Abstract
The entorhinal cortex (EC) is considered as the gate to control the flow of information into and out of the hippocampus. The EC is important for numerous physiological functions such as emotional control, learning and memory and pathological disorders including Alzheimer’s disease, schizophrenia and temporal lobe epilepsy. Serotonin is a classical neurotransmitter which may modify these physiological functions and pathology of neurological diseases. The EC receives profuse serotonergic innervations from the raphe nuclei in the brainstem and expresses high density of serotonergic receptors including 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT3 and 5-HT6. The prominent innervation by serotonergic neurons and the dense expression of serotonergic receptors in the EC suggest that serotonin is a major modulator in this brain region. Serotonin exerts inhibitory effects in the EC. Serotonin hyperpolarizes entorhinal neurons and inhibits the excitatory synaptic transmission via activation of 5-HT1A receptors but facilitates GABA release via activation of 5-HT2A receptors. Both 5-HT1A and 5-HT2A receptors are required for serotonin-induced inhibition of epileptiform activity although 5-HT3 receptors may be involved in serotonin-mediated inhibition of acetylcholine release in the EC. Furthermore, the functions of serotonin in the EC may be implicated in Parkinson’s disease, Alzheimer’s disease and depression. Thus, understanding the roles of serotonergic modulation in the EC is of major clinical importance. Here, I review recent findings concerning the effects of serotonin on neural circuitry activity in the EC.
Keywords: Glutamate, GABA, synaptic transmission, epilepsy, neurotransmitter, G-protein coupled receptor
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a classical neurotransmitter distributed in both the periphery and the central nervous system. Serotonin in the brain has extensive physiological functions including modulation of sleep, mood, emotion, learning and memory. Serotonergic signaling is altered in many neurological disorders such as Alzheimer’s disease, Parkinson’s disease, schizophrenia and depression. The cerebral cortices including the EC receive prominent serotonergic innervations from the raphe nuclei which are clustered along the midline of the brainstem. The EC mediates the majority of the connections between the hippocampus and other cortical areas [1,2]. Sensory inputs converge onto the superficial layers (layers II-III) of the EC [3] which give rise to dense projections to the hippocampus; the axons of the stellate neurons in layer II of the EC form the perforant path that innervates the dentate gyrus and CA3 [4] whereas those of the pyramidal neurons in layer III form the temporoammonic pathway that synapses onto the distal dendrites of pyramidal neurons in CA1 and the subiculum [2,4,5]. Reciprocally, neurons in the deep layers of the EC (layers V-VI) relay a large portion of hippocampal output projections back to the superficial layers of the EC [6-9] and to other cortical areas [1]. The EC is part of a network that aids in the consolidation and recall of memories [10-13]. Neuronal pathology and atrophy of the EC are commonly observed in Alzheimer’s disease [14,15] and schizophrenia [16-19]. Furthermore, the EC is closely related to the induction and maintenance of temporal lobe epilepsy [20,21].
In addition to being innervated by serotonergic fibers, the EC also expresses high density of serotonergic receptors. In the following sections, I will review the expression of different subtypes of serotonergic receptors and experimental evidence concerning the physiological functions and pathological roles of serotonin in the EC.
Serotonergic innervation and distribution of serotonergic receptors in the EC
Serotonin in the central nervous system is released majorly by raphe nuclei in the brainstem. With the technique of combined retrograde fluorescent tracing and immunohistochemistry, the cells that innervate the EC were found to be situated in the caudal half of the dorsal raphe nucleus, the medial part of the median raphe and throughout the rostrocaudal extension of the nucleus reticularis tegmenti pontis [22,23]. The distribution of 5-HT was detected in both the medial and lateral EC with antibodies against 5-HT in combination with fluorescence histochemistry [23,24]. Thin, varicose, branching fibers were found to be distributed in a relatively even, diffuse pattern throughout all layers with the highest innervation in layer I (molecular layer) of the EC. A dense network of 5-HT terminals was also observed in layer III. The EC contains the largest amount of 5-HT among all the monoamines [25].
Serotonin interacts with serotonergic receptors. According to their pharmacological, structural, and transductional characteristics, 5-HT receptors are classified into seven subfamilies, 5-HT1 t o 5 -HT7, which comprise 14 receptor subtypes associated with unique genes [26]. All the 5-HT receptors belong to the G proteincoupled receptor superfamily except 5-HT3 which is a ligand-gated ion channel [26]. The former 5-HT1C was renamed as 5-HT2C, based on its transductional properties and molecular structure.
