.7) [17]. Passive smoking also seems to increase the risk for a head and neck cancer [194]. Nicotine starvation reduces, however not eliminates the risk of cancer disease [154].
1.3.2 HPV
Since the discovery of HPV16 in the seventies of the last century, a role of oncogenic HPV in carcinogenesis was more and more confirmed. Because cultivation of HPV is not possible up to now, evidence for an infection with HPV can only be found by detection of parts of the viral DNA genome. The importance of oncogenic HPV for the development of head and neck cancer was recognized from 1980 on with increasing interest [47], [83]. Later on, HPV16 was discovered to be the dominant HPV subtype, and viral DNA was especially detected in carcinoma of palatine tonsils and base of the tongue. One of the earliest discoveries was an incomplete inverse correlation with mutations in the genomic section TP53, coding for the p53 tumor suppressor protein. The E6-protein of HPV16 inactivates p53, TP53 mutations are rarely found in uterine cervix cancer, which are usually HPV-associated. In 60 to 80% of head and neck cancers, however, mutations of TP53 are found, thus expecting HPV-infections in the remaining 20 to 40% of tumors with the wild type of p53. By means of checking gene expression of E6 and E7 in HPV-positive cases, predominantly wild type of p53 was found [187]. Finally it was shown that with special reference to early signs of carcinogenesis, different gene signatures exist for HPV-associated head and neck carcinoma [12]. In summary it reveals that HPV-associated OSCC represent a separate tumor entity. With case-control studies it was proven that oncogenic HPV acts as an independent risk factor for the development of OSCC [33].
It can be assumed that oral HPV-infections are acquired by sexual contacts. Nevertheless, alternative routes of infection are not excluded. Although the data with respect to transmission of oncogenic HPV into the oral cavity are insufficient and natural pathways of infection still need to be proven. Definitely it was shown for females, that an existing genital HPV-infection is an important predisposing factor for oral HPV-infection [177]. In a survey from Finland with a follow-up period of 2 years, oncogenic HPV was found in 10% of all children within the first 26 months of life. In the same publication the rate of persisting infections was 10% [142]. Of course, so far very little is known about the transmission pathway of oncogenic HPV, the preconditions for an oropharyngeal infection and its malignant transformation. The detection of oncogenic HPV in mouth and pharynx was successful in up to 14% of healthy probands [29]. Of course, it is important to differentiate between oncogenic and non-oncogenic HPV-types (see chapter 3). The contamination of the oral mucosal with HPV seems to be no rare event. However, in tumor-free tonsils the incidence of oncogenic HPV was only 1% [79]. The probability for detection of high-risk HPV in the oral cavity increases with the number of sexual partners [165]. Further risk factors for an infection with oncogenic HPV are young age, male sex, a HIV-infection as well as smoking [86]. In a case-control study of 2001 from Norway, an increased risk for head and neck tumors was identified by serological testing of probands with high seropositivity for HPV16 [109]. In a pooled analysis of case-controlled studies of 5,442 cases and 6,069 controls, the relationship between sexual behavior and the risk of head and neck cancers was calculated. A higher risk for OSCC correlated with a higher number of sexual partners and with practiced oral sex. Both, an early beginning of sex life and homosexual contacts in men had a high correlation with the development of OSCC [61]. In a retrospective study it was shown that the risk of an HPV-induced OSCC doubles, if the number of sexual partners with oral sex was higher than one and up to five, and increased even fivefold with 6 or more sexual partners [46]. Another risk factor for OSCC is genital condylomas in the patient’s anamnesis [174]. These results suggest that oral HPV-infection is transmitted by oral sexual practice. However, it is known that a significant number of patients with HPV-induced OSCC never had practiced oral sex. Oral sexual intercourse is a risk factor; however, it does not exclude an HPV-induced OSCC [33], [46].
1.3.4 HPV and nicotine
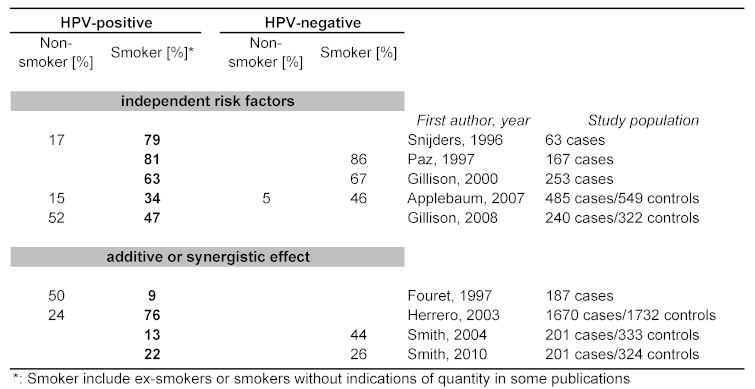
The discussion about association of HPV and smoking with the carcinogenesis of OSCC is controversial in literature. For uterine cervix cancer numerous publications support a synergistic effect [78]. Smoking by females prolongs infection time and therefore increases risk for progression of dysplasia to invasive carcinoma of cervix uteri [50]. Recently, a report was published about an interaction of nicotine with the transcription factor Brn-3, thus revealing an interaction between smoking and HPV-induction on the molecular level [117]. Different data have been published in regard to interaction of oncogenic HPV infection and tobacco-smoking for the development of OSCC [158]. Some authors report a lack of association [7], while others found evidence for an additive or synergistic effect [166]. In Table 2 (Tab. 2) recent original publications about HPV and smoking are listed. HPV is an established risk factor for OSCC in non-smokers. Although it is known that patients with HPV-associated OSCC have less heavy nicotine abuse, a significant amount of them are ex- or light smokers and smoking might act as an additional risk factor. However, in several publications additional nicotine abuse had no additive or synergistic effect. According to a publication by Gillison et al. smokers were more frequently in the subset of HPV-negative tumors, but differences were only marginal and not significant [46]. Furthermore positive serum antibodies against HPV E6/E7 revealed a 56.2-fold increased risk for OSCC for patients who had smoked [64]. In a further group of 201 patients with OSCC, serum antibodies against HPV were tested. Here, a higher risk for OSCC was calculated if the patients had smoking or drinking in their personal history, regardless if the patient had positive HPV antibody reactivity [166]. In addition, to the higher risk for OSCC in case of an HPVinfection and smoking, it was shown that smoking has an influence on the survival rate after therapy [54]. The following theoretical statements underline a possible effect of smoking as promoter:
Table 2. Smoking habits and HPV – independent risk factors or synergy?
Smoking is a feasible co-effector, because the exclusive infection with oncogenic HPV does not lead to a malignant tumor in most cases;
Smoking increases the susceptibility for an oral HPV-infection;
Smoking encroaches upon the immune reaction to an HPV-infection;
HPV-E6 inhibits the p53-protein, whereas smoking leads to mutations of TP53gene and consequently restoration of an intact p53-function fails;
Smoking leads to splintering of DNA which alleviates the integration of virus-DNA.
In summary, we have convincing evidence that an infection with oncogenic HPV and the development of head and neck cancer is supported by additive or even synergistic effects of nicotine abuse.
1.4. Prophylactic HPV-vaccination
The group of papilloma virus encloses several hundred different species, which infect human and animal epithelia. In most cases an infection with HPV turns into benign lesions. However, so-called high risk (HR) types of HPV may lead to malignant tumors. The prevalence of an HPV-association is almost 100% with cervical carcinoma. For OSCC between a third and half of tumors are associated with an infection with oncogenic HPV. Presence and participation of viral proteins at the beginning of premalignant lesions, or even carcinoma, offers the possibility for prevention of these lesions by active or passive vaccination [3], [125].
The active immunization is world wide in use. In Germany this is the case since 2006 with two different vaccines. Sanofi-Pasteur-MSD/Merck developed the product Gardasil® , a quadrivalent vaccine, which is effective against HPV6, HPV11, HPV16 and HPV18. After certification for Europe by EMEA the vaccine was established in October 2006. The second vaccine Cervarix® was developed by GlaxoSmithKline. This vaccine, preventing infection by type HPV16 and HPV18, contains the adjuvant AS04, which increases its immunogenicity. In Germany Cervarix® is on the market since October 2007. Both vaccines have to be applied intramuscular threefold, at the beginning of vaccination and after 1–2 and 6 months. Meanwhile both vaccines are licensed in several countries. In Germany the recommendation of the STIKO for a general vaccination of all girls at the age between 12 and 17 years was published in March 2007 [144]. In August 2009 a re-evaluation of both vaccines, considering the newest literature, was published by the STIKO. Unrestricted recommendation of a general vaccination of all girls between 12 and 17 year of age was confirmed [143]. If HPV infection already exists no protective or curative effect to prevent carcinogenesis can be expected from vaccination for the HPV concerned [65].
Both vaccines are based on the viral capsid-protein L1, which can be produced by recombinant techniques in bacteria, yeast and insect cells. L1 monomers are able to organize itself to so-called virus-like particles (VLPs) spontaneously. The biotechnological production of these VLPs almost completely prevents infections through contaminations with viral genetic material or other infectious DNA or RNA. For prophylactic immunization neutralizing antibodies need to recognize surface structures on the VLPs. This excludes the usage of L1-monomers for vaccination. Application of native VLPs, however, requires special demands on production, storing and delivery of the vaccines and stipulates a high virus-type specificity of the vaccines with only little protection against close related gene types [153].
In order to optimize vaccine protection and costs of manufacturing, special vaccines are in development, which consist of only five L1-monomers. A more broad and extensive vaccine protection is promised by using the second capsid-protein L2. It contains a protein region highly conserved in various types of HPV [16]. Both approaches may find application for the next generation of vaccines. However, it may take several decades of worldwide prophylactic vaccination to achieve a significant reduction of the prevalence rate of HPV.
Although prophylactic vaccinations are highly effective, they have no therapeutic or curative effects on already existing infections with HPV. Accordingly, the development of therapeutic vaccines for the treatment of premalignant lesions and HPV-associated carcinoma would be very useful and significant. The target proteins L1 and L2 are produced at late stage of the HPV-lifecycle in uppermost layers of the epithelia only. Both proteins are not expressed in the primarily infected basal layers, whereas the early viral proteins E6 and E7 are produced in the basal layers and are also responsible for the transformation of the host cells. Therefore these proteins will be proper targets for therapeutic vaccines. At the moment several therapeutic vaccines against E6 and E7 are in preclinical and clinical tests [92]. Another very promising approach is the introduction of “naked” DNA vaccines, which encode a genetic modified E7 protein. Such a mutated E7 protein will be expressed in the target cells and it will trigger the proper antigen-specific immune response. This modified E7 protein however, cannot interact with pRB and thus can not contribute to the cellular transformation. DNA vaccines have several advantages like low production costs, high stability and safe application, but further research is still needed to improve immunogenicity and methods of application [68].
Studies are in progress regarding a protective effect of vaccination for boys and men to prevent infection with HPV and HPV-associated diseases, like genital warts and perianal and penile dysplasia. For instance in Australia a nation-wide and financially supported vaccination program for all girls and women between 12 and 26 years and all boys and men between 9 and 26 years was carried out. This led to a significant reduction of genital warts in the male population [30]. Since October 2009 Gardasil® is admitted in the USA for the prevention of genital warts in men and boys. In a study with 4,065 boys and men this quadrivalent vaccine was found to be effective for the prevention of genital lesions [49]. Presently, a prospective clinical study is in progress with homosexual men to prevent condyloma and genital carcinoma.
The aim of a protective vaccination should also be the prevention of head and neck cancer. For this purpose also boys need to be vaccinated. This issue is discussed in Germany, its realization however is doubtful at the moment. Although estimates suggest that costs for treatment of HPV-associated head and neck carcinoma in Germany are more than 80 Million Euro per year (Klussmann, J.P.: 27th International Papillomavirus Conference and Clinical Workshop 2011).
2 Models of carcinogenesis for HNSCC
2.1 Heterogeneity
Different genetic and epigenetic changes may accumulate during lifetime of normal cells, which might cause development of neoplasia. Type and absolute number of these changes differ among tumor types, also for HNSCC. Similarities are observed only in regard to the mixture of cell types within one neoplasia [107]. The development of this heterogeneity respects to morphologic, molecular biologic and metabolic features of tumor cells. It is related to carcinogenesis and may be explained by both, stochastic and hierarchic model of carcinogenesis (chapter 2.6).
2.1.1 Gene expression profile
More than 95% of all HNSCC are squamous cell carcinoma, probably assuming a homogeneous disease. However, HNSCC are much more heterogeneous than expected. Often this is hindering clinical practice, since tissue samples do not necessarily represent the whole tumor. Different subclasses of HNSCC may be defined histologically, which is supported by DNA and RNA-profiles recently examined [160]. For example, four groups of HNSCC could be defined by analyzing 60 tumors samples using cDNA microarrays (EGFR-pathway signature, mesenchymal-enriched subtype, normal epithelium-like subtype, subtype with high levels of antioxidant enzymes). This study revealed a statistically significant correlation of disease free survival of patients, related to distinct gene expression patterns, which was confirmed by the same investigators by analyzing FFPE samples later on [20], [21].
2.1.2 Chromosomal aberrations
By karyotyping and checking status of ploidy it was demonstrated for one subgroup of HNSCC to be diploid or nearly diploid, while the vast majority shows different types of aneuploidy [63], [75]. Comparative genomic hybridization (CGH) experiments confirmed this data. However, about 20% of HPV-nonrelated OSCC displayed only few genetic changes and these tumors were almost normal in ploidy [162]. In another study, a series of 60 tumor samples was analyzed be using conventional CGH for critical genetic alterations. HPV-related OSCC showed fewer genetic alterations and amplifications, while especially deletions at chromosome 3p, 5q, 9p 15q and 18q, as well as amplifications at region 11q13 were significantly prevalent for HPV-unrelated OSCC [81].
Despite promising results, carcinoma of the head and neck still display a genetically heterogeneous picture. More studies are required to clarify this picture, especially in regard to connect genetic alterations more closely with changes in gene expression profiles, epigenetic changes and posttranscriptional control of gene expression. Rising acceptance for HPV-related OSCC, to be a specific subclass of head and neck carcinoma, underlines the heterogeneity of this disease and further sub classification of HPV-unrelated head and neck carcinoma with respect to future results of clinical and molecular biological research may be expected.
2.2. Field carcinogenesis
Preneoplastic processes of high carcinogenic potential at diverse sites of an area are summarized by the term field carcinogenesis. It is assumed that theses processes are at different states of progression and their individual development may be de- or accelerated, depending on cellular programs or other stimuli [159]. Especially the mucosa of the upper aerodigestive tract is damaged by high tobacco and alcohol usage, leading to carcinogenesis as a result of a multi-step process of accumulation of diverse mutation and premalignant degenerations. By molecular biological techniques, degenerations can often be detected in mucosa next to surgery margins, even if the mucosa appears to be healthy by microscopic inspection. This might be responsible for synchronous or metachronous secondary cancers (chapter 4.2). On the other hand local recrudescence is rare after treatment of HPV-induced OSCC. However, HPV-infections may cause multicentric tumor growth, known from the female genital tract. Neighboring mucosa of HPV-induced OSCC typically does not show degenerative alterations as described for field carcinogenesis according to Slaughter. Otherwise, multicentric tumor induction by HPV is also reported for the head and neck [105]. Three models for multicentric tumor induction by HPV are possible: First, a persisting infection causes a field effect with multiple tumors induced by the same HPV-type. Second, multiple HPV-infections and -reinfections cause several tumors by different HPV types. And third, a lesion caused by HPV creates clonal neoplasias, which migrate to different sites and grow up to form a secondary tumor. Until now well-founded field effects could not be shown for HPV-induced OSCC. The importance of reinfections or migrating neoplasias as a cause for secondary cancers and relapse will be analyzed by future studies. This will certainly give rise to translational approaches for the clinical routine concerning following aspects: Is tumor endoscopy reasonable to exclude secondary tumors for HPV-positive primary tumors? Is follow-up care required to the full extent? Are possibly more effective procedures available to improve follow up care?
2.3. Molecular models
The former three-step model for carcinogenesis of initiation, promotion and progression appears to be obsolete. Nowadays a more complex model of carcinogenesis is assumed. However, this model is not completely understood, but obviously cellular processes like cell cycle control, senescence and apoptosis have to become deregulated for developing cancer. Genes related to these mechanisms may be damaged by different physical (UV, asbestos), chemical (nicotine, alcohol) or biological agents (oncogenic viruses), which is compensated to a certain extend by repair mechanisms in healthy cells. If theses mechanisms are overstrained, mutations may accumulate and eventually led to an advantage of growth for certain cell and finally to the development of cancer.
