Abstract
The P2X7 receptor is an ATP-gated non-selective cation-permeable ionotropic receptor selectively expressed in neurons and glia in the brain. Activation of the P2X7 receptor has been found to modulate neuronal excitability in the hippocampus and it has also been linked to microglia activation and neuroinflammatory responses. Accordingly, interest developed on the P2X7 receptor in disorders of the nervous system, including epilepsy. Studies show that expression of the P2X7 receptor is elevated in damaged regions of the brain after prolonged seizures (status epilepticus) in both neurons and glia. P2X7 receptor expression is also increased in the hippocampus in experimental epilepsy. Recent data show that mice lacking the P2X7 receptor display altered susceptibility to status epilepticus and that drugs targeting the P2X7 receptor have potent anticonvulsant effects. Together, this suggests that P2X7 receptor ligands may be useful adjunctive treatments for refractory status epilepticus or perhaps pharmacoresistant epilepsy. This review summarizes the evidence of P2X7 receptor involvement in the pathophysiology of epilepsy and the potential of drugs targeting this receptor for seizure control.
Keywords: Adenosine 5’-triphosphate, anticonvulsant, epilepsy, hippocampal sclerosis, interleukin 1 beta, status epilepticus
Introduction
In addition to being the cell’s major energy currency, adenosine 5’-triphosphate (ATP) also serves a critical function in cell-to-cell communication. ATP was first proposed as a neurotransmitter in the seminal work by Burnstock et al. in experiments that showed ATP acted as neurotransmitter in non-cholinergic inhibitory nerves in the guinea-pig taenia coli [1]. The purinergic signalling system is now understood to have an early evolutionary basis and is a widespread route for cell-to-cell communication. Indeed, ATP is recognized to trigger a wide array of physiological effects in several different tissues, including the central nervous system (CNS), where it can act as a potent signalling molecule.
ATP release from cells of the nervous system
ATP is recognized as a co-transmitter in most nerves of the peripheral and central nervous system and has been shown to be released from neurons and astrocytes to act as either sole transmitter or as co-transmitter [2]. Indeed, ATP is probably present in almost every synaptic and secretory vesicle either co-stored with other classical neurotransmitters such as γ-aminobutyric acid (GABA) or glutamate, or in ATP-only vesicles [3]. ATP is stored in secretory and synaptic vesicles and released into the extracellular space by exocytosis or from damaged and dying cells [3]. ATP uptake into vesicles involves a chloride-dependent vesicular nucleotide transporter that is highly expressed in the brain [4].
Several potential mechanisms have been proposed to contribute to the release of ATP including its co-secretion in a calcium-dependant manner from synaptic vesicles containing neurotransmitters such as acetylcholine, noradrenaline, serotonin, GABA or glutamate [5]. ATP has been shown to be released in response to neuronal activity [6-8], stimulation of adenylate cyclase [9] and by direct activation of ATP-gated receptors by ATP itself [10]. Additional mechanisms have been proposed for ATP release including ATP-binding cassette transporters [11], connexin or pannexin hemichannels [12], plasmalemal voltage-dependent anion channels [13], mitochondrial porins [14] and stretch activated channels [5]. ATP release is not only a normal process in basal cellular activity but ATP can be induced by pathological changes, leading to a sharp increase in ATP concentrations in the extracellular environment. Extracellular ATP concentrations have been reported to increase after ischemia [15], spinal cord injury [16], stab wounds [17], and after high frequency neuronal activation during seizures [18-20]. After release, ATP and other nucleotides are rapidly degraded by ectonucleotidases into breakdown products adenosine 5’-diphosphate (ADP), adenosine 5’-monophosphate and adenosine. Several enzyme families are involved in this process including ecto-nucleoside triphosphate diphosphohydrolases, ectonucleotide pyrophosphatase, alkaline phosphatases, ecto-5′-nucleotidase and ecto-nucleoside diphosphokinase [21].