The EC expresses serotonergic receptors including 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT3 and 5-HT6. Experiments that detect the expression of serotonergic receptors usually include autoradiography using radiolabeled ligands for the receptors, measuring the mRNA for the receptors using in situ hybridization and immunostaining of the receptors with specific antibodies. High density of binding sites for 5-[3H] hydroxytryptamine which may label all the 5-HT receptors was found in layers I and II and layers IV through VI of the EC and moderate to low density of binding was observed in layer III of the EC [27].
Individual 5-HT receptor subtypes have been identified in the EC. The EC expresses high density of 5-HT1 receptors. High densities of binding sites for the selective 5-HT1A agonist tandospirone [28] and 5-HT1A antagonists 8-OH-DPAT [29] or WAY 100635 [30-32] have been detected in each layer of the EC. High level of 5-HT1A mRNA are found in the EC [33] which is one of the brain regions expressing the highest level of mRNA for 5-HT1A receptors [34,35]. The highest density of immunostaining for 5-HT1A receptors using 5-HT1A receptor antibody is also found in the limbic areas including the EC [36]. In addition to 5-HT1A, the EC also expresses 5-HT1D [37,38] and 5-HT1E [37] receptors.
For 5-HT2 receptors, very high density of ketanserin (selective 5-HT2A antagonist) binding sites is found in layers III and V of the EC [39]. Layer I and layer II of the EC are also labeled by spiperone, an antagonist for both 5-HT2A and D2-like receptors [40]. mRNAs for 5-HT2A [35] and 5-HT2C [41] are found in the EC. The EC also expresses 5-HT2A proteins [42].
For 5-HT3 receptors, the homogenates of rat EC contain high-affinity binding sites for 5-HT3 receptor antagonists, GR65630 [43], zacopride [44,45] and LY278584 [46]. Autoradiography has also detected high binding sites for GR65630 [47] and (S)-zacopride [48,49]. Whereas the potent 5-HT3 receptor antagonist (S)-zacopride only labels 5-HT3 receptor binding sites, the (R)-enantiomer, (R)-zacopride, labels these receptors and another class of high-affinity binding sites, named the R sites, in membranes from the rat cerebral cortex and NG 108-15 clonal cells [50]. Further study suggests that the R sites and 5-HT3 receptors are different molecular species [51]. Therefore data obtained by (R)-zacopride should include 5-HT3 receptors and another unidentified molecular entity. In situ hybridization demonstrates that high density of 5-HT3 receptor mRNA exists in the EC [52]. Furthermore, 5-HT6-like immunoreactive material is abundant in the EC [53].
Physiological functions of 5-HT in the EC
Activation of 5-HT3 receptors inhibits acetylcholine (ACh) release in the EC
5-HT3 receptors are ligand-gated cation channels. Whereas the binding sites for 5-HT3 ligands and 5-HT3 receptor mRNA have been detected in the EC, a direct functional identification of 5-HT3 in the entorhinal neurons has not been determined. Nonetheless, activation of 5-HT3 receptor has been shown to reduce ACh release in the EC [54]. Furthermore, applications of 5-HT3 receptor antagonists, ondansetron and granisetron, concentration-dependently increase both spontaneous and K+-evoked ACh release in the entorhinal slices whereas application of 5-HT3 receptor agonists exerts no effects on ACh release but fully blocks the ondansetron-induced enhancement in both spontaneous and K+-evoked ACh release [55]. These results suggest that activation of 5-HT3 receptors tonically inhibits ACh release in the EC. However, a late study demonstrates that no significant inhibition or increase in K+-evoked ACh release is observed with either 5-HT3 receptor agonists or antagonists [56] casting doubts on the effects of 5-HT3 receptor activation on ACh release in the EC.