2.3.1 Genetic aberrations
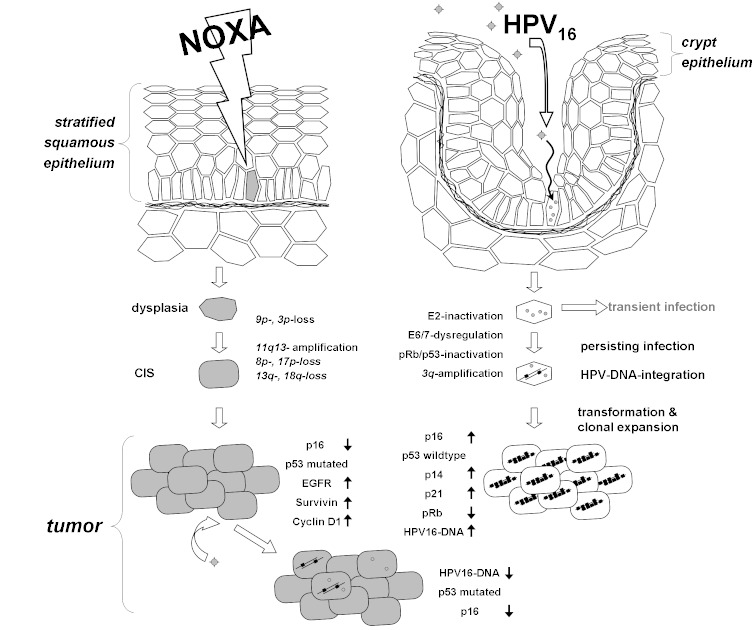
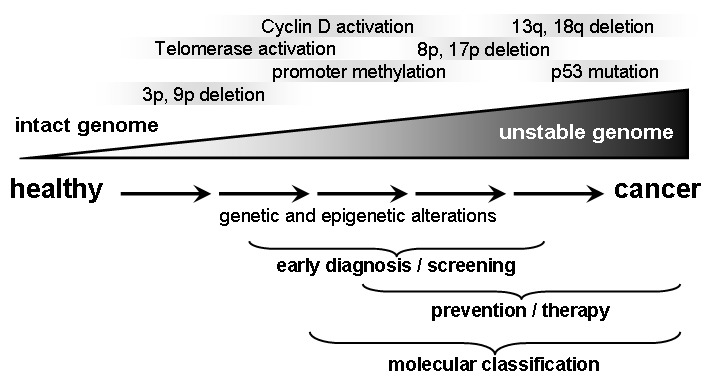
In different studies a multi-step process with accumulation of multiple genetic and epigenetic alterations could be shown to proceed for HNSCC development [100]. More than 20 years ago aneuploidy was discovered to be associated with carcinogenesis in the oral cavity [66]. Also, for premalignant lesions genetic alterations were correlated to poor prognosis like the development of a malign phenotype and a high risk for local tumor relapse [173]. One of the first and most abundant events for lesions of the head and neck is loss of heterozygosity (LOH) for alleles located at chromosomes 3p and 9p21, which can be detected already in hyperplasia, dysplasia and even normal appearing epithelial cells [100]. Genes of tumor suppressors like FHIT and p16 are coded at these locations and about half of the analyzed premalignant lesions developed a malign phenotype. This indicates involvement of the mentioned genes during the process of malign transformation. The risk for a lesion to undergo malign transformation is 33 times increased if further LOH occur at regions (4q, 8p, 11q, 13q, 14q and/or 7p), being also related to HNSCC [101], [147]. Furthermore, elevated expression of CyclinD1 was observed for early lesions [71] and early telomerase reactivation resulted in prolonged survival of genetically altered cells and accumulation of multiple genetic abnormalities [100], [111]. Progression of genetic changes typically starts with certain early events (like 9p21 LOH/p16-inactivation: → hyperplasia) and proceeds with later events (Figure 1 (Fig. 1)). However, the sequence of changes can not be considered to be consistent.
Figure 1. Multi-step progression of carcinogenesis in head and neck cancer: Chronic exposition with carcinogenic substances leads to progressive genetic and epigenetic changes that accumulate and finally lead to premalignant and malignant lesions.
2.3.2 Cell cycle regulation
The tumor suppressor protein p53 is of central importance for carcinogenesis and its overexpression was shown to be strongly correlated with chromosomal instability [157]. TP53 gene mutations can be detected for 60 to 80% of all HNSCC and are associated with inhibition of apoptosis and elevated resistance against chemotherapy and irradiation. Inactivation of the p53 protein occurs either by mutation of its gene (in case of HPV unrelated tumors) or via the effect of the viral protein E6 (see also chapter 2.4). Roughly the same is true for cell cycle control by the Rb/pRb system. Also, chromosomal alterations affecting the Rb/pRb system and therefore activating the cell cycle are common for HNSCC. For example, chromosomal region 9p21, encoding the cyclin-dependent kinase inhibiting protein p16, which usually controls cell cycle progression, is regularly affected by mutations, methylation or deletion [138]. On the other hand, about 80% of non HPV related OSCC contain amplifications at chromosome 11q3. At this region cyclin D is encoded, which is involved in the cell cycle by controlling G1/S phase transition [161].
2.3.3 Invasion and metastasis
Local invasion is a first step in epithelial-mesenchymal transition (EMT, chapter 2.5.3) of tumor cells, finally resulting in generation of regional or distant metastases. Morphological studies demonstrated that the pattern of invasion, perineural invasion and presence of immune cells correlate to the course of the disease for HNSCC [164]. Also, expression of EGFR (epidermal growth factor receptor) is regularly increased for HNSCC. Beside stimulating effects for cellular proliferation, EGFR also assists invasion of tumor cells by increasing cellular mobility, which has been shown for HNSCC cells in vitro [191]. This was confirmed in vivo by an animal model, showing that the ligand for EGFR (EGF) and the chemokine CXCL 12 are inducing invasion for HNSCC [164].
During further progression of tumor growth and reaching a certain tumor size, oxygen supply, as well as metabolite disposing becomes limiting and demands an own connection to the blood stream. Growth factors are frequently produced by solid tumors, leading to the outgrowth of blood vessels. One of the best known factors for angiogenesis is VEGF (vascular endothelial growth factor). Its expression has been correlated to the prognosis of HNSCC in several analyses. In a recent study VEGF was reviewed as a prognostic marker in regard to EGFR and the HPV status. VEGF was shown to be associated with the disease specific mortality rate for patients treated by irradiation. However, HPV status was demonstrated by the same study, to be a marker with stronger prognostic value for HNSCC than VEGF or EGFR [37].
2.3.4 Signal transduction pathways
Different pathways may be involved in development and progression of cancer. For HNSCC one of the most important ones is the TGF-ß pathway. Several growth inhibiting genes are targets of this pathway, like cyclin-dependent kinases (CDKN2B, coding for p15INK4B; CDKN1A coding for p21CIP and CDKN1C, coding for p57KIP), being involved in cell cycle control [70]. Binding of TGF-β ligand to its receptor leads to activation of SMAD2 and SMAD3, forming a protein complex together with SMAD4. This complex subsequently migrates into the nucleus, where it binds to transcription factors, coactivators and corepressors and finally to promoter regions of its target genes. A reduced expression of TGF-β receptor and losses at chromosome 18q, where genes for SMAD2, SMAD3, SMAD4 and TGF-β receptor II are locates, are regularly observed for HNSCC [135]. Also, it was recently shown for conditional knock out of SMAD4 in mice to cause HNSCC, pointing to the impact of SMAD4 as an oncogene for HNSCC [11]. TGF-β signaling seems to be linked to the NF-кB (nuclear factor-kappaB) pathway, since blocking of TGF-β signaling apparently is associated with NF-кB activation [22]. NF-кB is an important transcription factor integrating several signals for controlling cellular survival.
Another crucial signal transduction pathway is the EGFR-PI3K-Akt-mTOR pathway, which is activated for 90%–100% of all HNSCC. It governs important cellular processes like growth, proliferation, apoptosis, cellular survival, differentiation, as well as cell cycle and metabolic control. Diverse genetic and epigenetic alterations for this pathway have been reported, finally leading to the activation of oncogenes or inactivation of tumor suppressors. For example, activation of the AKT-mTOR branch yields activation of the eukaryotic initiation factor 4E (eIF-4E). Elevated expression levels of eIF-4E can subsequently activate different oncogenes [115], [127]. The tumor suppressor protein PTEN (Phosphatase and TENsin homolog) acts as an inhibitor for the AKT pathway and mutations or homozygote deletion of the gene coding for PTEN is described for about 10% of HNSCC [118]. Central components of the mentioned pathways, like EGFR (see also chapter 4.2.7), have recently emerged to be interesting targets for directed cancer therapies [77]. Several approaches for HNSCC are in clinical and preclinical testing. However, details of these pathways still remain not understood and need to be further analyzed in future.
2.3.5 Summary
Despite research progress in the field of molecular Dysregulation, genetic and epigenetic alterations, a distinct model for carcinogenesis can not be demonstrated for HNSCC. On the one hand, heterogeneity of the disease (chapter 2.1) shows more than one mechanism for carcinogenesis to be involved for HNSCC, which probably overlap and engage. So, for each subset of HNSCC a separate model may be necessary to comprise the complexity of the disease. On the other hand, in regard to the molecular background and the connection to clinical data, further analyses are required. Taken together, several chromosomal alterations and instabilities respectively, are inducing molecular dysregulation, mostly affecting regulation of cell cycle and thereby driving carcinogenesis.
2.4 HPV-induced carcinogenesis
More than 120 human papilloma viruses (HPV) are known, the vast majority infecting cutaneous epithelia (β-group). Oral or anogenital mucosa is infected by about 30 to 40 HPV types, being summarized in the α-group. This subgroup can be divided into non-oncogenic, or low risk (LR) HPV-types like HPV-6 and -11, and oncogenic, or high-risk (HR) HPV-types, like HPV16 and -18. LR-HPV-types can cause condyloma and papilloma, whereas HR-HPV induced lesions may emerge to dysplasia and carcinoma [28], [195]. Coherence of HPV-infections and carcinogenesis is known for a long time and in particular analyzed for the cervix uteri. HPV-infections are rapidly cleared by the immune system in most of cases. But for about 10% a persisting infection may occur, lasting for several years and probably developing a high grade neoplastic lesion and finally an invasive carcinoma [126].
2.4.1 Viral life cycle
The viral life cycle starts with an acute infection of basal cells of an epithelium. For completion of the life cycle, differentiation of the epithelial cell is essential (Figure 2 (Fig. 2)). Viral DNA replication is independent of cellular DNA replication and disabled until proteins, required for viral replication, are produced in sufficient amounts. On the other hand, expression of viral gene products changes in parallel with the grade of differentiation and the migration of infected epithelial cells towards the surface (with differences in timing, depending on the virus type). In certain cases, e.g. an HPV16 infection of the cervix, it may result in an abortive infection. Here, the viral life cycle is not completed and a predisposition for carcinogenesis may evolve [31].
Figure 2. Carcinogenesis during exposition with carcinogenic agents, e.g. smoking: Accumulation of genetic alterations leads to a loss of cell cycle control and unimpeded growth (left). During HPV-induced Carcinogenesis (right) the sentinel of cell cycle, the p53-protein, is knocked down by viral E6- and E7-proteins. This process is advanced by integration of viral DNA. Both pathways may overlap.
2.4.2 E6 & E7 – viral oncoproteins
Expression of the viral proteins E6 and E7 in lower epithelial layers accounts for turning the cells into S-phase of the cell cycle and creating a well suited environment for viral replication. The viral E2 protein regulates E6 and E7 expression and thus coordinates viral replication in a time depending manner. During persisting infections with HPV viral genes can also be expressed from proliferating cells of lower epithelial layers, the viral genome resides epigenetic in the beginning. Frequently, elevated expression levels are also observed, especially for the oncogenes E6 and E7, which are important for further progression of the disease and inactivation of cellular tumor suppressor proteins p53 and Rb (without affecting the respective genes). Through this, massive dysregulation of host DNA synthesis takes place, as well as loss of cell cycle control. Finally, consequences are destabilizing of the genome, abnormal centrosome numbers and further chromosomal aberrations [34], [35], [179], [180].
2.4.3 Integration
With the development of a malign phenotype an increased rate of viral integration is observed. In the majority of cases, integration takes place by linearization of the circular, episomal viral genome at a region coding for the viral E1 and E2-proteins. By recombination of this region with cellular sequences, the viral E2 gene is commonly inactivated, which results in loss of transcriptional control of the viral proteins E6 and E7 and an elevated oncogenic potential of these proteins [76], [146]. Additionally, by integration at the E4 region, viral mRNA transcripts lose their polyadenylation signal. Resulting virus-host fusion transcripts possess a higher stability compared to viral transcripts, which add to the effect of viral oncoproteins [73]. For cervical cancer, integration sites of HPV-DNA seem to be distributed throughout the whole genome, with differences between clinical samples [99], [185]. Specific integration sites analogous to retroviral insertion mutagenesis could not be detected until now. However, certain preferences for chromosomal fragile sites seem to exist [14], [25]. For cervical cancer the activity of viral oncogene expression was shown to be affected by the surrounding area of the integration site [179] and several studies investigating the physical status of the HPV-DNA have been published. For HNSCC much less information is available. Unpublished data of our group suggest integration of viral DNA to be less important for tumor progression for OSCC compared to cervical cancer. Additionally, malignant tumors of the head and neck are molecular and genetically diverse (chapter 2.1). Hence, knowledge about carcinogenesis and tumor progression of HPV-infected cervical cancers may not be directly transferred to HNSCC. However, in cell culture experiments it has also been shown that inhibition of the viral E6 protein recovers p53 expression and a continuous expression of E6 and E7 is essential for repression of apoptosis and retention of a malign phenotype for OSCC [38], [137].
2.5 Molecular differences – HPV-unrelated vs. HPV-related carcinogenesis
HPV-induced carcinogenesis is generally differing from carcinogenesis induced by physical or chemical noxa. Here, stepwise accumulation of mutations creates a growth benefit for cells, in case of affecting oncogenes or genes of tumor suppressor proteins (chapter 2.3). For HPV-induced cancers, basically the oncogenic activity of the viral proteins E6 and E7 are responsible for carcinogenesis. In this aspect HPV-positive HNSCC resemble cervical cancer, because in both cases genes for p53 and Rb are not mutated in general. However, continuous expression of the viral E6 and E7 proteins disables the activity of cellular tumor suppressor proteins [45], [56]. As mentioned before, it was shown that repression of the viral E6 protein recovers p53 expression and a continuous expression of E6 and E7 is essential for repression of apoptosis and retention of a malign phenotype for OSCC [38], [137]. Cellular mechanisms usually interfering with carcinogenesis (like intact and activated p53) are suppressed by the activity of viral proteins for HPV-induced tumors. On the contrary for non-HPV related tumors, the proper genetic information coding for the respective mechanisms has been lost. Mutations in tumor suppressor proteins or oncogenes are rare in HPV-induced tumors, which we could confirm by analyzing a set of 60 OSCC using conventional comparative genomic hybridization (CGH) [81]. Of the reviewed samples 29 were HPV-positive and the remaining 31 were HPV-negative. Corresponding clinical and pathological features, tobacco and alcohol usage, as well as disease specific survival was analyzed with statistical methods and correlated with results from CGH analysis. Generally, the number of genetic alterations was increased for all chromosomes, with significantly higher numbers for HPV-negative compared to HPV-positive tumors. For example gains at 11q13 significantly correlated with tobacco and alcohol usage, whereas 11q13 amplification was uncommon for HPV-associated OSCC. On the other hand losses at chromosome 11p were frequently detected for HPV-positive tumors. Furthermore, amplifications at 3q, 11q and 18p were correlated to poor prognosis, whereas deletion at 16q correlated to a good prognosis for survival of patients [81].