Purinergic receptors
Separate membrane receptors for adenosine and ATP were identified in 1978 and called P1 and P2 receptors, respectively [2]. The P2 receptor family was further subdivided into P2X and P2Y receptors based on mechanism of action, pharmacology and molecular cloning [22]. Purinergic receptor subtypes are widely distributed throughout the CNS, expressed in neurons and glia including astrocytes, oligodendrocytes and microglia, as demonstrated by in situ hybridization, real-time PCR studies and immunohistochemistry [23]. Currently, there are four subtypes of the P1 receptor, 7 subtypes of the ionotropic P2X receptor (P2X1-7) and 8 subtypes of the metabotropic P2Y receptors (P2Y1,2,4,6,11,12,13,14) recognized [23].
The P2Y receptors are G protein-coupled and respond to ATP and UTP (uracil 5’-triphosphate). P2Y receptors share the seven-transmembrane-domain topology of G-protein coupled receptors and based on phylogenetic similarities are subdivided into two further subfamilies. P2Y1,2,4,6,11 use mainly Gq/G11 to activate the phospholipase C/inositol triphosphate endoplasmic reticulum Ca2+-release pathway, and the P2Y12,13,14 receptors couple to Gi/O inhibiting adenylyl cyclase and modulating ion channels [3]. P2Y receptors are largely involved in sloweracting presynaptic functions, as well as mediating long-term (trophic) signaling in cell proliferation, differentiation and death during development and regeneration [2].
P2X receptors are trimeric ligand-gated ion channels which respond to ATP and are involved in fast synaptic transmission and synaptic plasticity. P2X receptors allow the rapid and nonselective passage of cations (Na+, K+, Ca2+) across the cell membrane, resulting in depolarizing responses [3]. The P2X7 receptor is also Ca2+ permeable, meaning its activation leads to an increase in the intracellular calcium concentration [22]. The P2X receptor subtypes consist of intracellular C and N termini and two transmembrane domains and are composed of homomeric or heteromeric assemblies of three or six subunits. All isoforms identified to date are expressed in the CNS and mainly assemble as heteromeric channels [24]. P2X receptors have been described to be localized in neurons both pre- and postsynaptically and in glial cells.
P2X7 receptor
Initially thought to be exclusively expressed in immunocompetent cells, the P2X7 receptor is now understood to be expressed throughout the brain in neurons as well as in glial cells [24]. The P2X7 receptor was first isolated from the rat superior cervical ganglia and medial habenula [25]. The gene codes for a 595 amino acid protein that has 35-40% homology with the other six members of the P2X receptor family. The P2X7 receptor is structurally very similar to the other members of the P2X subfamily, comprising two transmembrane domains, a large extracellular loop containing 10 similarly spaced cysteines and glycosylation sites within the ATP binding site, and intracellular amino and carboxyl termini, except with a much longer carboxy terminal domain [25]. Genes encoding the human [26], mouse [27] and Xenopus laevis [28] P2X7 receptor have also been identified. Recently, splice variants of the human P2X7 receptor with a deleted cytoplasmic tail have been characterized [29]. P2X7 receptors mainly function as trimers in a homomeric form [30], although a more recent study proposes the formation of P2X7/P2X4 heteromers [31].
P2X7 receptors have a relatively low affinity for ATP, which is above 100 μM, compared to other P2X receptor subunits which are activated by ATP in the low micromolar range. P2X7 receptors belong to the slowly desensitizing type, showing little or no desensitization during several seconds of application. The P2X7 receptor gates a non-selective inward cation current which is similar to inward currents caused by the activation of other P2X receptor subunits. Sustained activation of the P2X7 receptor by ATP has also been reported to promote the formation of a reversible plasma membrane pore permeable to hydrophilic solutes up to 800 Da, which has been suggested to be involved in the cytotoxic effects of P2X7 receptor activation [24,25]. However, this latter property of the P2X7 receptor continues to be a matter of some debate in the field.
A variety of signalling pathways are modulated following P2X7 receptor activation. This includes activation of caspase-1 [32] and induction of the cytokines interleukuin-1β [33-35] and TNFα [32]. Also, activation of kinases such as c-Jun N-terminal kinases 1 and 2, extracellular signal-regulated kinases (ERC1/2) and p38 MAPK [36], inhibition of glycogen synthase kinase-3 [37] and the activation of transcription factors such as CREB, nuclear factor кB and the activator protein 1 [36]. P2X7 receptor stimulation also directly activates microglia and promotes their proliferation [38].