The mechanisms underlying 5-HT3 receptor-mediated inhibition of ACh release are unclear. The release of ACh in the EC is Ca2+-dependent and tetrodotoxin-sensitive. Application of GABAA receptor antagonists bicuculline and flumazenil by themselves remarkably potentiates ACh release in the EC [55]. The GABAA receptor antagonists potentiates ondansetron-induced increases in ACh release in a tetrodotoxin-sensitive manner but does not modify the facilitatory effects of MDL 72222 and granisetron, other two 5-HT3 receptor antagonists [57]. Application of the GABAA antagonists in a superfusion medium deficient in Cl- also potentiates ACh efflux. ACh release is also increased by the nonspecific K+-channel blockers TEA and Ba2+ but bicuculline does not modify the effects of TEA and Ba2+. These results support the functional interaction of ondansetron with GABAergic interneurons in the rat EC and GABA-independent mechanisms may be involved in the regulation of cortical cholinergic function by other 5-HT3 receptor antagonists.
There are further controversies as to the subtypes of 5-HT receptors and the mechanisms involved in the inhibitory effects of serotonin on ACh release in the EC. Serotonin inhibits ACh release induced by depolarization evoked electrically or by high K+ (20 mM) via activation of 5-HT1B receptors located on cholinergic terminals [58]. However, this inhibition requires the functional elimination of the substance P-containing GABAergic interneurons which express 5-HT2A receptors as shown by in situ hybridization. Activation of these somadendritically located 5-HT2A receptors facilitates the release of substance P which in turn, stimulates ACh release through NK1 receptors present on cholinergic terminals [58].
Modulation of membrane conductance of EC neurons
Serotonin has been shown to induce membrane hyperpolarization of layer II stellate neurons and pyramidal neurons in layer II and III resulting in inhibition of action potential firing frequency (Figure 1) [59-63]. Serotonin-induced hyperpolarization is accompanied with the generation of an outward current and a reduction of input resistance suggesting that serotonin opens a conductance to produce membrane hyperpolarization. It is generally agreed that serotonin activates a background K+ channel to hyperpolarize entorhinal neurons. The involved K+ channels belong to the family of the two-pore domain K+ channels [63]. However, the contribution of other ionic channels cannot be completely excluded because serotonin has been shown to evoke a biphasic response consisting of a moderately short latency and large amplitude hyperpolarization followed by a slowly developing, long lasting, and small amplitude depolarization in layer II projection neurons [59]. The 5-HT-induced depolarization is accompanied with an inward current which is sensitive to ZD7288, a blocker for the hyperpolarization-activated channels (H-channel) suggesting multiple ionic mechanisms underlying the effects of serotonin in the EC.
Figure 1.
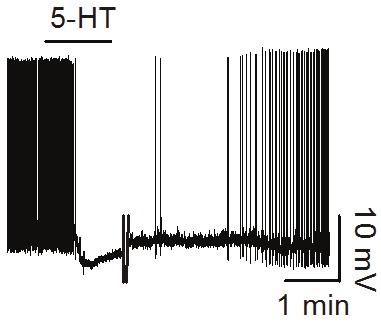
5-HT depresses the firing frequency of action potentials recorded from a stellate neuron in layer II of the EC (unpublished data).
Consistent with the abundant expression of 5-HT1A receptors in the EC, 5-HT-induced hyperpolarization is mediated via activation of 5-HT1A receptors [59-63] whereas 5-HT-mediated late depolarization in layer II projection neurons is independent of 5-HT1A receptors [59].
Modulation of excitatory synaptic transmission in the EC
Glutamate is the major excitatory neurotransmitter in the EC. An initial in vivo experiment suggests that 5-HT may facilitate synaptic transmission in the EC. Intraperitoneal injections of 5-HT precursor, 5-hydroxytryptophan, and the 5-HT1A receptor agonist, 8-OH-DPAT, into the urethane-anesthetized rats facilitate synaptic transmission between the EC and the dentate gyrus in vivo [64]. However, in vitro experiments from entorhinal slices demonstrate that 5-HT inhibits synaptic transmission in the EC. Iontophoretic application of 5-HT reduces the depolarization evoked by exogenous application of glutamate but has no apparent action on neuronal responses to iontophoretically ejected GABA in the pyramidal neurons of layers II/III in entorhinal slices [65]. The 5-HT-mediated attenuation of glutamate response persists in the medium containing CdCl2 to block synaptic transmission. Serotonin has no effects on the release of endogenous glutamate measured by a fluorometric enzyme assay. This study suggests that the depressant effect of 5-HT on the response evoked by exogenous application of glutamate is not mediated by modulation of presynaptic glutamate release but due to an effect on glutamate receptors. However, further studies demonstrate that 5-HT decreases presynaptic glutamate release (see below).