2.5.1 p16 – biomarker for transcriptionally active HPV infections
Beside chromosomal alterations, changes in expression patterns of cellular and viral proteins are of particular diagnostic and prognostic importance, since they can be detected quite easily by histological and molecular biological techniques. In the past years several studies were published trying to classify HNSCC by the use of different biomarkers. Classification of OSCC by HPV-status was done by different studies. However, to state the transcriptional activity of HPV more precisely is very important for OSCC. Detection of HPV-DNA alone is no stringent proof for HPV-induced carcinogenesis. Only transcriptionally active HPV-DNA is biologically and clinically relevant for carcinogenesis [133]. To analyze expression of cellular p16 protein has been shown to be helpful in this regard. The transcriptional inhibitor of p16 is Rb. In the case of an active HPV-infection Rb is inactivated by the viral E7 protein, which subsequently results in a strong expression of p16 (Figure 3 (Fig. 3)). A combination of positive staining for p16, together with the detection of HPV-DNA may be useful to detect a transcriptionally active HPV-infection, since toxin-associated OSCC do not display positive p16 staining in general.
Figure 3. HPV-unrelated carcinogenesis (A): p16 is not activated and phosphorylation of Rb is unblocked. Progression of cell cycle is not inhibited by mutated p53 and apoptosis cannot be initiated. HPV-related carcinogenesis (B): Binding of E7 to pRB leads to activation of E2F, which induces cell cycle progression and induces p16-expression. E6-protein is responsible for degradation of p53. The attendance of noxious agents for HPV-related carcinogenesis is not mandatory, however might occur in addition.
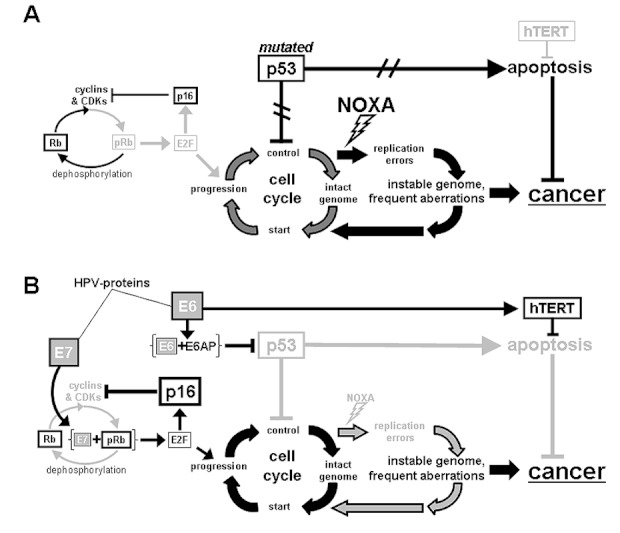
Several studies illustrate similarities for HPV-positive and transcriptionally active OSCC with cervical cancer, like non-mutated TP53, low Rb- and high p16-expression [24]. Recently, OSCC have been grouped into three subsets: HPV16-positive/p16-positive (HPVactive), HPV16-positive/p16-negative (HPVinactive) and HPV16-negative/p16-negative (HPV-negative). For the HPVactive subgroup a significantly different protein expression pattern was identified compared to the two other groups. Additionally, an elevated expression of β-Catenin (and probably also EGFR and VEGF) was detected for the same group [184].
2.5.2 EMT and metastasis
Cellular changes in regard to adhesion molecules during the process of metastasis have been analyzed for HPV-associated and HPV-unrelated primary tonsillar tumors by our group, recently. The clinical observation of an early metastasis of HPV-associated compared to not-HPV-associated OSCC could be confirmed by histological data of the study (Figure 4 (Fig. 4)). A markedly reduced expression of the adhesion molecule E-cadherin was already detectable for primary tumors of HPV-associated OSCC, while its loss was only observed for lymph node metastasis of HPV-unrelated OSCC, but not for respective primary tumors. Results of this study suggest an early induction of an epithelial mesenchymal transition for HPV-associated OSCC [168].
Figure 4. HPV-unrelated carcinogenesis (left): HPV-negative primary is located at the base of tongue, FDG-PET depicts no evidence for regional metastasis (A), p16 expression is absent (C). β-Catenin (green) is located at HNSCC culture-cell surfaces, nuclei are DAPI blue-labeled (E).
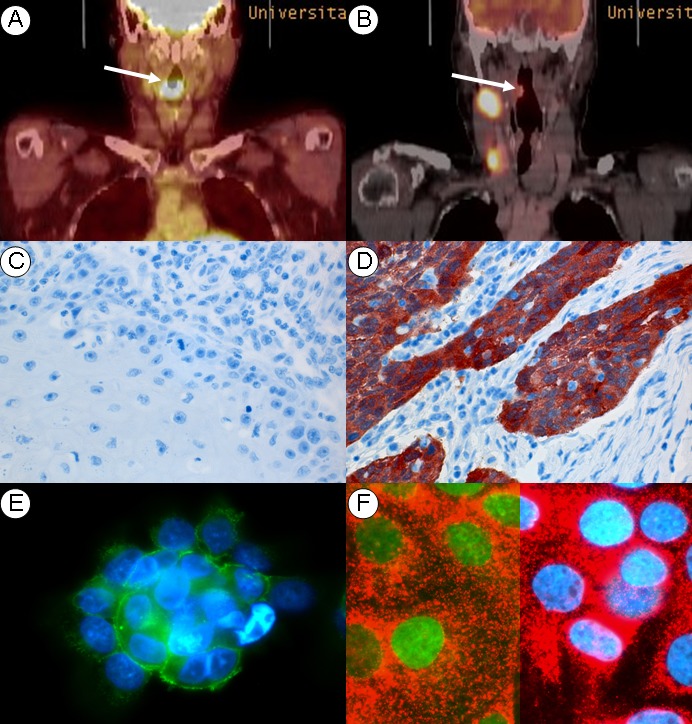
HPV-related carcinogenesis (right): HPV-associated primary of the tonsil, FDG-PET depicts neck metastasis (B). p16 expression is strong (D) during fluorescence labeling nucleus (blue) and p16 protein (red) are shown. β-Catenin (green) is located to the nucleus assigning EMT phenotype (F).
2.5.3 Epigenetic changes
Besides well known genetic changes, epigenetic alterations seem to play a role in carcinogenesis, too. With growing insights about mechanisms, how chromatin organization affects gene expression, also epigenetic changes gain importance for HNSCC. Methylation is probably the best known modification of DNA and already accepted as an epigenetic marker. Most likely, this might also becoming important for HNSCC in future. A recently published study analyzed epigenetic signatures of tumor stem cells. Fractions of HNSCC cell lines were sorted for the stem cell marker protein CD44 and subsequently analyzed in regard of epigenetic changes using the “Illumina BeadChip Array” technology. For CD44-positive fractions of five different OSCC cell lines 17 hypometylated and 9 hypermethylated genes were found, suggesting specific methylation patterns to be required to sustain stem cell characteristics and pluripotency of the analyzed OSCC tumor stem cells [42]. In another study, specific methylation patterns were detected for HPV-positive and HPV-negative cell cultures, which are significantly consistent to the methylation profile of the respective primary tumor [151].
Patterns of hypo- and hypermethylation may be helpful for further classification of existing OSCC subgroups. For example, hypomethylation of integrated viral genomes was shown to be present for the vast majority of HPV-positive OSCC. This correlated with the expression of viral oncogenes E6 and E7. It was assumed for this subgroup, that expression of E6 and E7 is essential to sustain a malign phenotype and detection of methylation of viral genomes from serum or salivary samples may be of diagnostic relevance [122].
2.5.4 miRNA
MicroRNAs (miRNAs) are non-coding, regulatory RNAs, playing an important role in different diseases and in particular in carcinogenesis. The length of miRNAs ranges between 18 and 24 nucleotides. A perfect base pairing of an miRNA and its target usually results in degradation of the target, while imperfect base pairing inhibits transcription of the target mRNA. In carcinogenesis miRNA may thereby act as an oncogene, as well as a tumor suppressor. Expression profiles of miRNAs seem to be specific for certain tumors and tissues. For head and neck carcinoma they have been correlated with pathogenesis, metastasis and resistance for chemotherapy [18], [95], [96], [193]. Significant differences in miRNA profiles for HPV-positive vs. HPV-negative OSCC samples have been detected already, pointing to the importance of miRNA for HNSCC. For example miRNA-363 is induced by the viral oncoprotein E6 and its expression was found to be increased in HPV-positive cell lines, as well as in HPV-positive tumor samples [91], [181]. An elevated expression of miRNA-135a has been linked in vivo and in vitro with resistance against the chemotherapeutic agent Paclitaxel, with a function in Wnt signaling and with the inhibition of β-Catenin depletion [67], [112]. The role of miRNA-9, which was found to be strongly expressed in the same study, and its antisense miRNA-9* has been discussed controversially. Expression of miRNA-9 is thought to be brain specific. However, precursor molecules of miRNA-9 were detected in different cell lines, neither of them originating from brain tissue. Early diagnosis of carcinogenesis, as well as diagnostics of metastasizing diseases could be applications where miRNAs might be helpful in future. Circulating miRNAs, being increasingly expressed have already been found in plasma and salivary samples of patients with HNSCC [94], [123]. Also, miRNA profiles were used to differentiate between HPV-positive and HPV-negative HNSCC in a recent study [91].
In the past year several studies have been published, aiming to classify HNSCC with regard to relevant prognostic and diagnostic biomarkers. Despite promising approaches, some kind of a general tendency to classify HNSCC with prognostic significance is not visible. Future studies are required to get more into detail of the molecular relationships and to build a closer linkage between basic science and clinical data of the disease.
2.6 Cancer stem cells
Stem cells have the ability to self-renew and to differentiate into all (pluripotent embryonic stem cells) or certain cell types (adult stem cells). These cells are of central importance for the generation and regeneration of tissues and may be classified according to two of their functions being especially important for carcinogenesis: Infiltrating stem cells may enter the tumor from the surrounding tissue and are only indirectly participating in carcinogenesis, but can help tumor progression, for example by supporting angiogenesis. Although HNSCC are regularly infiltrated by stem cells, their role in carcinogenesis is still largely uncertain [189]. However, it was shown that production of GM-CSF (granulocyte macrophage colony-stimulating factor) by HNSCC was activating CD34-positive stem cells from bone marrow [44], [120] and being chemotactically attracted with the aid of VEGF [192]. In contrast, cancer-initiating stem cells (CSC) are able to grow up forming a new tumor, which resembles the tumor the CSC originated from, histologically. This was recently shown in a study by sorting primary OSCC for the cell surface antigen CD44 by flow cytometry. The primary tumor typically contained a small fraction of cells expressing CD44 (<10%). CD44-positive cells only were able to outgrow and form new tumors in mice. These secondary tumors, as well as tumors after several further passages, displayed the same heterogenic composition like the primary tumor (from which CD44-positive cells were originating from). CD44 expressing cells did also show a nuclear enrichment of BMI1. BMI1 is important for tumorigenesis and self-renewal of other stem cell types, indicating the acquisition of several characteristics specific for CSC by CD44 expressing OSCC cells [189].
Beside expression of CD44, export of the vital stain Hoechst 33342 has been discusses as a universal marker for stem cells. This has been linked to expression of transport molecules, which are related to the development of resistance to chemotherapeutic drugs (multiple drug resistance transporter proteins). Hoechst 33342 dye exclusion was detected for “side population (SP) cells” of several normal tissues and also for OSCC it was shown for these cells, to own properties of CSC [175].
Several details for carcinogenesis are still not understood, however, it becomes clearer that a small portion of tumor cells are CSC, which undergo differentiation and redifferentiation constantly during carcinogenesis. The course of carcinogenesis seems to be essentially influenced by properties of CSC and processes like metastasizing or relapse after non-surgical tumor treatment may be in close relationship to CSC. Since HPV is infecting epithelial cells of the basal layer, it is nearby to assume that epithelial stem cells are infected, which may subsequently be transformed to CSC via the consequences of the infection [102].
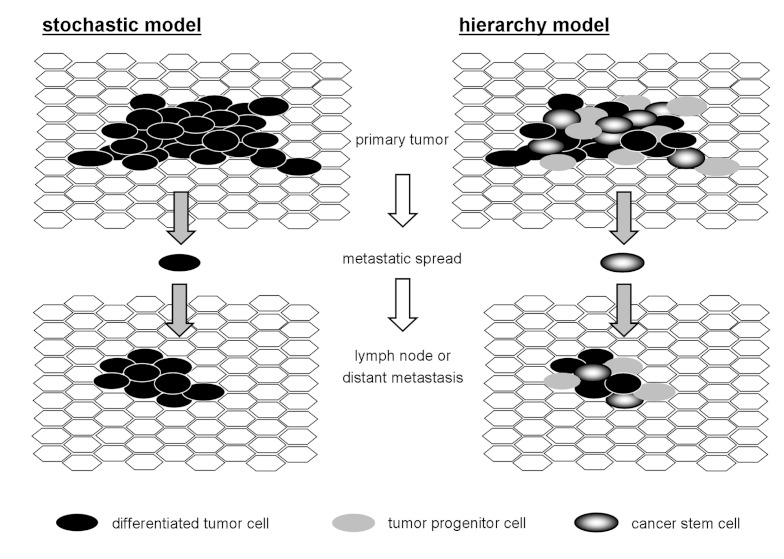
The definition for properties of CSC includes unlimited self-renewal, ability to differentiate to different cell types, loss of cell cycle control, ability to perform equal cell division and to grow up to a malign tumor starting from one single cell and to express certain marker proteins specific for CSC. For the origin of CSC, three models are currently discussed. First, healthy stem cells could develop a malign phenotype. Second, differentiated cells could accumulate oncogenic mutations and be transformed back to the level of stem cells. And third, CSC might derive from a fusion of stem cells and tumor cells [139]. Further on, to explain the growth behavior of the tumor two models are possible (Figure 5 (Fig. 5)). For the stochastic model equal cell division creates daughter cells with identical ability to support tumor growth. Here, each cell is able to initiate new tumors and cellular heterogeneity of the tumors is a result of spontaneous alterations during division of single tumor cells. For the hierarchical model a tumor initiating CSC is discriminated from differentiated daughter cells, originating from the CSC. CSCs have the ability for unlimited self renewal and to initiate new tumors, while differentiated daughter cells have lost this ability. In theory, like for the stochastic model, a single CSC would be enough to outgrow to a new, histologically consistent tumor.
Figure 5. Mitotic cells generate daughter cells with identical capability of tumor growth (left). In hierarchic model of tumorigenesis (right) tumor initiating stem cells (CSC) can be distinguished from daughter cells.
Which model discussed above may apply to HNSCC remains unknown. Future therapies for HNSCC will be targeted for CSC with high probability, since this cell type seems to be of great importance for a relapse after conventional treatment. However, until now no approach of this kind is in translational implementation. During development of CSC specific drugs, problems arise due to the selective accessibility of CSC, since big overlaps are present with healthy stem cells.
3 Naturally occurring HPV-infections
Epithelia of amphibians, reptiles, birds and mammals are infected by widely spread papilloma viruses. An extreme specificity of the virus for a certain host is most probably conditioned by coevolution, which excludes in infection of even closely related hosts by the same virus type. Also, to complete the viral life cycle a full differentiation of the squamous epithelium is required.
Capsids of papilloma viruses are about 55nm in diameter. They are composed of the two structural proteins L1 and L2 and do not posses a membrane envelope. The viral genome is encoded by a circular double stranded DNA of about 8 kilo base pairs. Until now, more than 120 human papilloma viruses are identified, which either infect cutaneous (β-group) or anogenital and oral mucosa (α-group) [188], [195]. (see also chapter 2.4)
3.1 Oropharyngeal infections of healthy adults
The infection rate is high in the adult population and may reach up to 30% in younger age cohorts [23]. However, infections become clinically apparent only in low percentage. An infection with HPV always starts at dividable cells of the basal layer of stratified epithelia and needs a micro injury in order to reach the susceptible cells. Upon a successful infection, viral replication generates about 20–100 episomal copies of the viral genome in the nucleus. Approximately 85% of all adults may undergo an HPV infection once in their life span [152]. An infection with HPV is not sufficient to cause cancer, since malignant tumors only arise if an HPV-induced lesion persists for several years. The vast majority of infections are rapidly cleared by the immune system in healthy people.
An infection with HPV may be latent for several years and a persisting infection with the same type of HPV is accepted to be the cause for development of primary stages of cervical cancer. However, it is known that only a small portion of these primary stages proceed to invasive carcinoma. The progression rate to carcinoma of the cervix is estimate to be about 20% [136]. On the other hand, a “persisting infection” is not well defined and was equalized simply by a repeated detection of HPV in most studies, comprising a source of methodic errors (Figure 6 (Fig. 6)).