There has been significant interest in the role of the P2X7 receptor in disorders of the CNS and the P2X7 receptor has been proposed as a potential drug target in acute and chronic diseases of the nervous system, including spinal cord injury [39], neuropathic pain [24,40], ischemia [41,42], traumatic brain injury [43], Alzheimer’s disease [44], Huntington’s disease [45], and depression [36].
Localization of the P2X7 receptor in brain
Although the P2X7 receptor was first cloned from the rat brain [25], P2X7 receptor expression was originally suggested to be predominantly on antigen-presenting immune cells and epithelia. This was consistent with data showing the P2X7 receptor was able to regulate various aspects of immunocompetent cells such as the expression and secretion of inflammatory mediators including IL-1β, IL-2, IL-4, IL-6, IL-8 and TNFα [32,46], and the formation of multinuclear giant cells [47] or mycobacterium killing [48]. Early studies on P2X7 receptor expression in the CNS reported the P2X7 receptor was present on astrocytes [49] and microglia [46]. In situ hybridization studies also supported P2X7 receptor in brain macrophages rather than neurons [50]. Other work in mice also argued against significant expression of the receptor in the brain [51]. However, technical advances in detecting the P2X7 receptor transcript and protein with high sensitivity and specificity by independent groups now support the presence of P2X7 receptor mRNA and protein in neurons as well as glia and in various regions of the CNS, including cortex and striatum [45,52], brainstem [53], nucleus accumbens [54], cerebellum [55], and hippocampus [52,56-59]. Evidence of functional P2X7 receptors in various CNS cell types has been convincingly demonstrated, including in oligodendrocytes [60] and neurons [56,58,61,62]. The P2X7 receptor expression is also found to be enriched in the axonal growth cone, the precursor structure of the pre-synapse, controlling dynamic axonal growth, suggesting important roles in brain development [58]. The P2X7 receptor appears to maintain a presynaptic presence in the adult hippocampus, where it is found on the glutamatergic terminals of mossy fibers [52,63].
In the human brain, seven different splice variants of the P2X7 receptor have been confirmed [29]. P2X7 receptor-deficient mice have been generated, although a functional splice variant of the P2X7 receptor has been reported to escape gene inactivation [64]. Consistent with this, P2X7 receptor knock-out mice have been reported to show some P2X7-like receptor responses [65]. As an alternative resource for studying P2X7 receptor expression which avoids the use of P2X7 receptor-deficient mice and concerns about the specificity of P2X7 receptor antibodies, there is a transgenic mouse generated with the egfp (enhanced green fluorescence protein) gene upstream of the ATG start codon of the p2rx7 gene [66]. Analysis of the brain of these mice confirms expression of the P2X7 receptor in glial cells, oligodendrocytes and neurons in brain areas previously identified with immunological techniques (see Figure 1).
Figure 1.
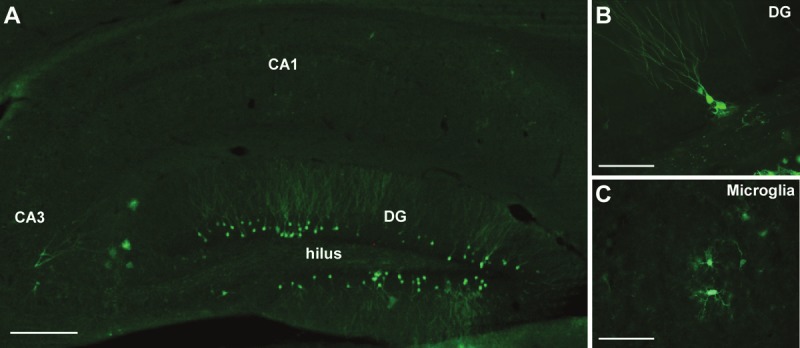
P2X7 receptor expression in hippocampal neurons and microglia. A. Representative photomicrograph showing a field view of the adult mouse hippocampus from a P2X7 receptor reporter mouse which expresses enhanced green fluorescent protein (EGFP) immediately downstream of the P2rx7 promoter. Sections were stained with primary antibodies against GFP. Note, GFP-positive cells are mainly localized within the dentate gyrus (DG). B. Higher magnification view of GFP-positive granule neurons of the dentate gyrus. C. Higher magnification view of GFP-positive cells with morphological features of microglia. Scale bar; 250 μm in A, and 50 μm in B and C.