The actions of serotonin on excitatory synaptic transmission in the EC were further probed by recording synaptic responses in the entorhinal slices. Serotonin reduces stimulus-evoked EPSPs/EPSCs recorded by whole-cell patchclamp and intracellular recordings from layers II and III principal neurons [61] and field potentials recoded in the superficial layers [66] of the EC. The depressant effects of 5-HT are presynaptic based on the following lines of evidence. 1) both NMDA and AMPA receptor-mediated responses are reduced to similar extents by 5-HT [60-62]; 2) 5-HT-induced depression is similar in whole-cell patch-clamp versus intracellular recordings, does not require intracellular GTP, and is not visible in glutamate applications to excised patches; 3) 5-HT reduces the frequency with no effects on the amplitude of miniature EPSCs recorded in the presence of tetrodotoxin and bicuculline; 4) 5-HT-mediated depression of field potentials is associated with a significant increase in paired-pulse facilitation [66]. Therefore, 5-HT suppresses excitatory synaptic transmission by reducing presynaptic glutamate release not by inhibiting the functions of postsynaptic glutamate receptors.
The 5-HT1A receptors are also identified to be responsible for 5-HT-induced depression of excitatory synaptic transmission in the EC [61,66,67]. The inhibitory effects of 5-HT on EPSPs are mimicked by 5-HT1A receptor agonists but reduced by 5-HT1A receptor antagonists. However, the ionic and signaling mechanisms underlying 5-HT-induced depression of excitatory synaptic transmission in the EC still need to be elucidated.
Modulation of GABAergic transmission in the EC
The principal neurons in the EC receive GABAergic innervations. The effects of 5-HT on GABAergic transmission in the EC have also been explored in the EC. Initial study demonstrate that 5-HT has no apparent action on neuronal responses to iontophoretically ejected GABA in the EC suggesting that 5-HT has no effects on postsynaptic GABAA receptors [65]. In the entorhinal slices, 5-HT increases both the frequency and amplitude of spontaneous IPSCs recorded from the principal neurons with no effects on the frequency and amplitude of miniature IPSCs recorded in the presence of tetrodotoxin [68]. However, 5-HT reduces the amplitude of IPSCs evoked by extracellular field stimulation and in synaptically connected interneuron and pyramidal neuron pairs. Another study also demonstrates that 5-HT inhibits evoked IPSPs in the EC [67]. Because 5-HT does not modulate the miniature IPSCs which suggests that 5-HT has no effects on postsynaptic GABAA receptors, the effects of 5-HT on GABAergic transmission should be presynaptic in origin. Because the spontaneous, miniature and evoked IPSCs represent different status of inhibitory synaptic circuitry, these results suggest that 5-HT exerts diverse functions according to the distinct physiological conditions of neural circuit. 5-HT generates membrane depolarization and increases action potential firing frequency but reduces the amplitude of action potentials recorded directly from presynaptic GABAergic interneurons (Figure 2) suggesting that 5-HT increases GABA release whereas the depressant effects of 5-HT on evoked IPSCs could be explained by 5-HT-induced reduction in action potential amplitude [68]. The depolarizing effect of 5-HT is mediated by inhibition of TASK-3 K+ channels in interneurons and requires the functions of 5-HT2A receptors and Gαq/11 proteins but is independent of phospholipase C activity [68]. Consistent with the electrophysiological data, double immunofluorescence confocal microscopy demonstrates the colocalization of 5-HT2A receptors with GABA in the EC [42].
Figure 2.
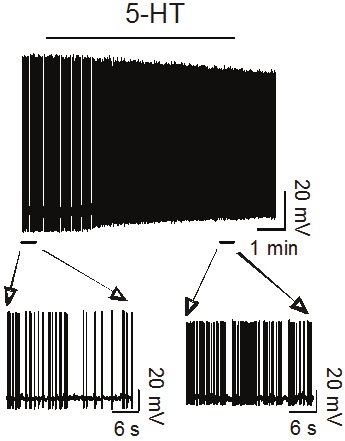
5-HT enhances the firing frequency of action potentials recorded from an interneuron in the EC (unpublished data).