Figure 6. Depending upon time point of repeated HPV testing infections can be referred as persisting (A) or non-persisting infection (B).
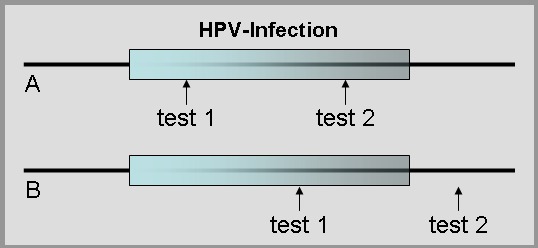
Consequently, many studies concluding a persisting infection is required for the development of a neoplasia may adhere to this bias. Certainly, duration of an infection increases the risk of an oncogenic transformation for infected host cells. Nevertheless, it has been shown for cellular transformation to take place, even shortly after infection [182]. The impact of a persisting infection for oncogenic transformation is emphasized, because viral DNA integration into the host’s genome is essential for progression to dysplasia and carcinoma [186]. This step seems to happen by accident and probably may require many years to take place.
About 30 of more than 100 described HPV types have been detected in the oral cavity and the oropharynx. Oral infections rarely cause symptoms. However, immune suppressed patients frequently develop condyloma and papilloma. It is assumed that orogenital sexual contact is the most important source of infection. Also, oral to oral infections or infections transmitted from mother to child, taking place in the birth canal, have been reported. For example, the risk for juvenile laryngeal papillomatosis is decreased for Cesarean sections. On the other hand, in a large, multicentric study, no elevated risk to develop HNSCC could be demonstrated for tumor free mucosa of the upper aerodigestive tract, if only HPV-DNA was detected [64]. However, results were obtained mostly by using non-invasive sampling (e.g., brush biopsy). In an own study, analyzing tonsillar samples from 262 patients without HNSCC, we detected positive immunogenic staining for the p16-protein in 28% of cases. In healthy tissue, this is not related to an HPV-infection, but is a physiological sign for protein expression during cellular aging. The rate of HR-HPV infections was determined to be less than 1% for the same group of samples [79]. This low infection rate for healthy adults suggests a distinct role of HPV for carcinogenesis of the upper aerodigestive tract compared to the cervix uteri.
3.2 Disease transmission
Compared to extensive date concerning transmission of genital HPV-infections by sexual contact, little is known about transmission of oral HPV-infections. For example, the mean period of latency between infection and development of HPV-associated OSCC is completely unknown. To uncover predisposing factors for oral HPV-infections may be helpful for primary prevention of HPV-associated OSCC and for consulting couples, affected by HPV-associated OSCC. In 2009, for example, two cases of heterosexual couples have been reported, which each developed HPV-associated OSCC in a short period. Genetic signatures of the virus types were conform for each couple, suggesting a horizontal virus transmission by the partner [4].
The increase of incidences for HPV-associated OSCC is probably in line with changing sexual practices. HPV-DNA was detected in the female oropharynx to a significantly higher percentage, when a genital lesion caused by HPV was present at the same time. Oral sex could not be directly related to an expansion of genital to oral infection [48]. On the other hand, the question for oral sex was affirmed three times more often by the group with HPV-associated OSCC, than by the group with HPV-negative OSCC in a study by Herrero et al. [64]. A large Swedish index of tumors between 1958 and 1996 was analyzed for secondary carcinoma of husbands of wife’s having cervical carcinoma. The risk for OSCC was significantly elevated for man. Also, for women with carcinoma in situ of the cervix, the risk for developing HPV-associated OSCC was elevated [62]. Genital HPV-infections are frequent for man. A prevalence of 2/3 of the male population between 18 and 70 years of age is estimated in several studies. The number of sexual partners seems to be the most important risk factor and HPV-infection persist longer for females than for males. Main interests were focused on the transmission between men and woman and the role for cervical cancer development of in the past. A transmission path of oral HPV-infections of men could not be experimentally demonstrated coherently so far.
Despite identification of HPV as a risk factor for OSCC, only few population based studies about the oropharyngeal transmission of HPV do exist. At the beginning, risk factors for patients with OSCC to have HPV-positive tested tumors have been lacking tobacco abuse, early life time and HIV-infection [165]. In a recent review from Termine et al., prevalence of oral HPV-infections of women with cervical HPV-infections was determined and a meta-analysis of available literature was performed [177]. The pooled prevalence of oral HPV-infection is indicated with 18.1% and the only significant determinant of oral HPV infection was younger age at sexual debut. An association of oral sex for instance, could not be demonstrated by the study. In another analysis, not only the number of vaginal or oral sex partners, but also number of partners sharing kisses was demonstrated as risk factors for oral HPV-infections [32]. In conclusion, evidence for oral HPV-infections to be related to sexual behavior seems to be sufficient for women and most probably also for men.
3.3. Tonsillar crypt epithelium – correlation to cervical crypts?
Tonsilla palatina is a well known localization for the replication of viruses (e.g., Epstein-Barr virus), and possibly also serving as a reservoir for HPV. However, prevalence of HPV was estimated to be only 1% for healthy test persons by using brush biopsies [79]. For detailed understanding of their role, tonsillar samples with apparent risk for malign transformation would be required. In contrast to the cervical transformation zone, precursors of tonsillar carcinoma (dysplasia, carcinoma in-situ) are almost unknown.
The mucosa at the tonsillar area is morphologically in close contact to lymphatic tissue of the Waldeyer's ring. A unique, reticular stratified epithelium with basaloid differentiation covers the invaginations in the depth of the crypts and enables transport and direct contact to lymphatic tissue. Basal cells and basal membrane are partially permeable and admit passage of lymphocytes and antigen presenting cells at the bottom side of the epithelium [119]. Probably, the majority of HPV-positive OSCC originates from tonsillar crypts, which might be preferential sites for infection by HPV. At the cervical transformation zone, an injury is required to expose the basal lamina for infection by HPV [145], this is rather not the case within the tonsillar crypts.
Integration of HPV-DNA has been located to the basaloid, single- or double-layered epithelia of the tonsillar crypts and is mostly definable from stratified epithelia of the tonsillar surface above. In a recent study, samples from 176 patients with head and neck carcinoma were analyzed by in-situ hybridization and immunohistochemistry for the p16-protein. It was shown, that HPV-DNA detection was limited to abnormal and dysplastic epithelia of tonsillar crypts, while p16 was also detected in the surrounding, healthy tissue of the crypts. Therefore the authors conclude, that integration of HPV-DNA is not a field effect [8]. In summary from today’s state of knowledge, the reason for a strong association between HPV-infection and tonsillar carcinoma seems to be primary founded in the susceptibility, because of the special microanatomy of the Tonsilla palatina. Probably, local cytokine expression may also be involved, by stimulating viral transcription and cellular transformation. Consequently, crypt epithelia of the Tonsilla palatina may be considered as correlate of the transformation zone of cervix uteri.
4 Prognosis
Risk assessment in the field of oncology yields two different categories of markers:
Prognostic markers provide insight into aggressiveness of the disease concerning recurrence-free and progression-free survival
Predictive markers predict response or resistance concerning therapy
HNSCC are a heterogeneous group of diseases, depending upon localization and diameter of the tumor, a major difference in prognosis might result. The aim of new investigation often is, whether biomarkers or other confounders have influence towards the outcome. Independent prognostic significance, however, can best be assessed using multivariate analysis, considering classical risk factors. Determination of new clinical or molecular markers therefore is of limited value when the sample size is small.
4.1 Classical prognostic factors
4.1.1 Subsites
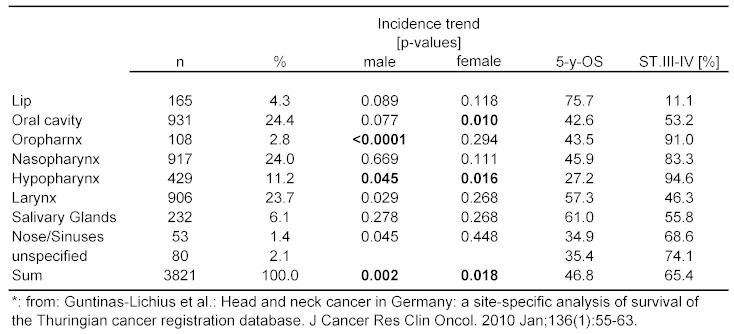
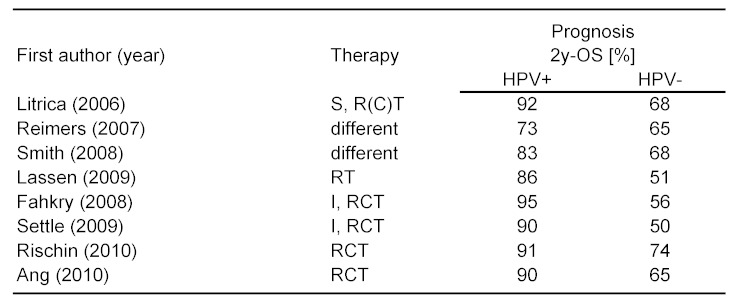
Subsite analysis is problematic in the field of otorhinolaryngology (ORL), because there are so many. Even large databases, e.g., EUROCARE-3, often provide limited information concerning subsite localization [149]. It is further known that due to variable smoking habits differences exist depending upon geographic localization of samples [124]. Incidence of HNSCC for example is higher in Southern Europe. Subsite descriptions might be erroneous, e.g., bas of tongue cancer might in US-publications be called oral/tongue cancer. Recently, population-bases analysis of 3,821 HNSCC patients of Thuringia/Germany including subsites have been published (Table 3 (Tab. 3)).
Table 3. Survival in Thuringia according to subsites, 1996-2005*.
A 5-year-OS below 50% was demonstrated for the whole sample, the same paper provided hint to increasing incidence of HNSCC in men, mainly resulting from incidence rise in cancers of the oro- and hypopharynx. Most frequent subsites affected were oral cavity and larynx (~25%). The best, respectively worst prognosis was related to cancer of the lip and hypopharynx [53]. It was concluded that, in ORL, different subsite show favorable discriminatory power in cancer of the lip and hypopharynx only.
4.1.2 Tumor staging
US-American (AJCC) und European (UICC) staging systems with the help of TNM categories exist. The TNM-system was developed in France by Pierre Denoix from 1943–1952. It was based to statistical evaluation of such as tumor size and prognosis of the patients. Currently, the predominantly congruent 7th editions have been published by UICC and AJCC. Minor modifications affect the field of ORL, including cancer of the naso- and oropharynx [183]. T3-categorie in cancer of the oropharynx now is:
T3: Tumor >4 cm in largest diameter or extension to lingual surface of the epiglottis
Extension to lingual surface of the epiglottis is not infiltration of the larynx in base of tongue and/or vallecula cancer and therefore must not be classified T4a. Minor changes affect cancers of the nasopharynx:
T1: Tumor confined to nasopharynx or extends to oropharynx and/or nasal cavity
T2: Tumor with parapharyngeal extension
Prognostic significance of TNM categories have been evaluated in the sample of Thuringia. Respective Hazard ratios for overall survival were as follows: T4 (2.6) → N3 (2.1) → M1 (2.0) → T3 (1.7) → N2 (1.7) → T2 (1.4) → N1 (1.3). All differences were significant [53]. Overall survival also has been evaluated in a study from Cologne. Odd’s ratios concerning T- (p=0.016; OR 0.52), N- (p=0.02; OR 0.52) and M-category (p<0.0001, OR 0.37) differed significantly [130].
Some peculiarities of TNM-classification should be addressed. T-category showed to be able to predict the outcome, e.g., after transoral laser surgery of the larynx [129]. 6th edition of TNM provided the new T4a und T4b category. After retrospective analysis of 163 patients, both categories turned out to provide discriminatory power (32.4 vs. 6.7% DSS) [132]. The value of T-categories in cancer of the hypopharynx is limited since less than 3% of the patients are stage I–II when presenting for treatment. In Canada, alternative staging systems of cancers of the hypopharynx have been examined and published to be superior, when compared to T-categories [57].
Oral cavity cancers have been examined and UICC-stages showed discriminatory power concerning prognosis. T4a and T4b-category, however, showed no significant difference towards the outcome [88]. Groome and co-workers demonstrated superiority of alternative stage groupings for cancers in 642 patients with cancer of the oropharynx [51]. The power of TNM staging is obvious. It is easy, accepted, long-standing, user-friendly and therefore effective. Validity towards prognosis is proven. However, improvements might be proposed for each subsite of cancer. In conclusion, stage grouping according to TNM categories can be regarded as gold standard to predict prognosis of our patients.
4.1.3 Resection margins
Favorable outcome after complete surgical excision has manifold been demonstrated. After cutting through a tumor as soon as 1978 a recurrence rate of 80% versus 12–18% (free margins) could be demonstrated in a large sample [15]. A safety margin of ≥5 mm is frequently declared to be appropriate. There is limited knowledge whether larger margins result in better prognosis. There is uneven data comparing “tumor free margins” versus “close margins“, definition of close margin often is blurry. Free margins can be monitored “online” using frozen sections. The drawback concerning survival, however, most likely cannot completely be corrected, once the pathologist tells the surgeon to cut more out, since tumor tissue is present in frozen sections [52].
4.1.4 Tumor thickness/infiltration depth
Tumor thickness and infiltration depth are accepted risk factors for occult neck metastasis in early-stage oral cavity cancer. Infiltration depth of <2 mm has recently been demonstrated to correlate with recurrence-free survival in 216 patients with tongue cancer [43]. Many publications cite larger cut-off values, it might be appropriate to perform elective neck dissection at 4 mm infiltration depth in early stage tongue cancer. In floor of mouth cancer occult metastasis has been shown to exceed 30% depending upon tumor thickness of >1.5 mm [108].
4.1.5 Others
Demographic and other patient related markers (age, gender, comorbidity, smoking habits...) are meaningful. In the Thuringia patients, the negative impact of male gender and age above 60 years was demonstrated [53]. However, in contrast to Karnofsky performance score, age and gender did not show influence on prognosis in a large Eastern European study [74]. After evaluation of the RTOG 0129-Study (Platinum + accelerated [concomitant boost] radiotherapy versus standard-fractioning) HPV and smoking habits were proven as independent risk factors for prognosis [6]. In a study from the Netherlands 208 patients with HNSCC were evaluated and cognitive factors and family status showed influence on survival. Unwed patients had 1.7 respectively 1.9-fold higher risk of recurrence or death during follow-up [26]. As further example blood hemoglobin (Hb) concentration is known to influence the outcome after radiotherapy. In a retrospective study, 214 patients showed survival of ≥15 months of 25%, 50%, and 75% when Hb-values of <11.2 g/dl, <12.7 g/dl, und <13.9 g/dl were reached. Hb-concentration seems to be a good predictive factor for the effectivity of radiotherapy and not an independent prognostic factor. Many other examples could be given.
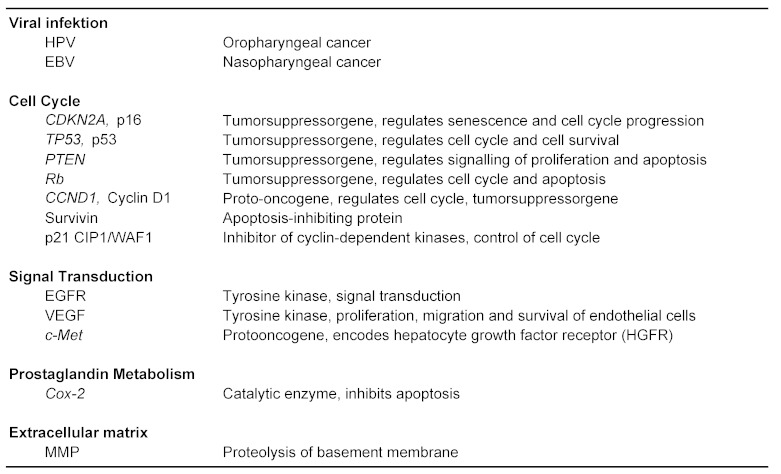
4.2 Molecular markers
Different cellular signaling networks can be involved in tumor growth through deregulation and activation. Many potential links of signals lead to networks that can be up regulated in relation to specific tumor entities. Table 4 (Tab. 4) contains signal paths and biomarkers with relevance towards prognosis in HNSCC.
Table 4. Molecular markers in head and neck cancer.