Pathologic roles of 5-HT in the EC
The EC is an indispensable structure participating in the induction and maintenance of temporal lobe epilepsy [20,21]. The actions of 5-HT on epilepsy have been studied mainly in entorhinal slices. Application of 5-HT to entorhinal slices attenuated the length of epileptiform bursts induced by bath application of the GABAA receptor inhibitor bicuculline [65]. Lowering the concentration of Mg2+ in the extracellular solution in entorhinal slices generates epileptiform activity characterized by an initial expression of seizure-like events followed by late recurrent discharges and bath application of 5-HT blocks the epileptiform activity induced by low Mg2+ [68,69]. Furthermore, application of the 5-HT-releasing agent fenfluramine reversibly blocks epileptiform activity induced by omission of the extracellular Mg2+ [70]. 5-HT-induced depression of epileptiform activity is related to 5-HT1A [68,70] and 5-HT2A [68] receptors. The antiepileptic effects of 5-HT likely represent its inhibitory effects on entorhinal neuronal excitability [59-63] and excitatory synaptic transmission [60-62,66] and its facilitatory effects on GABA release [68].
The EC is also a predilection site for the pathological alterations underlying Parkinson’s disease [71,72]. The functions of 5-HT are also implicated in Parkinson’s disease. A reduction of serotonin level was observed in Parkinson’s disease [73] and serotonin fibers in the EC are dystrophic in the brains of individuals with Parkinson’s disease [74]. Furthermore, the maximal density of the binding of the selective 5-HT3 antagonist GR 65630 was reduced in the entorhinal homogenates on the side lesioned with 6-hydroxydopamine [75] suggesting a role of 5-HT in the neuropathology of Parkinson’s disease.
Pathological alterations of Alzheimer’s disease first occur in the EC [14,15]. Electrochemical oxidation of 5-HT produces 4,5-diketotryptamine (4,5-DKT) and administration of 4,5-DKT into the lateral ventricles of rats results in cell death and terminal degeneration in the EC [76,77]. Furthermore, the density of 5-HT2 receptors was reduced to 45% in postmortem patients with Alzheimer’s disease [78]. A significant reduction in serotonin transporter sites was also observed in the EC in Alzheimer’s disease [79].
The EC is an important limbic structure involved in emotional control and it is well-known that serotonin plays an important role in emotional control. The level of serotonin is reduced in the EC of demented patients with depression [80] suggesting that the modulatory effects of 5-HT in the EC contribute to depression as well.
Future directions
The EC is a brain region receiving the most abundant serotonergic innervations from the raphe nuclei. The EC also expresses numerous subtypes of serotonergic receptors including 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT3 and 5-HT6. Whereas the functions of 5-HT1A (inhibition of neuronal excitability and excitatory synaptic transmission), 5-HT2A (facilitation of GABA release), 5-HT3 (inhibition of ACh release) receptors are beginning to be revealed, the functions of other 5-HT receptor subtypes have not been determined. Moreover, the EC is an indispensable structure involved in learning and memory and undergoes synaptic plasticity. However, the potential roles of 5-HT in the modulation of long-term potentiation (LTP), long-term depression (LTD), learning and memory in the EC have not been determined. Whereas there is convincing evidence demonstrating an antiepileptic action of 5-HT in in vitro slice models of epilepsy, the in vivo roles of 5-HT in the EC in antagonizing epilepsy have not been determined. This area is worthy of further investigation because the temporal lobe epilepsy is usually resistant to most antiepileptic drugs and agonists for 5-HT1A and 5 -HT2A could be novel antiepileptic drugs. Furthermore, more direct evidence is required to define the roles of 5-HT in other neurological diseases including Parkinson’s disease, Alzheimer’s disease and depression.
Acknowledgements
This work was supported by the National Institutes of Health (R01MH082881).
References
- 1.Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- 2.Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 4.Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- 5.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 6.Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246:149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- 7.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- 9.van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- 10.Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- 11.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 12.Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 15.Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer’s disease. J Mol Neurosci. 2001;17:225–232. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- 16.Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region--a morphometric study. Biol Psychiatry. 1988;24:515–521. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- 18.Joyal CC, Laakso MP, Tiihonen J, Syvälahti E, Vilkman H, Laakso A, Alakare B, Räkköläinen V, Salokangas RK, Hietala J. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–1007. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- 19.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 20.Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 21.Avoli M, D’Antuono M, Louvel J, Köhling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 22.Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- 23.Kohler C, Chan-Palay V, Steinbusch H. The distribution and orientation of serotonin fibers in the entorhinal and other retrohippocampal areas. An immunohistochemical study with anti-serotonin antibodies in the rats brain. Anat Embryol (Berl) 1981;161:237–264. doi: 10.1007/BF00301824. [DOI] [PubMed] [Google Scholar]
- 24.Kohler C, Chan-Palay V, Haglund L, Steinbusch H. Immunohistochemical localization of serotonin nerve terminals in the lateral entorhinal cortex of the rat: demonstration of two separate patterns of innervation from the midbrain raphe. Anat Embryol (Berl) 1980;160:121–129. doi: 10.1007/BF00301855. [DOI] [PubMed] [Google Scholar]
- 25.Reader TA, Dewar KM, Grondin L. Distribution of monoamines and metabolites in rabbit neostriatum, hippocampus and cortex. Brain Res Bull. 1989;23:237–247. doi: 10.1016/0361-9230(89)90153-6. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 27.Kohler C. The distribution of serotonin binding sites in the hippocampal region of the rat brain. An autoradiographic study. Neuroscience. 1984;13:667–680. doi: 10.1016/0306-4522(84)90087-3. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Shimizu H, Kumasaka Y, Hirose A, Tatsuno T, Nakamura M. Autoradiographic localization and pharmacological characterization of [3H] tandospirone binding sites in the rat brain. Brain Res. 1991;546:181–189. doi: 10.1016/0006-8993(91)91479-k. [DOI] [PubMed] [Google Scholar]
- 29.Kohler C, Radesater AC, Lang W, Chan-Palay V. Distribution of serotonin-1A receptors in the monkey and the postmortem human hippocampal region. A quantitative autoradiographic study using the selective agonist [3H] 8-OH-DPAT. Neurosci Lett. 1986;72:43–48. doi: 10.1016/0304-3940(86)90615-4. [DOI] [PubMed] [Google Scholar]
- 30.Laporte AM, Lima L, Gozlan H, Hamon M. Selective in vivo labelling of brain 5-HT1A receptors by [3H] WAY 100635 in the mouse. Eur J Pharmacol. 1994;271:505–514. doi: 10.1016/0014-2999(94)90812-5. [DOI] [PubMed] [Google Scholar]
- 31.Hume SP, Ashworth S, Opacka-Juffry J, Ahier RG, Lammertsma AA, Pike VW, Cliffe IA, Fletcher A, White AC. Evaluation of [O-methyl-3H] WAY-100635 as an in vivo radioligand for 5-HT1A receptors in rat brain. Eur J Pharmacol. 1994;271:515–523. doi: 10.1016/0014-2999(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 32.Khawaja X. Quantitative autoradiographic characterisation of the binding of [3H] WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res. 1995;673:217–225. doi: 10.1016/0006-8993(94)01416-f. [DOI] [PubMed] [Google Scholar]
- 33.Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 34.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 36.Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Vergé D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Barone P, Jordan D, Atger F, Kopp N, Fillion G. Quantitative autoradiography of 5-HT1D and 5-HT1E binding sites labelled by [3H] 5-HT, in frontal cortex and the hippocampal region of the human brain. Brain Res. 1994;638:85–94. doi: 10.1016/0006-8993(94)90636-x. [DOI] [PubMed] [Google Scholar]