4.2.1 p53
A keynote change in cancer cells is deregulation of the cell cycle. Hereby endless mitotic activity can be achieved. Relevant genes encode the p53- and retinoblastoma (pRb/Rb)-Signal pathway proteins. Mutations of TP53 gene can be shown in 60–80% of HNSCC and 50% of the specimen show loss of the chromosomal region 17p, where the TP53 gene is located. 560 specimen of HNSCC were evaluated for TP53 mutation and presence of mutation was correlated to a poor survival [128].
4.2.2 Prevention
It is unknown why dysplasia proceeds to carcinoma, morphologic changes are not valid. WHO grading of dysplasia is not very reliable. Molecular markers that help to identify dangerous dysplasia have been described as combined allelic loss (loss of heterozygosity, LOH) on chromosomes 3p and 9p in oral cavity cancer [101]. Ploidy has been examined in leukoplakia of the oral cavity in 150 patients and it was shown that resection status had no influence on progression to carcinoma [173]. Biomarker detection in saliva seems promising for secondary prevention [119].
4.2.3 Resection margins
Traditional definition of resection margins might be newly defined by molecular methods in future. TP53 mutation [13], LOH [150], promoter hypermethylation [103] and eIF4E-protoncogene-overexpression (eukaryotic initiation factor 4E – eIF4E) have been examined in free resection margins [116]. Recurrence rate was enhanced if the respective changes were detectable. Direct visualization of changes by autofluorescence or live dyes has the advantage to be available online in the operating room. However, processing times in molecular pathology will be significantly reduced towards real-time analysis in future [60].
4.2.4 Prognostic relevance
Many publications deal with immunohistochemistry of apoptosis- or proliferation markers and EGFR-expression turned out to have limited prognostic significance. None of these markers is used in routine histopathology. Heterogeneity of the disease and methodological problems might explain this handicap. For example TP53 mutations lead to different structural changes influencing p53 analysis. Merely mutations that alter the core region of the p53 protein and influence DNA-binding of the protein turned out to be of prognostic significance in HNSCC [128].
4.2.5 Oncogenic HPV
Association of oncogenic HPV and prognosis is well-established since years. Diseases-specific and overall survival is significantly better compared to HPV unrelated cancer [6], [47], [82]. The first prospective trial showed a 73% reduced risk of tumor progression and a 63% reduced risk of death after induction chemotherapy and radiotherapy when patients had HPV-positive tumors [36]. Fundamental reasons for the better outcome might be combined effects of immune response and intact apoptosis networks being activated during irradiation. Enhanced radioresistance might also be explained by E6 mediated action that could be shown in colony forming assay in cell culture experiments [121]. HPV-associated OSCC reveal specific overexpression of p16 protein. Intact p16 blocks progression from G1 to S-phase in healthy cells and hereby acts as a tumor suppressor. In HPV-related HNSCC binding of viral E7 to pRb leads to release of transcription factor E2F. E2F promotes cell cycle progression and induces p16 expression. This acts as an immunohistochemical check mark of HPV-related versus HPV-unrelated tumorigenesis. Comparing classical prognostic factors (TNM) showed inferiority according to p16-expression [39]. HPV association with DNA integration turned out to be an even better prognostic marker in comparison to neck metastasis. Notably, surgery followed by irradiation revealed no difference towards prognosis when compared to chemoradiation alone [170].
The effect of surgery alone in HPV-related OSCC was not shown to date since most tumors show advanced regional metastasis. However, strong clues that surgery followed by adjuvant therapy and chemoradiation in resectable OSCC at least are on a par exist [40]. The benefit of surgery has not been demonstrated in HPV-unrelated tumors in a prospective setting, however, there is a great need for this since in Germany it can be regarded as “standard of care”. Publications concerning prognosis of OSCC are summarized in Table 5 (Tab. 5). The 2-year-OS is given in relation to HPV status. Survival benefit can be estimated as 30% in HPV-related OSCC. Possibly, in HPV-unrelated OSCC, a survival benefit exists after surgery followed by chemoradiation compared to chemoradiation alone [93]. In any case the effect of surgery must not be denied, though OSCC is treated in many countries with chemoradiation, independent of resectability. Until now is has not been shown conclusively that chemoradiation alone is the best treatment for all patients with OSCC. Further cell cycle components with association to Rb- und p53 signaling (Ki67, cyclin D1, Rb, p14ARF, MDM2, p53, p21Cip1/WAF1, and p27KIP1) have been examined in OSCC. Coexistence of HPV-infection with p14ARF und p21Cip1/WAF1, and inverse correlation of Rb and cyclin D1 in comparison to HPV-negative tumors has been demonstrated. Down-regulation of cyclin D1 und p21Cip1/WAF1 turned out to show independent prognostic significance after multivariate analysis [55].
Table 5. Prognosis of OSCC depending upon HPV status.
4.2.6 Survivin
HPV-related tumorigenesis seems to be associated with expression of survivin, a key protein of apoptosis. Survivin eliminates attempt of apoptosis and regulates the cell cycle. It cannot be detected in the majority of ripe cells. However, it is expressed in fetal tissues and nearly all human malignant tumors. OSCC yield connections between survivin expression and HPV-infection. It is assumed that HPV16 E6 und E7 regulate the promoter region of the survivin gene according to p53 status. Prognostic impact has been shown for several human tumors. Survivin expression respectively is correlated with poor outcome. In OSCC surviving expression has been shown to be inversely correlated with HPV-relation [131].
4.2.7 EGFR
The receptor for epidermal growth factor (EGFR) is part of the ErbB family of tyrosine kinase receptors that regulate epidermal cell growth. Specific ligands are EGF, TGF-α heparin-binding EGF-like growth factor, amphiregulin, betacellulin, epiregulin and epigen. Upregulated EGFR-signaling has been associated with cell proliferation, invasion, angiogenesis, metastasis, cell migration and apoptosis. EGFR is upregulated in many epithelial tumors, in HNSCC up to 80% of the cases, depicting an early event in carcinogenesis [5]. However, autocrine as well as downstream-effects of EGFR are influenced by tyrosine-phosphorylation and cross-phosphorylation of EGFR. The demonstration of EGFR expression during immunohistochemistry covers methodological problems, however, it can be concluded that overexpression of EGFR probably is linked with a poor prognosis. Consistently, antidromic data on EGFR-expression and HPV-infection are available [89], [140]. Mutations of the EGFR-gene, e.g., EGFRvIII has been identified in 42% of HNSCC, and correlated with inefficient anti-EGFR-therapy [167]. Later these results were not confirmed [58]. Gene amplification can lead to EGFR activation and was shown in 30% of patients with oral cavity cancer [113], [155]. HGFR (hepatocyte growth factor receptor) activates AKT and Ras signaling and has recently been described in HNSCC [84].
A vast number of molecular markers and candidate genes exist, however valid data concerning prognosis is lacking. Serum-biomarkers (crP, HPV16-proteom), markers of invasion and metastasis (MMP, CSMD1-Gen, EMT-Marker), angiogenesis (VEGF), miRNA, PI3K-PTEN-AKT-signalling, radioresistance (hypoxia, ataxia telangiectasia mutated protein – ATM) and chemoresistance (ERCC1) might be mentioned. The quest for biomarkers and validating their prognostic impact is the crucial step towards individualized tumor therapy. The prediction of radiosensitivity is of utmost importance in future. An option to validate predictive value of a biomarker is response to induction chemotherapy. For clinical routine, HPV-association is a valid prognostic marker in HNSCC, illustrating classical prognostic factors to be less important in OSCC.
4.3 HPV testing in clinical practice
In 1985 detection of HPV16 DNA succeeded in a patient with oral cavity cancer [98]. To date, no standard is available for the verification of HPV-association in HNSCC. Basically amplification-techniques for detection of viral DNA and mRNA transcripts and protein-labeling can be distinguished (Table 6 (Tab. 6)). The choice of method depends on the material (fresh or fixed tissue), time on your hands, human resources, and financial aspects. Each method has its limitations. PCR-based methods carry the risk of false positive results. For the detection of biologically active HPV-infection mRNA detection of E6 and E7 oncogene products with quantitative RT-PCR is mandatory. However it is time-consuming and expensive. The combination of two methods is meaningful. Conveniently, immunohistochemical detection of p16-protein is followed by PCR-based assays [163].
Table 6. Detection of HPV infection.
5 Targeted therapy
Conventional therapy is undirected and may damage otherwise healthy tissue. Targeted therapy is directed to microenvironment, vasculature, and specific proteins and signaling pathways that are involved in carcinogenesis. Ideally, selective components of tumor cells that are absent in healthy cells are targeted. Several techniques for selective tumor therapy are under development. Gene therapy, monoclonal antibodies, antibody/toxin-conjugates, small molecules, antisense molecules and tumor vaccine hereunder can be summarized. Decoding the role of HER/EGFR-family for carcinogenesis, detection of EGFR-overexpression in HNSCC patients with poor prognosis as well as the absence of an essential role of EGFR signal transduction in healthy tissue accentuates the EGFR network as a favorable goal of targeted therapy. Other potential signal transducers comprise the ras oncogene, the STAT family, fibroblast and hepatocyte growth factor, and the nuclear factor kappaB (NF-κB). NF-κB normally advances cell growth in inflammatory conditions.
5.1 Extracellular blocking of HER/EGFR
The strategy of extracellular blocking of HER/EGFR-family contains antibody against HER2-receptor, Trastuzumab and Pertuzumab. Trastuzumab (Herceptin®) is approved in breast cancer, when HER-2 is expressed. Pertuzumab is a humanized monoclonal antibody and exhibits a new class of HER dimerization-inhibitors. Pertuzumab is the first developed drug for the inhibition of pair production of HER2-receptor and other HER-receptors (EGFR/HER1, HER3 und HER4). Further anti-HER-1/EGFR-antibodies are involved in preclinical and clinical studies.
5.2 Monoclonal antibodies against EGFR
Many solid tumors including HNSCC show overexpression of EGFR by means of immunohistochemistry. Overexpression, as well as aberrant copy numbers of the EGFR gene was reported to be associated with poor disease-specific and overall survival. The first phase-III-study demonstrating effectiveness of EGFR-antibody has been published by Bonner et al. [10]. Cetuximab (Erbitux®) plus irradiation versus irradiation alone have been compared and local tumor control (24.4 vs. 14.9 months), as well as overall survival (49 vs. 29.3 months) were significantly improved [10]. During palliation Vermorken and coworkers published that the addition of Cetuximab to platinum-based chemotherapy resulted in 3 months longer survival of the patients [178]. Various other indications for Cetuximab (e.g., Cetuximab-maintenance/ACCRA-HN-study) currently are under clinical investigation. The application of another EGFR-antibody (Panitumumab, Vectibix®) is also currently under clinical investigation in a phase-III-study (EORTC 22071-24071), however, to date Panitumumab is approved for colorectal cancer only.
A further aspect is mutation of EGFR: EGFRvIII (epidermal growth factor receptor variant III). EGFRvIII does not exhibit EGF binding site, but contains tyrosine kinase activity. In a phase-II-study effectivity of a specific peptide vaccination has been approved successfully in glioblastoma patients. EGFRvIII has been detected in almost 50% of patients with HNSCC and reduced activity of Cetuximab in this regard, has been reported in a mouse model [167]. Another predictive factor for effectivity of EGFR targeted therapy is an acne-like rash during therapy. Enhanced activity of EGFR targeted therapy has manifold been reported, if a pronounced rash occurred. In future the identification of patients that will benefit from anti-EGFR targeted therapy will be of importance, to date this is not possible. Certainly, the demonstration of EGFR overexpression in immunohistochemistry alone, will not predict effectivity of anti-EGFR targeted therapy. Recently, copy numbers of EGFR-gene amplification has been reported to act as predictive marker for anti-EGFR targeted therapy.
5.3 Intracellular blocking of the HER/EGFR-network
Inhibitors of tyrosine kinase (TKI) of EGFR have the theoretical advantage of blocking intracellular signaling, too. In preclinical studies, the addition of TKI to EGFR-antibody targeted therapy has been effective [69]. Numerous small molecules with TKI-activity are under clinical investigation. Erlotinib (Tarceva®) and Gefitinib (Iressa®) are applicable as oral drugs and have been approved, yet not in HNSCC. In a phase-III-study with Gefitinib in recurrent HNSCC the addition of Gefitinib to chemotherapy has failed to result in longer overall survival [169]. A further TKI, Lapatinib, inhibits EGFR- and HER-2-signalling. A phase-I-study showed promising results, however, the following phase-II-study did not [1]. Until now, there is insufficient impact of TKI-effectivity in HNSCC. A deeper insight into networks of signaling and valid predictive markers would be mandatory in future.
5.4 Others
Deregulation of the cell cycle is a potential target for anti-tumor related therapy. Restoration of intact p53 has been evaluated by adenoviral gene transfer in vitro, but not in clinical trials. The INK-gene family (p15, p16, p18, p19) regulates G1-phase of cells, animal experiments and in vitro studies have also been performed to restore intact p16. By means of transfection of antisense-cyclinD1-DNA growth inhibition could be reached in animal experiments. Induction of apoptosis by stimulation of extracellular receptors (TRAIL-R1, TRAIL-R2) is another potential target and is subject of experimental studies. Telomerase of cells yields another future target.
Selective inhibition of angiogenesis has been attempted by antisense-VEGF-mRNA in vitro. Vaccines have been established in animal models to inhibit angiogenesis. Bevacizumab, a humanized anti-VEGF-antibody has been studied in a phase-III clinical trial in colorectal cancer. For HNSCC patients a phase-III-study currently is performed (ECOG-E1305). For the inhibition of invasion and metastasis monoclonal antibodies against EpCAM (Epithelial cell adhesion molecule) have been evaluated [134]. Interleukin-13-receptor has been identified as a further target by gene transfer and subsequent therapy with antibody-toxin-conjugate in cell lines, further examples are numerous.
Molecular targeted therapy will play a major role in future. To date only EGFR targeting is established in clinical routine. Numerous targets are currently under clinical investigation. The identification of new molecular markers will push further clinical studies. Oncogenic HPV as a risk factor has opened the floodgates for primary prophylaxis as well as selective targeted therapy directed against oncogenic HPV.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Abidoye OO, Cohen EE, Wong SJ, et al. A phase II study of lapatinib (GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) Proc Am Soc Clin Oncol. 2006;24:297S. [Google Scholar]
- 2.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D'Souza G, Gravitt PE, Westra W, Psyrri A, Kast WM, Koutsky LA, Giuliano A, Krosnick S, Trotti A, Schuller DE, Forastiere A, Ullmann CD. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009 Nov;31(11):1393–1422. doi: 10.1002/hed.21269. Available from: http://dx.doi.org/10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 3.Albers AE, Hoffmann TK, Klussmann JP, Kaufmann AM. Prophylaktische und therapeutische Vakzinen gegen humane Papillomviren. [Prophylactic and therapeutic vaccines against human papilloma virus]. HNO. 2010 Aug;58(8):778–790. doi: 10.1007/s00106-010-2118-6. (Ger). Available from: http://dx.doi.org/10.1007/s00106-010-2118-6. [DOI] [PubMed] [Google Scholar]
- 4.Andrews E, Shores C, Hayes DN, Couch M, Southerland J, Morris D, Seaman WT, Webster-Cyriaque J. Concurrent human papillomavirus-associated tonsillar carcinoma in 2 couples. J Infect Dis. 2009 Sep;200(6):882–887. doi: 10.1086/605442. Available from: http://dx.doi.org/10.1086/605442. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002 Dec;62(24):7350–7356. [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul;363(1):24–35. doi: 10.1056/NEJMoa0912217. Available from: http://dx.doi.org/10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Applebaum KM, Furniss CS, Zeka A, Posner MR, Smith JF, Bryan J, Eisen EA, Peters ES, McClean MD, Kelsey KT. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007 Dec;99(23):1801–1810. doi: 10.1093/jnci/djm233. Available from: http://dx.doi.org/10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 8.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005 Aug;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. Int J Cancer. 2011 Aug;129(3):733–741. doi: 10.1002/ijc.25699. Available from: http://dx.doi.org/10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006 Feb;354(6):567–578. doi: 10.1056/NEJMoa053422. Available from: http://dx.doi.org/10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, Andersen P, Gross N, Olson S, Deng C, Lu SL, Wang XJ. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 2009 Nov;119(11):3408–3419. doi: 10.1172/JCI38854. Available from: http://dx.doi.org/10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, Brakenhoff RH. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004 Jul;96(13):998–1006. doi: 10.1093/jnci/djh183. Available from: http://dx.doi.org/10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 13.Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995 Feb;332(7):429–435. doi: 10.1056/NEJM199502163320704. Available from: http://dx.doi.org/10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 14.Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000 Mar;21(3):405–426. doi: 10.1093/carcin/21.3.405. Available from: http://dx.doi.org/10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- 15.Byers RM, Bland KI, Borlase B, Luna M. The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978 Oct;136(4):525–528. doi: 10.1016/0002-9610(78)90275-1. Available from: http://dx.doi.org/10.1016/0002-9610(78)90275-1. [DOI] [PubMed] [Google Scholar]
- 16.Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010 Feb;84(3):1214–1220. doi: 10.1128/JVI.01927-09. Available from: http://dx.doi.org/10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellsagué X, Quintana MJ, Martínez MC, Nieto A, Sánchez MJ, Juan A, Monner A, Carrera M, Agudo A, Quer M, Muñoz N, Herrero R, Franceschi S, Bosch FX. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int J Cancer. 2004 Feb;108(5):741–749. doi: 10.1002/ijc.11627. Available from: http://dx.doi.org/10.1002/ijc.11627. [DOI] [PubMed] [Google Scholar]
- 18.Cervigne NK, Reis PP, Machado J, Sadikovic B, Bradley G, Galloni NN, Pintilie M, Jurisica I, Perez-Ordonez B, Gilbert R, Gullane P, Irish J, Kamel-Reid S. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum Mol Genet. 2009 Dec;18(24):4818–4829. doi: 10.1093/hmg/ddp446. Available from: http://dx.doi.org/10.1093/hmg/ddp446. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008 Feb;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. Available from: http://dx.doi.org/10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 20.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, Ang KK, El-Naggar AK, Zanation AM, Cmelak AJ, Levy S, Slebos RJ, Yarbrough WG. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006 Aug;66(16):8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 21.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X, Shockley WW, Weissler MC, Dressler LG, Shores CG, Yarbrough WG, Perou CM. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004 May;5(5):489–500. doi: 10.1016/S1535-6108(04)00112-6. Available from: http://dx.doi.org/10.1016/S1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J, Chen Z, Lu SL, Yang XP, Arun P, Ehsanian R, Brown MS, Lu H, Yan B, Diallo O, Wang XJ, Van Waes C. Attenuated transforming growth factor beta signaling promotes nuclear factor-kappaB activation in head and neck cancer. Cancer Res. 2009 Apr;69(8):3415–3424. doi: 10.1158/0008-5472.CAN-08-3704. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-08-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, McGoogan E, Menon U, Terry G, Edwards R, Brooks C, Desai M, Gie C, Ho L, Jacobs I, Pickles C, Sasieni P. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003 Dec;362(9399):1871–1876. doi: 10.1016/S0140-6736(03)14955-0. Available from: http://dx.doi.org/10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 24.Dai M, Clifford GM, le Calvez F, Castellsagué X, Snijders PJ, Pawlita M, Herrero R, Hainaut P, Franceschi S. Human papillomavirus type 16 and TP53 mutation in oral cancer: matched analysis of the IARC multicenter study. Cancer Res. 2004 Jan;64(2):468–471. doi: 10.1158/0008-5472.CAN-03-3284. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-03-3284. [DOI] [PubMed] [Google Scholar]
- 25.Dall KL, Scarpini CG, Roberts I, Winder DM, Stanley MA, Muralidhar B, Herdman MT, Pett MR, Coleman N. Characterization of naturally occurring HPV16 integration sites isolated from cervical keratinocytes under noncompetitive conditions. Cancer Res. 2008 Oct;68(20):8249–8259. doi: 10.1158/0008-5472.CAN-08-1741. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-08-1741. [DOI] [PubMed] [Google Scholar]
- 26.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001 Feb;37(3):332–339. doi: 10.1016/S0959-8049(00)00385-3. Available from: http://dx.doi.org/10.1016/S0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 27.de Souza DL, de Camargo Cancela M, Pérez MM, Curado MP. Trends in the incidence of oral cavity and oropharyngeal cancers in Spain. Head Neck. 2012 May;34(5):649–654. doi: 10.1002/hed.21793. Available from: http://dx.doi.org/10.1002/hed.21793. [DOI] [PubMed] [Google Scholar]
- 28.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004 Jun;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. Available from: http://dx.doi.org/10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Do Sacramento P, Babeto E, Colombo J, et al. The prevalence of human papillomavirus in the oropharynx in healthy individuals in a Brazilian population. J Med Virol. 2006;78:614–618. doi: 10.1002/jmv.20583. Available from: http://dx.doi.org/10.1002/jmv.20583. [DOI] [PubMed] [Google Scholar]
- 30.Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, Wand H, Fairley CK. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011 Jan;11(1):39–44. doi: 10.1016/S1473-3099(10)70225-5. Available from: http://dx.doi.org/10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 31.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005 Mar;32 Suppl 1:S7–15. doi: 10.1016/j.jcv.2004.12.006. Available from: http://dx.doi.org/10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 32.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009 May 1;199(9):1263–1269. doi: 10.1086/597755. Available from: http://dx.doi.org/10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. Available from: http://dx.doi.org/10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 34.Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA. 2000 Aug;97(18):10002–10007. doi: 10.1073/pnas.170093297. Available from: http://dx.doi.org/10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duensing S, Münger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol. 2003 Nov;77(22):12331–12335. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. Available from: http://dx.doi.org/10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 37.Fei J, Hong A, Dobbins TA, Jones D, Lee CS, Loo C, Al-Ghamdi M, Harnett GB, Clark J, O'Brien CJ, Rose B. Prognostic significance of vascular endothelial growth factor in squamous cell carcinomas of the tonsil in relation to human papillomavirus status and epidermal growth factor receptor. Ann Surg Oncol. 2009 Oct;16(10):2908–2917. doi: 10.1245/s10434-009-0579-1. Available from: http://dx.doi.org/10.1245/s10434-009-0579-1. [DOI] [PubMed] [Google Scholar]
- 38.Ferris RL, Martinez I, Sirianni N, Wang J, López-Albaitero A, Gollin SM, Johnson JT, Khan S. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005 Mar;41(5):807–815. doi: 10.1016/j.ejca.2004.11.023. Available from: http://dx.doi.org/10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Fischer CA, Kampmann M, Zlobec I, Green E, Tornillo L, Lugli A, Wolfensberger M, Terracciano LM. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010 Oct;21(10):1961–1966. doi: 10.1093/annonc/mdq210. Available from: http://dx.doi.org/10.1093/annonc/mdq210. [DOI] [PubMed] [Google Scholar]
- 40.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010 Mar;126(5):1256–1262. doi: 10.1002/ijc.24842. Available from: http://dx.doi.org/10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 41.Franceschi S, Levi F, La Vecchia C, Conti E, Dal Maso L, Barzan L, Talamini R. Comparison of the effect of smoking and alcohol drinking between oral and pharyngeal cancer. Int J Cancer. 1999 Sep;83(1):1–4. doi: 10.1002/(SICI)1097-0215(19990924)83:1<1::AID-IJC1>3.0.CO;2-8. Available from: http://dx.doi.org/10.1002/(SICI)1097-0215(19990924)83:1<1::AID-IJC1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Furusawa J, Zhang H, Vural E, Stone A, Fukuda S, Oridate N, Fang H, Ye Y, Suen JY, Fan CY. Distinct epigenetic profiling in head and neck squamous cell carcinoma stem cells. Otolaryngol Head Neck Surg. 2011 Jun;144(6):900–909. doi: 10.1177/0194599811398786. Available from: http://dx.doi.org/10.1177/0194599811398786. [DOI] [PubMed] [Google Scholar]
- 43.Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer. 2012 Jan;118(1):101–111. doi: 10.1002/cncr.26229. Available from: http://dx.doi.org/10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 44.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997 Nov;73(5):663–669. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 45.Gasco M, Crook T. The p53 network in head and neck cancer. Oral Oncol. 2003 Apr;39(3):222–231. doi: 10.1016/S1368-8375(02)00163-X. Available from: http://dx.doi.org/10.1016/S1368-8375(02)00163-X. [DOI] [PubMed] [Google Scholar]
- 46.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008 Mar;100(6):407–420. doi: 10.1093/jnci/djn025. Available from: http://dx.doi.org/10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 47.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000 May;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 48.Giraldo P, Gonçalves AK, Pereira SA, Barros-Mazon S, Gondo ML, Witkin SS. Human papillomavirus in the oral mucosa of women with genital human papillomavirus lesions. Eur J Obstet Gynecol Reprod Biol. 2006 May;126(1):104–106. doi: 10.1016/j.ejogrb.2005.09.009. Available from: http://dx.doi.org/10.1016/j.ejogrb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011 Feb;364(5):401–411. doi: 10.1056/NEJMoa0909537. Available from: http://dx.doi.org/10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, Papenfuss MR, Abrahamsen M, Inserra P. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States) Cancer Causes Control. 2002 Nov;13(9):839–846. doi: 10.1023/A:1020668232219. Available from: http://dx.doi.org/10.1023/A:1020668232219. [DOI] [PubMed] [Google Scholar]
- 51.Groome PA, Schulze KM, Mackillop WJ, Grice B, Goh C, Cummings BJ, Hall SF, Liu FF, Payne D, Rothwell DM, Waldron JN, Warde PR, O'Sullivan B. A comparison of published head and neck stage groupings in carcinomas of the tonsillar region. Cancer. 2001 Sep;92(6):1484–1494. doi: 10.1002/1097-0142(20010915)92:6<1484::AID-CNCR1473>3.0.CO;2-W. Available from: http://dx.doi.org/10.1002/1097-0142(20010915)92:6<1484::AID-CNCR1473>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 52.Guillemaud JP, Patel RS, Goldstein DP, Higgins KM, Enepekides DJ. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2010 Aug;39(4):370–377. [PubMed] [Google Scholar]
- 53.Guntinas-Lichius O, Wendt T, Buentzel J, Esser D, Lochner P, Mueller A, Schultze-Mosgau S, Altendorf-Hofmann A. Head and neck cancer in Germany: a site-specific analysis of survival of the Thuringian cancer registration database. J Cancer Res Clin Oncol. 2010 Jan;136(1):55–63. doi: 10.1007/s00432-009-0636-y. Available from: http://dx.doi.org/10.1007/s00432-009-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008 Jun;122(12):2656–2664. doi: 10.1002/ijc.23458. Available from: http://dx.doi.org/10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 55.Hafkamp HC, Mooren JJ, Claessen SM, Klingenberg B, Voogd AC, Bot FJ, Klussmann JP, Hopman AH, Manni JJ, Kremer B, Ramaekers FC, Speel EJ. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009 May;22(5):686–698. doi: 10.1038/modpathol.2009.23. Available from: http://dx.doi.org/10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 56.Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, Hopman AH, Manni JJ. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003 Nov;107(3):394–400. doi: 10.1002/ijc.11389. Available from: http://dx.doi.org/10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 57.Hall SF, Groome PA, Irish J, O'Sullivan B. TNM-based stage groupings in head and neck cancer: application in cancer of the hypopharynx. Head Neck. 2009 Jan;31(1):1–8. doi: 10.1002/hed.20917. Available from: http://dx.doi.org/10.1002/hed.20917. [DOI] [PubMed] [Google Scholar]
- 58.Hama T, Yuza Y, Saito Y, O-uchi J, Kondo S, Okabe M, Yamada H, Kato T, Moriyama H, Kurihara S, Urashima M. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist. 2009 Sep;14(9):900–908. doi: 10.1634/theoncologist.2009-0058. Available from: http://dx.doi.org/10.1634/theoncologist.2009-0058. [DOI] [PubMed] [Google Scholar]
- 59.Hammarstedt L, Dahlstrand H, Lindquist D, Onelöv L, Ryott M, Luo J, Dalianis T, Ye W, Munck-Wikland E. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007 Sep;127(9):988–992. doi: 10.1080/00016480601110170. Available from: http://dx.doi.org/10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 60.Harden SV, Thomas DC, Benoit N, Minhas K, Westra WH, Califano JA, Koch W, Sidransky D. Real-time gap ligase chain reaction: a rapid semiquantitative assay for detecting p53 mutation at low levels in surgical margins and lymph nodes from resected lung and head and neck tumors. Clin Cancer Res. 2004 Apr;10(7):2379–2385. doi: 10.1158/1078-0432.CCR-03-0405. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-03-0405. [DOI] [PubMed] [Google Scholar]
- 61.Heck JE, Berthiller J, Vaccarella S, Winn DM, Smith EM, Shan'gina O, Schwartz SM, Purdue MP, Pilarska A, Eluf-Neto J, Menezes A, McClean MD, Matos E, Koifman S, Kelsey KT, Herrero R, Hayes RB, Franceschi S, Wünsch-Filho V, Fernández L, Daudt AW, Curado MP, Chen C, Castellsagué X, Ferro G, Brennan P, Boffetta P, Hashibe M. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010 Feb;39(1):166–181. doi: 10.1093/ije/dyp350. Available from: http://dx.doi.org/10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemminki K, Dong C. Cancer in husbands of cervical cancer patients. Epidemiology. 2000 May;11(3):347–349. doi: 10.1097/00001648-200005000-00022. Available from: http://dx.doi.org/10.1097/00001648-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 63.Hermsen M, Guervós MA, Meijer G, Baak J, van Diest P, Marcos CA, Sampedro A. New chromosomal regions with high-level amplifications in squamous cell carcinomas of the larynx and pharynx, identified by comparative genomic hybridization. J Pathol. 2001 Jun;194(2):177–182. doi: 10.1002/path.862. Available from: http://dx.doi.org/10.1002/path.862. [DOI] [PubMed] [Google Scholar]