- 38.Bonaventure P, Voorn P, Luyten WH, Jurzak M, Schotte A, Leysen JE. Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: clues for function. Neuroscience. 1998;82:469–484. doi: 10.1016/s0306-4522(97)00302-3. [DOI] [PubMed] [Google Scholar]
- 39.Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain--IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 40.Kohler C. Autoradiographic mapping of spiro-decanone binding-sites in the hippocampal region of the rat. Evidence for a localization on intrinsic neurons. Neurosci Lett. 1984;46:179–184. doi: 10.1016/0304-3940(84)90438-5. [DOI] [PubMed] [Google Scholar]
- 41.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 42.Bombardi C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res Bull. 2012;87:259–273. doi: 10.1016/j.brainresbull.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- 44.Barnes NM, Costall B, Naylor RJ. [3H] zacopride: ligand for the identification of 5-HT3 recognition sites. J Pharm Pharmacol. 1988;40:548–551. doi: 10.1111/j.2042-7158.1988.tb05300.x. [DOI] [PubMed] [Google Scholar]
- 45.Barnes JM, Barnes NM, Costall B, Jagger SM, Naylor RJ, Robertson DW, Roe SY. Agonist interactions with 5-HT3 receptor recognition sites in the rat entorhinal cortex labelled by structurally diverse radioligands. Br J Pharmacol. 1992;105:500–504. doi: 10.1111/j.1476-5381.1992.tb14283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H] LY278584 binding sites in human brain. J Neurochem. 1993;60:730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 47.Kilpatrick GJ, Jones BJ, Tyers MB. The distribution of specific binding of the 5-HT3 receptor ligand [3H] GR65630 in rat brain using quantitative autoradiography. Neurosci Lett. 1988;94:156–160. doi: 10.1016/0304-3940(88)90287-x. [DOI] [PubMed] [Google Scholar]
- 48.Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterisation and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H] -(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–1045. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 49.Laporte AM, Koscielniak T, Ponchant M, Vergé D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I] iodo-zacopride and [3H] zacopride as radioligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- 50.Kidd E, Bouchelet de Vendegies I, Levy JC, Hamon M, Gozlan H. The potent 5-HT3 receptor antagonist (R)-zacopride labels an additional high affinity site in the central nervous system. Eur J Pharmacol. 1992;211:133–136. doi: 10.1016/0014-2999(92)90276-a. [DOI] [PubMed] [Google Scholar]
- 51.Kidd FJ, Levy JC, Nielsen M, Hamon M, Gozlan H. Characterisation of the non-5-HT3 high-affinity ‘R’ binding site for (R)-zacopride in brain and other tissues. Eur J Pharmacol. 1993;247:45–56. doi: 10.1016/0922-4106(93)90136-w. [DOI] [PubMed] [Google Scholar]
- 52.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gérard C, Martres MP, Lefèvre K, Miquel MC, Vergé D, Lanfumey L, Doucet E, Hamon M, el Mestikawy S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- 54.Barnes JM, Barnes NM, Costall B, Naylor RJ, Tyers MB. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissue. Nature. 1989;338:762–763. doi: 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez MJ, Cenarruzabeitia E, Lasheras B, Del Rio J. Involvement of GABA systems in acetylcholine release induced by 5-HT3 receptor blockade in slices from rat entorhinal cortex. Brain Res. 1996;712:274–280. doi: 10.1016/0006-8993(95)01471-3. [DOI] [PubMed] [Google Scholar]
- 56.Johnson RM, Inouye GT, Eglen RM, Wong EH. 5-HT3 receptor ligands lack modulatory influence on acetylcholine release in rat entorhinal cortex. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:241–247. doi: 10.1007/BF00167441. [DOI] [PubMed] [Google Scholar]
- 57.Diez-Ariza M, Ramirez MJ, Lasheras B, Del Rio J. Differential interaction between 5-HT3 receptors and GABAergic neurons inhibiting acetylcholine release in rat entorhinal cortex slices. Brain Res. 1998;801:228–232. doi: 10.1016/s0006-8993(98)00562-9. [DOI] [PubMed] [Google Scholar]
- 58.Feuerstein TJ, Gleichauf O, Landwehrmeyer GB. Modulation of cortical acetylcholine release by serotonin: the role of substance P interneurons. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:618–626. doi: 10.1007/BF00170837. [DOI] [PubMed] [Google Scholar]