- 64.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernández L, Idris A, Sánchez MJ, Nieto A, Talamini R, Tavani A, Bosch FX, Reidel U, Snijders PJ, Meijer CJ, Viscidi R, Muñoz N, Franceschi S. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003 Dec;95(23):1772–1783. doi: 10.1093/jnci/djg107. Available from: http://dx.doi.org/10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 65.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007 Aug;298(7):743–753. doi: 10.1001/jama.298.7.743. Available from: http://dx.doi.org/10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 66.Högmo A, Munck-Wikland E, Kuylenstierna R, Lindholm J, Auer G. Nuclear DNA content and p53 immunostaining in metachronous preneoplastic lesions and subsequent carcinomas of the oral cavity. Head Neck. 1996 Sep-Oct;18(5):433–440. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<433::AID-HED6>3.0.CO;2-6. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199609/10)18:5<433::AID-HED6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Holleman A, Chung I, Olsen RR, Kwak B, Mizokami A, Saijo N, Parissenti A, Duan Z, Voest EE, Zetter BR. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene. 2011 Oct;30(43):4386–4398. doi: 10.1038/onc.2011.148. Available from: http://dx.doi.org/10.1038/onc.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang CF, Monie A, Weng WH, Wu T. DNA vaccines for cervical cancer. Am J Transl Res. 2010;2(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- 69.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004 Aug;64(15):5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 70.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010 Jun;10(6):415–424. doi: 10.1038/nrc2853. Available from: http://dx.doi.org/10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 71.Izzo JG, Papadimitrakopoulou VA, Liu DD, den Hollander PL, Babenko IM, Keck J, El-Naggar AK, Shin DM, Lee JJ, Hong WK, Hittelman WN. Cyclin D1 genotype, response to biochemoprevention, and progression rate to upper aerodigestive tract cancer. J Natl Cancer Inst. 2003;95:198–205. doi: 10.1093/jnci/95.3.198. Available from: http://dx.doi.org/10.1093/jnci/95.3.198. [DOI] [PubMed] [Google Scholar]
- 72.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan-Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. Available from: http://dx.doi.org/10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 73.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci USA. 1995 Feb;92(5):1654–1658. doi: 10.1073/pnas.92.5.1654. Available from: http://dx.doi.org/10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeremić B, Milicić B. Pretreatment prognostic factors of survival in patients with locally advanced nonmetastatic squamous cell carcinoma of the head and neck treated with radiation therapy with or without concurrent chemotherapy. Am J Clin Oncol. 2009 Apr;32(2):163–168. doi: 10.1097/COC.0b013e31818254cc. Available from: http://dx.doi.org/10.1097/COC.0b013e31818254cc. [DOI] [PubMed] [Google Scholar]
- 75.Jin C, Jin Y, Wennerberg J, Annertz K, Enoksson J, Mertens F. Cytogenetic abnormalities in 106 oral squamous cell carcinomas. Cancer Genet Cytogenet. 2006 Jan;164(1):44–53. doi: 10.1016/j.cancergencyto.2005.06.008. Available from: http://dx.doi.org/10.1016/j.cancergencyto.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene. 1996 Jul;13(2):427–431. [PubMed] [Google Scholar]
- 77.Kim JS, Crooks H, Foxworth A, Waldman T. Proof-of-principle: oncogenic beta-catenin is a valid molecular target for the development of pharmacological inhibitors. Mol Cancer Ther. 2002 Dec;1(14):1355–1359. [PubMed] [Google Scholar]
- 78.Kjaer SK, Engholm G, Dahl C, Bock JE. Case-control study of risk factors for cervical squamous cell neoplasia in Denmark. IV: role of smoking habits. Eur J Cancer Prev. 1996 Oct;5(5):359–365. doi: 10.1097/00008469-199610000-00008. Available from: http://dx.doi.org/10.1097/00008469-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Klingenberg B, Hafkamp HC, Haesevoets A, Manni JJ, Slootweg PJ, Weissenborn SJ, Klussmann JP, Speel EJ. p16 INK4A overexpression is frequently detected in tumor-free tonsil tissue without association with HPV. Histopathology. 2010;56:957–967. doi: 10.1111/j.1365-2559.2010.03576.x. Available from: http://dx.doi.org/10.1111/j.1365-2559.2010.03576.x. [DOI] [PubMed] [Google Scholar]
- 80.Klussmann JP, Dinh S, Guntinas-Lichius O, Wittekindt C, Weissenborn S, Wieland U, Dienes HP, Hoffmann T, Smith E, Turek L, Speel EJ, Pfister HJ. HPV-assoziierte Tonsillenkarzinome. Ein Update. [HPV-associated tonsillar cancer. An update]. HNO. 2004 Mar;52(3):208–218. doi: 10.1007/s00106-004-1069-1. (Ger). Available from: http://dx.doi.org/10.1007/s00106-004-1069-1. [DOI] [PubMed] [Google Scholar]
- 81.Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I, Preuss SF, Straetmans JM, Manni JJ, Hopman AH, Speel EJ. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009 Mar;15(5):1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 82.Klussmann JP, Preuss SF, Speel EJ. Humane Papillomviren und Oropharynxkarzinome. Molekulare Interaktion und klinische Auswirkung. [Human papillomavirus and cancer of the oropharynx. Molecular interaction and clinical implications]. HNO. 2009 Feb;57(2):113–122. doi: 10.1007/s00106-008-1867-y. (Ger). Available from: http://dx.doi.org/10.1007/s00106-008-1867-y. [DOI] [PubMed] [Google Scholar]
- 83.Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Kolligs J, Jungehuelsing M, Eckel HE, Dienes HP, Pfister HJ, Fuchs PG. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001 Dec;92(11):2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::AID-CNCR10130>3.0.CO;2-7. Available from: http://dx.doi.org/10.1002/1097-0142(20011201)92:11<2875::AID-CNCR10130>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 84.Knudsen BS, Vande Woude G. Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev. 2008 Feb;18(1):87–96. doi: 10.1016/j.gde.2008.02.001. Available from: http://dx.doi.org/10.1016/j.gde.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999 Oct;109(10):1544–1551. doi: 10.1097/00005537-199910000-00002. Available from: http://dx.doi.org/10.1097/00005537-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, Shah KV, Gillison ML. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004 Feb;189(4):686–698. doi: 10.1086/381504. Available from: http://dx.doi.org/10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 87.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005 Feb;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. Available from: http://dx.doi.org/10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 88.Kreppel M, Eich HT, Kübler A, Zöller JE, Scheer M. Prognostic value of the sixth edition of the UICC's TNM classification and stage grouping for oral cancer. J Surg Oncol. 2010 Oct;102(5):443–449. doi: 10.1002/jso.21547. Available from: http://dx.doi.org/10.1002/jso.21547. [DOI] [PubMed] [Google Scholar]
- 89.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D'Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008 Jul;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. Available from: http://dx.doi.org/10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdhan A, Shah JP, Singh B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003 Jan;129(1):106–112. doi: 10.1001/archotol.129.1.106. Available from: http://dx.doi.org/10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 91.Lajer CB, Nielsen FC, Friis-Hansen L, Norrild B, Borup R, Garnæs E, Rossing M, Specht L, Therkildsen MH, Nauntofte B, Dabelsteen S, von Buchwald C. Different miRNA signatures of oral and pharyngeal squamous cell carcinomas: a prospective translational study. Br J Cancer. 2011 Mar;104(5):830–840. doi: 10.1038/bjc.2011.29. Available from: http://dx.doi.org/10.1038/bjc.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li YL, Qiu XH, Shen C, Liu JN, Zhang J. Vaccination of full-length HPV16 E6 or E7 protein inhibits the growth of HPV16 associated tumors. Oncol Rep. 2010 Nov;24(5):1323–1329. doi: 10.3892/or_00000989. [DOI] [PubMed] [Google Scholar]
- 93.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantù G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006 Dec;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. Available from: http://dx.doi.org/10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 94.Liu CJ, Kao SY, Tu HF, Tsai MM, Chang KW, Lin SC. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010 May;16(4):360–364. doi: 10.1111/j.1601-0825.2009.01646.x. Available from: http://dx.doi.org/10.1111/j.1601-0825.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009 Dec;286(2):217–222. doi: 10.1016/j.canlet.2009.05.030. Available from: http://dx.doi.org/10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H, Zhou X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics Proteomics. 2009 May-Jun;6(3):131–139. [PMC free article] [PubMed] [Google Scholar]
- 97.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003 Feb;39(2):106–114. doi: 10.1016/S1368-8375(02)00026-X. Available from: http://dx.doi.org/10.1016/S1368-8375(02)00026-X. [DOI] [PubMed] [Google Scholar]
- 98.Löning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, zur Hausen H. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol. 1985 May;84(5):417–420. doi: 10.1111/1523-1747.ep12265517. [DOI] [PubMed] [Google Scholar]
- 99.Luft F, Klaes R, Nees M, Dürst M, Heilmann V, Melsheimer P, von Knebel Doeberitz M. Detection of integrated papillomavirus sequences by ligation-mediated PCR (DIPS-PCR) and molecular characterization in cervical cancer cells. Int J Cancer. 2001 Apr;92(1):9–17. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1144>3.0.CO;2-L. Available from: http://dx.doi.org/10.1002/1097-0215(200102)9999:9999<::AID-IJC1144>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 100.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004 Apr;5(4):311–316. doi: 10.1016/S1535-6108(04)00090-X. Available from: http://dx.doi.org/10.1016/S1535-6108(04)00090-X. [DOI] [PubMed] [Google Scholar]
- 101.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996 Jun;2(6):682–685. doi: 10.1038/nm0696-682. Available from: http://dx.doi.org/10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 102.Martens JE, Arends J, Van der Linden PJ, De Boer BA, Helmerhorst TJ. Cytokeratin 17 and p63 are markers of the HPV target cell, the cervical stem cell. Anticancer Res. 2004 Mar-Apr;24(2B):771–775. [PubMed] [Google Scholar]
- 103.Martone T, Gillio-Tos A, De Marco L, Fiano V, Maule M, Cavalot A, Garzaro M, Merletti F, Cortesina G. Association between hypermethylated tumor and paired surgical margins in head and neck squamous cell carcinomas. Clin Cancer Res. 2007 Sep;13(17):5089–5094. doi: 10.1158/1078-0432.CCR-07-0119. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 104.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010 Aug;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. Available from: http://dx.doi.org/10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGovern SL, Williams MD, Weber RS, Sabichi A, Chambers MS, Martin JW, Chao KS. Three synchronous HPV-associated squamous cell carcinomas of Waldeyer's ring: case report and comparison with Slaughter's model of field cancerization. Head Neck. 2010 Aug;32(8):1118–1124. doi: 10.1002/hed.21171. Available from: http://dx.doi.org/10.1002/hed.21171. [DOI] [PubMed] [Google Scholar]
- 106.Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J, Dillner J, Heideman DA, Snijders PJ. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009 Feb;124(3):516–520. doi: 10.1002/ijc.24010. Available from: http://dx.doi.org/10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila) 2010;3:1361–1364. doi: 10.1158/1940-6207.CAPR-10-0234. Available from: http://dx.doi.org/10.1158/1940-6207.CAPR-10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohit-Tabatabai M, Sobel H, Rush B, et al. Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg. 1986;152:351–353. doi: 10.1016/0002-9610(86)90303-X. Available from: http://dx.doi.org/10.1016/0002-9610(86)90303-X. [DOI] [PubMed] [Google Scholar]
- 109.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L, Lehtinen M, Dillner J. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001 Apr;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. Available from: http://dx.doi.org/10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 110.Mork J, Møller B, Dahl T, Bray F. Time trends in pharyngeal cancer incidence in Norway 1981-2005: a subsite analysis based on a reabstraction and recoding of registered cases. Cancer Causes Control. 2010 Sep;21(9):1397–1405. doi: 10.1007/s10552-010-9567-9. Available from: http://dx.doi.org/10.1007/s10552-010-9567-9. [DOI] [PubMed] [Google Scholar]
- 111.Mutirangura A, Supiyaphun P, Trirekapan S, Sriuranpong V, Sakuntabhai A, Yenrudi S, Voravud N. Telomerase activity in oral leukoplakia and head and neck squamous cell carcinoma. Cancer Res. 1996 Aug;56(15):3530–3533. [PubMed] [Google Scholar]
- 112.Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, Meijer GA, Agami R. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008 Jul;68(14):5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 113.Nakata Y, Uzawa N, Takahashi K, Sumino J, Michikawa C, Sato H, Sonoda I, Ohyama Y, Okada N, Amagasa T. EGFR gene copy number alteration is a better prognostic indicator than protein overexpression in oral tongue squamous cell carcinomas. Eur J Cancer. 2011 Oct;47(15):2364–2372. doi: 10.1016/j.ejca.2011.07.006. Available from: http://dx.doi.org/10.1016/j.ejca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 114.Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, Ramqvist T, Lindholm J, Sparén P, Ye W, Dahlstrand H, Munck-Wikland E, Dalianis T. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009 Jul;125(2):362–366. doi: 10.1002/ijc.24339. Available from: http://dx.doi.org/10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 115.Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, Zhou H, Smith M, Kimberly D, Glass J. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004 Sep;10(17):5820–5827. doi: 10.1158/1078-0432.CCR-03-0483. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 116.Nathan CO, Liu L, Li BD, Abreo FW, Nandy I, De Benedetti A. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997 Jul;15(5):579–584. doi: 10.1038/sj.onc.1201216. Available from: http://dx.doi.org/10.1038/sj.onc.1201216. [DOI] [PubMed] [Google Scholar]
- 117.Ndisang D, Khan A, Lorenzato F, Sindos M, Singer A, Latchman DS. The cellular transcription factor Brn-3a and the smoking-related substance nicotine interact to regulate the activity of the HPV URR in the cervix. Oncogene. 2010 May;29(18):2701–2711. doi: 10.1038/onc.2010.33. Available from: http://dx.doi.org/10.1038/onc.2010.33. [DOI] [PubMed] [Google Scholar]
- 118.Okami K, Wu L, Riggins G, Cairns P, Goggins M, Evron E, Halachmi N, Ahrendt SA, Reed AL, Hilgers W, Kern SE, Koch WM, Sidransky D, Jen J. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 1998 Feb;58(3):509–511. [PubMed] [Google Scholar]
- 119.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. Available from: http://dx.doi.org/10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pandit R, Lathers DM, Beal NM, Garrity T, Young MR. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000 Aug;109(8 Pt 1):749–754. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 121.Pang E, Delic NC, Hong A, Zhang M, Rose BR, Lyons JG. Radiosensitization of oropharyngeal squamous cell carcinoma cells by human papillomavirus 16 oncoprotein E6∗I. Int J Radiat Oncol Biol Phys. 2011 Mar;79(3):860–865. doi: 10.1016/j.ijrobp.2010.06.028. Available from: http://dx.doi.org/10.1016/j.ijrobp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 122.Park IS, Chang X, Loyo M, Wu G, Chuang A, Kim MS, Chae YK, Lyford-Pike S, Westra WH, Saunders JR, Sidransky D, Pai SI. Characterization of the methylation patterns in human papillomavirus type 16 viral DNA in head and neck cancers. Cancer Prev Res (Phila) 2011 Feb;4(2):207–217. doi: 10.1158/1940-6207.CAPR-10-0147. Available from: http://dx.doi.org/10.1158/1940-6207.CAPR-10-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009 Sep;15(17):5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. Available from: http://dx.doi.org/10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 125.Pathirana D, Hillemanns P, Petry KU, Becker N, Brockmeyer NH, Erdmann R, Gissmann L, Grundhewer H, Ikenberg H, Kaufmann AM, Klusmann J, Kopp I, Pfister H, Rzany B, Schneede P, Schneider A, Smola S, Winter-Koch N, Wutzler P, Gross G. Short version of the German evidence-based Guidelines for prophylactic vaccination against HPV-associated neoplasia. Vaccine. 2009 Jul;27(34):4551–4559. doi: 10.1016/j.vaccine.2009.03.086. Available from: http://dx.doi.org/10.1016/j.vaccine.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 126.Petry KU, Scheffel D, Bode U, Gabrysiak T, Köchel H, Kupsch E, Glaubitz M, Niesert S, Kühnle H, Schedel I. Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer. 1994 Jun;57(6):836–840. doi: 10.1002/ijc.2910570612. Available from: http://dx.doi.org/10.1002/ijc.2910570612. [DOI] [PubMed] [Google Scholar]
- 127.Pickering BM, Willis AE. The implications of structured 5' untranslated regions on translation and disease. Semin Cell Dev Biol. 2005 Feb;16(1):39–47. doi: 10.1016/j.semcdb.2004.11.006. Available from: http://dx.doi.org/10.1016/j.semcdb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 128.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, Westra W, Sidransky D, Koch WM. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007 Dec;357(25):2552–2561. doi: 10.1056/NEJMoa073770. Available from: http://dx.doi.org/10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Preuss SF, Cramer K, Klussmann JP, Eckel HE, Guntinas-Lichius O. Transoral laser surgery for laryngeal cancer: outcome, complications and prognostic factors in 275 patients. Eur J Surg Oncol. 2009 Mar;35(3):235–240. doi: 10.1016/j.ejso.2008.01.012. Available from: http://dx.doi.org/10.1016/j.ejso.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 130.Preuss SF, Klussmann JP, Wittekindt C, Damm M, Semrau R, Drebber U, Guntinas-Lichius O. Long-term results of the combined modality therapy for advanced cervical metastatic head and neck squamous cell carcinoma. Eur J Surg Oncol. 2007 Apr;33(3):358–363. doi: 10.1016/j.ejso.2006.10.043. Available from: http://dx.doi.org/10.1016/j.ejso.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 131.Preuss SF, Weinell A, Molitor M, Stenner M, Semrau R, Drebber U, Weissenborn SJ, Speel EJ, Wittekindt C, Guntinas-Lichius O, Hoffmann TK, Eslick GD, Klussmann JP. Nuclear survivin expression is associated with HPV-independent carcinogenesis and is an indicator of poor prognosis in oropharyngeal cancer. Br J Cancer. 2008 Feb;98(3):627–632. doi: 10.1038/sj.bjc.6604192. Available from: http://dx.doi.org/10.1038/sj.bjc.6604192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Psychogios G, Waldfahrer F, Bozzato A, Iro H. Evaluation of the revised TNM classification in advanced laryngeal cancer. Eur Arch Otorhinolaryngol. 2010 Jan;267(1):117–121. doi: 10.1007/s00405-009-0970-3. Available from: http://dx.doi.org/10.1007/s00405-009-0970-3. [DOI] [PubMed] [Google Scholar]