- 59.Ma L, Shalinsky MH, Alonso A, Dickson CT. Effects of serotonin on the intrinsic membrane properties of layer II medial entorhinal cortex neurons. Hippocampus. 2007;17:114–129. doi: 10.1002/hipo.20250. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz D, Empson RM, Gloveli T, Heinemann U. Serotonin reduces synaptic excitation of principal cells in the superficial layers of rat hippocampal-entorhinal cortex combined slices. Neurosci Lett. 1995;190:37–40. doi: 10.1016/0304-3940(95)11494-h. [DOI] [PubMed] [Google Scholar]
- 61.Schmitz D, Gloveli T, Empson RM, Draguhn A, Heinemann U. Serotonin reduces synaptic excitation in the superficial medial entorhinal cortex of the rat via a presynaptic mechanism. J Physiol. 1998;508:119–129. doi: 10.1111/j.1469-7793.1998.119br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grünschlag CR, Haas HL, Stevens DR. 5-HT inhibits lateral entorhinal cortical neurons of the rat in vitro by activation of potassium channel-coupled 5-HT1A receptors. Brain Res. 1997;770:10–17. doi: 10.1016/s0006-8993(97)00738-5. [DOI] [PubMed] [Google Scholar]
- 63.Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72:208–218. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- 64.Klancnik JM, Baimbridge KG, Phillips AG. Increased population spike amplitude in the dentate gyrus following systemic administration of 5-hydroxytryptophan or 8-hydroxy-2-(din-propylamino) tetralin. Brain Res. 1989;505:145–148. doi: 10.1016/0006-8993(89)90126-1. [DOI] [PubMed] [Google Scholar]
- 65.Sizer AR, Kilpatrick GJ, Roberts MH. A postsynaptic depressant modulatory action of 5-hydroxytryptamine on excitatory amino acid responses in rat entorhinal cortex in vitro. Neuropharmacology. 1992;31:531–539. doi: 10.1016/0028-3908(92)90184-q. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz D, Gloveli T, Empson RM, Heinemann U. Potent depression of stimulus evoked field potential responses in the medial entorhinal cortex by serotonin. Br J Pharmacol. 1999;128:248–254. doi: 10.1038/sj.bjp.0702788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitz D, Gloveli T, Empson RM, Heinemann U. Serotonin reduces polysynaptic inhibition via 5-HT1A receptors in the superficial entorhinal cortex. J Neurophysiol. 1998;80:1116–1121. doi: 10.1152/jn.1998.80.3.1116. [DOI] [PubMed] [Google Scholar]
- 68.Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci. 2008;39:273–284. doi: 10.1016/j.mcn.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitz D, Empson RM, Gloveli T, Heinemann U. Serotonin blocks different patterns of low Mg2+-induced epileptiform activity in rat entorhinal cortex, but not hippocampus. Neuroscience. 1997;76:449–458. doi: 10.1016/s0306-4522(96)00302-8. [DOI] [PubMed] [Google Scholar]
- 70.Gentsch K, Heinemann U, Schmitz B, Behr J. Fenfluramine blocks low-Mg2+-induced epileptiform activity in rat entorhinal cortex. Epilepsia. 2000;41:925–928. doi: 10.1111/j.1528-1157.2000.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 71.Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- 72.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 73.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- 74.Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res. 2008;1217:185–194. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cicin-Sain L, Jenner P. Reduction in cortical 5-HT3 binding sites following a unilateral 6-hydroxydopamine lesion of the medial forebrain bundle in rats. J Neurol Sci. 1993;115:105–110. doi: 10.1016/0022-510x(93)90074-9. [DOI] [PubMed] [Google Scholar]
- 76.Volicer L, Chen JC, Crino PB, Vogt BA, Fishman J, Rubins J, Schenepper PW, Wolfe N. Neurotoxic properties of a serotonin oxidation product: possible role in Alzheimer’s disease. Prog Clin Biol Res. 1989;317:453–465. [PubMed] [Google Scholar]
- 77.Crino PB, Vogt BA, Chen JC, Volicer L. Neurotoxic effects of partially oxidized serotonin: tryptamine-4,5-dione. Brain Res. 1989;504:247–257. doi: 10.1016/0006-8993(89)91364-4. [DOI] [PubMed] [Google Scholar]
- 78.Jansen KL, Faull RL, Dragunow M, Synek BL. Alzheimer’s disease: changes in hippocampal N-methyl-D-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors--an autoradiographic study. Neuroscience. 1990;39:613–627. doi: 10.1016/0306-4522(90)90246-z. [DOI] [PubMed] [Google Scholar]
- 79.Tejani-Butt SM, Yang J, Pawlyk AC. Altered serotonin transporter sites in Alzheimer’s disease raphe and hippocampus. Neuroreport. 1995;6:1207–1210. doi: 10.1097/00001756-199505300-00033. [DOI] [PubMed] [Google Scholar]
- 80.Zubenko GS, Moossy J, Kopp U. Neurochemical correlates of major depression in primary dementia. Arch Neurol. 1990;47:209–214. doi: 10.1001/archneur.1990.00530020117023. [DOI] [PubMed] [Google Scholar]