- 133.Psyrri A, Prezas L, Burtness B. Oropharyngeal cancer. Clin Adv Hematol Oncol. 2008 Aug;6(8):604–612. [PubMed] [Google Scholar]
- 134.Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E, Jones D, Dethling J, Colman J, Coward L, MacGregor S. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002 Aug;360(9334):671–677. doi: 10.1016/S0140-6736(02)09836-7. Available from: http://dx.doi.org/10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 135.Qiu W, Schönleben F, Li X, Su GH. Disruption of transforming growth factor beta-Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett. 2007 Jan;245(1-2):163–170. doi: 10.1016/j.canlet.2006.01.003. Available from: http://dx.doi.org/10.1016/j.canlet.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raffle AE, Alden B, Quinn M, Babb PJ, Brett MT. Outcomes of screening to prevent cancer: analysis of cumulative incidence of cervical abnormality and modelling of cases and deaths prevented. BMJ. 2003 Apr;326(7395):901. doi: 10.1136/bmj.326.7395.901. Available from: http://dx.doi.org/10.1136/bmj.326.7395.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009 Mar 18;101(6):412–423. doi: 10.1093/jnci/djp017. Available from: http://dx.doi.org/10.1093/jnci/djp017. [DOI] [PubMed] [Google Scholar]
- 138.Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, Bartek J, Sidransky D. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996 Aug;56(16):3630–3633. [PubMed] [Google Scholar]
- 139.Regenbrecht CR, Lehrach H, Adjaye J. Stemming cancer: functional genomics of cancer stem cells in solid tumors. Stem Cell Rev. 2008 Dec;4(4):319–328. doi: 10.1007/s12015-008-9034-0. Available from: http://dx.doi.org/10.1007/s12015-008-9034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ, Guntinas-Lichius O, Klussmann JP. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007 Apr;120(8):1731–1738. doi: 10.1002/ijc.22355. Available from: http://dx.doi.org/10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 141.Ribeiro KB, Levi JE, Pawlita M, Koifman S, Matos E, Eluf-Neto J, Wunsch-Filho V, Curado MP, Shangina O, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Daudt A, Menezes A, Bencko V, Mates D, Fernandez L, Fabianova E, Gheit T, Tommasino M, Boffetta P, Brennan P, Waterboer T. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011 Apr;40(2):489–502. doi: 10.1093/ije/dyq249. Available from: http://dx.doi.org/10.1093/ije/dyq249. [DOI] [PubMed] [Google Scholar]
- 142.Rintala MA, Grénman SE, Järvenkylä ME, Syrjänen KJ, Syrjänen SM. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: experience from the Finnish HPV Family Study. Clin Infect Dis. 2005 Dec;41(12):1728–1733. doi: 10.1086/498114. Available from: http://dx.doi.org/10.1086/498114. [DOI] [PubMed] [Google Scholar]
- 143.Robert-Koch-Institut. Impfungen gegen HPV - Aktuelle Bewertung der STIKO. Epid Bull. 2009;32:9. [Google Scholar]
- 144.Robert-Koch-Institut. Impfungen gegen humane Papillomaviren (HPV) für Mädchen und Frauen von 12-17 Jahren - Empfehlung und Begründung. Epid Bull. 2007;12:7. [Google Scholar]
- 145.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007 Jul;13(7):857–861. doi: 10.1038/nm1598. Available from: http://dx.doi.org/10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 146.Romanczuk H, Howley PM. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc Natl Acad Sci USA. 1992 Apr;89(7):3159–3163. doi: 10.1073/pnas.89.7.3159. Available from: http://dx.doi.org/10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosin MP, Cheng X, Poh C, Lam WL, Huang Y, Lovas J, Berean K, Epstein JB, Priddy R, Le ND, Zhang L. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000 Feb;6(2):357–362. [PubMed] [Google Scholar]
- 148.Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998 Nov-Dec;18(6B):4779–4786. [PubMed] [Google Scholar]
- 149.Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, Möller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D EUROCARE Working Group. EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003;14 Suppl 5:v61–118. doi: 10.1093/annonc/mdg754. Available from: http://dx.doi.org/10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 150.Sardi I, Franchi A, Ferriero G, Frittelli A, Bruschini L, Montali E, Gallo O. Prediction of recurrence by microsatellite analysis in head and neck cancer. Genes Chromosomes Cancer. 2000 Nov;29(3):201–206. doi: 10.1002/1098-2264(2000)9999:9999<::AID-GCC1031>3.0.CO;2-X. Available from: http://dx.doi.org/10.1002/1098-2264(2000)9999:9999<::AID-GCC1031>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 151.Sartor MA, Dolinoy DC, Jones TR, Colacino JA, Prince ME, Carey TE, Rozek LS. Genome-wide methylation and expression differences in HPV(+) and HPV(-) squamous cell carcinoma cell lines are consistent with divergent mechanisms of carcinogenesis. Epigenetics. 2011 Jun;6(6):777–787. doi: 10.4161/epi.6.6.16216. Available from: http://dx.doi.org/10.4161/epi.6.6.16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. Available from: http://dx.doi.org/10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 153.Schiller JT, Lowy DR. Prospects for cervical cancer prevention by human papillomavirus vaccination. Cancer Res. 2006 Nov;66(21):10229–10232. doi: 10.1158/0008-5472.CAN-06-0630. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-06-0630. [DOI] [PubMed] [Google Scholar]
- 154.Schlecht NF, Franco EL, Pintos J, Kowalski LP. Effect of smoking cessation and tobacco type on the risk of cancers of the upper aero-digestive tract in Brazil. Epidemiology. 1999 Jul;10(4):412–418. doi: 10.1097/00001648-199907000-00012. Available from: http://dx.doi.org/10.1097/00001648-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 155.Sheu JJ, Hua CH, Wan L, Lin YJ, Lai MT, Tseng HC, Jinawath N, Tsai MH, Chang NW, Lin CF, Lin CC, Hsieh LJ, Wang TL, Shih IeM, Tsai FJ. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 2009 Mar;69(6):2568–2576. doi: 10.1158/0008-5472.CAN-08-3199. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-08-3199. [DOI] [PubMed] [Google Scholar]
- 156.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005 May;103(9):1843–1849. doi: 10.1002/cncr.20998. Available from: http://dx.doi.org/10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 157.Shin DM, Charuruks N, Lippman SM, Lee JJ, Ro JY, Hong WK, Hittelman WN. p53 protein accumulation and genomic instability in head and neck multistep tumorigenesis. Cancer Epidemiol Biomarkers Prev. 2001 Jun;10(6):603–609. [PubMed] [Google Scholar]
- 158.Sinha P, Logan HL, Mendenhall WM. Human papillomavirus, smoking, and head and neck cancer. Am J Otolaryngol. 2012 Jan-Feb;33(1):130–136. doi: 10.1016/j.amjoto.2011.02.001. Available from: http://dx.doi.org/10.1016/j.amjoto.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953 Sep;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. Available from: http://dx.doi.org/10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 160.Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, Shyr Y, Murphy BM, Cmelak AJ, Burkey BB, Netterville JL, Levy S, Yarbrough WG, Chung CH. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006 Feb;12(3 Pt 1):701–709. doi: 10.1158/1078-0432.CCR-05-2017. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 161.Smeets SJ, Braakhuis BJ, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, Meijer GA, Leemans CR, Brakenhoff RH. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006 Apr;25(17):2558–2564. doi: 10.1038/sj.onc.1209275. Available from: http://dx.doi.org/10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 162.Smeets SJ, Brakenhoff RH, Ylstra B, van Wieringen WN, van de Wiel MA, Leemans CR, Braakhuis BJ. Genetic classification of oral and oropharyngeal carcinomas identifies subgroups with a different prognosis. Cell Oncol. 2009;31(4):291–300. doi: 10.3233/CLO-2009-0471. Available from: http://dx.doi.org/10.3233/CLO-2009-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007 Dec;121(11):2465–2472. doi: 10.1002/ijc.22980. Available from: http://dx.doi.org/10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 164.Smirnova T, Adomako A, Locker J, Van Rooijen N, Prystowsky MB, Segall JE. In vivo invasion of head and neck squamous cell carcinoma cells does not require macrophages. Am J Pathol. 2011 Jun;178(6):2857–2865. doi: 10.1016/j.ajpath.2011.02.030. Available from: http://dx.doi.org/10.1016/j.ajpath.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, Haugen TH, Turek LP. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004 Feb;108(5):766–772. doi: 10.1002/ijc.11633. Available from: http://dx.doi.org/10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 166.Smith EM, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010 Sep;21(9):1369–1378. doi: 10.1007/s10552-010-9564-z. Available from: http://dx.doi.org/10.1007/s10552-010-9564-z. [DOI] [PubMed] [Google Scholar]
- 167.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, Grandis JR. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006 Sep;12(17):5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 168.Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJ, Odenthal M, Klussmann JP. Nuclear translocation of β-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: an early event in human papillomavirus-related tumour progression? Histopathology. 2011 Jun;58(7):1117–1126. doi: 10.1111/j.1365-2559.2011.03805.x. Available from: http://dx.doi.org/10.1111/j.1365-2559.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 169.Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Hirsch FR, Varella-Garcia M, Ghiorghiu S, Hargreaves L, Armour A, Speake G, Swaisland A, Vokes EE. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected. J Clin Oncol. 2009 Apr 10;27(11):1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 170.Straetmans JM, Olthof N, Mooren JJ, de Jong J, Speel EJ, Kremer B. Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope. 2009 Oct;119(10):1951–1957. doi: 10.1002/lary.20593. Available from: http://dx.doi.org/10.1002/lary.20593. [DOI] [PubMed] [Google Scholar]
- 171.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011 Jun;9(6):665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 172.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007 Oct;110(7):1429–1435. doi: 10.1002/cncr.22963. Available from: http://dx.doi.org/10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 173.Sudbø J, Lippman SM, Lee JJ, Mao L, Kildal W, Sudbø A, Sagen S, Bryne M, El-Naggar A, Risberg B, Evensen JF, Reith A. The influence of resection and aneuploidy on mortality in oral leukoplakia. N Engl J Med. 2004 Apr;350(14):1405–1413. doi: 10.1056/NEJMoa033374. Available from: http://dx.doi.org/10.1056/NEJMoa033374. [DOI] [PubMed] [Google Scholar]
- 174.Syrjänen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010 Oct;21 Suppl 7:vii243–vii245. doi: 10.1093/annonc/mdq454. Available from: http://dx.doi.org/10.1093/annonc/mdq454. [DOI] [PubMed] [Google Scholar]
- 175.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. Head and neck cancer stem cells: the side population. Laryngoscope. 2011 Mar;121(3):527–533. doi: 10.1002/lary.21032. Available from: http://dx.doi.org/10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Talamini R, Bosetti C, La Vecchia C, Dal Maso L, Levi F, Bidoli E, Negri E, Pasche C, Vaccarella S, Barzan L, Franceschi S. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control. 2002 Dec;13(10):957–964. doi: 10.1023/A:1021944123914. Available from: http://dx.doi.org/10.1023/A:1021944123914. [DOI] [PubMed] [Google Scholar]
- 177.Termine N, Giovannelli L, Matranga D, Caleca MP, Bellavia C, Perino A, Campisi G. Oral human papillomavirus infection in women with cervical HPV infection: new data from an Italian cohort and a metanalysis of the literature. Oral Oncol. 2011 Apr;47(4):244–250. doi: 10.1016/j.oraloncology.2011.02.011. Available from: http://dx.doi.org/10.1016/j.oraloncology.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 178.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008 Sep;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. Available from: http://dx.doi.org/10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 179.von Knebel Doeberitz M, Bauknecht T, Bartsch D, zur Hausen H. Influence of chromosomal integration on glucocorticoid-regulated transcription of growth-stimulating papillomavirus genes E6 and E7 in cervical carcinoma cells. Proc Natl Acad Sci USA. 1991 Feb;88(4):1411–1415. doi: 10.1073/pnas.88.4.1411. Available from: http://dx.doi.org/10.1073/pnas.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.von Knebel Doeberitz M, Oltersdorf T, Schwarz E, Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988 Jul;48(13):3780–3786. [PubMed] [Google Scholar]
- 181.Wald AI, Hoskins EE, Wells SI, Ferris RL, Khan SA. Alteration of microRNA profiles in squamous cell carcinoma of the head and neck cell lines by human papillomavirus. Head Neck. 2011 Apr;33(4):504–512. doi: 10.1002/hed.21475. Available from: http://dx.doi.org/10.1002/hed.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wallin KL, Wiklund F, Angström T, Bergman F, Stendahl U, Wadell G, Hallmans G, Dillner J. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med. 1999 Nov;341(22):1633–1638. doi: 10.1056/NEJM199911253412201. Available from: http://dx.doi.org/10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 183.Weber A, Schmid KW, Tannapfel A, Wittekind C. Neuerungen der TNM-Klassifikation der Kopf-Hals-Tumoren. [Changes in the TNM classification of head and neck tumors]. Pathologe. 2010 Sep;31(5):339–343. doi: 10.1007/s00292-010-1302-5. (Ger). Available from: http://dx.doi.org/10.1007/s00292-010-1302-5. [DOI] [PubMed] [Google Scholar]
- 184.Weinberger PM, Yu Z, Kountourakis P, Sasaki C, Haffty BG, Kowalski D, Merkley MA, Rimm DL, Camp RL, Psyrri A. Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: validation of three-class hypothesis. Otolaryngol Head Neck Surg. 2009 Sep;141(3):382–389. doi: 10.1016/j.otohns.2009.04.014. Available from: http://dx.doi.org/10.1016/j.otohns.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 185.Wentzensen N, Ridder R, Klaes R, Vinokurova S, Schaefer U, Doeberitz MK. Characterization of viral-cellular fusion transcripts in a large series of HPV16 and 18 positive anogenital lesions. Oncogene. 2002 Jan 17;21(3):419–426. doi: 10.1038/sj.onc.1205104. Available from: http://dx.doi.org/10.1038/sj.onc.1205104. [DOI] [PubMed] [Google Scholar]
- 186.Wentzensen N, Vinokurova S, von Knebel Doeberitz M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004 Jun;64(11):3878–3884. doi: 10.1158/0008-5472.CAN-04-0009. Available from: http://dx.doi.org/10.1158/0008-5472.CAN-04-0009. [DOI] [PubMed] [Google Scholar]
- 187.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002 Feb;21(10):1510–1517. doi: 10.1038/sj.onc.1205214. Available from: http://dx.doi.org/10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 188.Wittekindt C, Wagner S, Klussmann JP. Humane Papillomaviren bei Kopf-Hals-Karzinomen. Molekulare und translationale Grundlagen. [HPV-associated head and neck cancer. The basics of molecular and translational research]. HNO. 2011 Sep;59(9):885–892. doi: 10.1007/s00106-011-2357-1. (Ger). Available from: http://dx.doi.org/10.1007/s00106-011-2357-1. [DOI] [PubMed] [Google Scholar]
- 189.Wollenberg B. Bedeutung von Stammzellen in der Biologie und Therapie von Kopf-Hals-Karzinomen. [Implication of stem cells in the biology and therapy of head and neck cancer]. Laryngorhinootologie. 2011 Mar;90 Suppl 1:S110–S119. doi: 10.1055/s-0030-1270442. (Ger). Available from: http://dx.doi.org/10.1055/s-0030-1270442. [DOI] [PubMed] [Google Scholar]
- 190.Wynder EL, Bross IJ. Aetiological factors in mouth cancer; an approach to its prevention. Br Med J. 1957 May;1(5028):1137–1143. doi: 10.1136/bmj.1.5028.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yeudall WA, Miyazaki H, Ensley JF, Cardinali M, Gutkind JS, Patel V. Uncoupling of epidermal growth factor-dependent proliferation and invasion in a model of squamous carcinoma progression. Oral Oncol. 2005 Aug;41(7):698–708. doi: 10.1016/j.oraloncology.2005.03.004. Available from: http://dx.doi.org/10.1016/j.oraloncology.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 192.Young MR, Petruzzelli GJ, Kolesiak K, Achille N, Lathers DM, Gabrilovich DI. Human squamous cell carcinomas of the head and neck chemoattract immune suppressive CD34(+) progenitor cells. Hum Immunol. 2001 Apr;62(4):332–341. doi: 10.1016/S0198-8859(01)00222-1. Available from: http://dx.doi.org/10.1016/S0198-8859(01)00222-1. [DOI] [PubMed] [Google Scholar]
- 193.Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT, Zhang CP. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol. 2010 Apr;46(4):317–322. doi: 10.1016/j.oraloncology.2010.02.002. Available from: http://dx.doi.org/10.1016/j.oraloncology.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 194.Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Hsu TC, Schantz SP. Environmental tobacco smoking, mutagen sensitivity, and head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2000 Oct;9(10):1043–1049. [PubMed] [Google Scholar]
- 195.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002 May;2(5):342–350. doi: 10.1038/nrc798. Available from: http://dx.doi.org/10.1038/nrc798. [DOI] [PubMed] [Google Scholar]