Effects of P2X7 receptors on neuronal excitability
In the rat hippocampus, P2X7 receptors are reported to be expressed presynaptically on mossy fiber terminals [67]. There are two main types of these axon terminals, which arise from dentate granule neurons. The large synaptic boutons terminate on CA3 neurons whereas the thin, filopodial-like terminals synapse on GABAergic (inhibitory) interneurons [68]. The consequences of mossy fiber stimulation differs markedly depending on which fibers are stimulated and the frequency of stimulation, producing a range of effects from synaptic facilitation to synaptic depression [68]. Armstrong and colleagues investigated the effects of P2X7 receptor activation on the mossy fiber-CA3 pathway. They stimulated dentate granule cells in hippocampal slices from immature rats (10-30 days), and recorded field potentials from area CA3. They found that evoked field potentials were strongly reduced by application of the P2X7 receptor agonist Bz-ATP, suggesting P2X7 receptor activation produces synaptic depression in the hippocampus [67]. The effect could be blocked by a P2X7 receptor selective antagonist but not by PPADS, a non-selective blocker of other P2X receptors [67]. The mechanism by which the reduction occurred was not explored. Since activation of P2X7 receptors gates depolarizing sodium and calcium entry this could either facilitate transmitter release or, if prolonged, perhaps lead to inactivation of those same channels leading to reduced transmitter release from mossy terminals. The overall effect of reduced transmitter release from mossy fiber terminals would also depend on whether CA3 neurons or the interneurons were most affected. Further evidence that P2X7 receptors function presynaptically was provided in paired-pulse facilitation experiments. In this paradigm of synaptic plasticity, the delivery of two stimulations to the mossy fiber pathway results in augmentation of the release of neurotransmitter, such that the second excitatory postsynaptic currents recorded in CA3 neurons was 174% of the first [67]. Armstrong et al. showed that application of a P2X7 receptor agonist increased the size of the second excitatory post-synaptic current to 214% of control. Together, these in vitro data suggest that activation of P2X7 receptor on mossy fiber terminals can either depress excitation or enhance it, depending on the stimulation paradigm.
Epilepsy
Interest has developed in the role of P2X7 receptors in the pathophysiology of epilepsy, particular following recent studies that revealed potent seizure-modulating effects of drugs that target the P2X7 receptor. Epilepsy is a common, chronic neurological disorder that is characterized by recurrent unprovoked seizures. It affects about 50 million people worldwide, with a prevalence of about 0.9% although the lifetime associated risk is 3%. Across Europe, epilepsy affects 6 million people and has an estimated socioeconomic cost of €15.5 Billion [69]. Seizures are the result of a transient dysfunction within the brain caused by hypersynchronous firing of neurons. This may occur in both sides of the brain simultaneously (a generalized seizure) or be localized (focal or partial seizures) [70]. Seizures produce transient disturbances of consciousness, motor, cognitive, autonomic and sensory functions.
The cause of epilepsy is often unknown. Epilepsy can result from mal-development of the brain. For a small number of cases epilepsy results from a mutation in a gene encoding an ion channel or other protein involved in neuronal function or neurotransmission [70]. The genetic contribution in sporadic epilepsy has been explored using genome-wide association studies looking at polymorphisms and other genetic differences but this has failed to explain most cases [71,72]. Epilepsy may develop following injury to the brain, and infection and tumor are also common causes of acquired/symptomatic epilepsies [73]. Epileptogenesis - the process of epilepsy development - is characterized by acute and chronic neuron loss and activation of surrounding glia [74,75]. There is strong support for an important contribution of neuroinflammation, particularly interleukin-1β which is a potent pro-convulsant [76]. Epileptogenesis also features changes in expression of coding and non-coding RNA, altered neuropeptides and ion channel levels, synaptic plasticity and neurogenesis [73,77,78]. Ultimately, the changes occurring during epileptogenesis contribute to network imbalances between excitation and inhibition which result in the enduring predisposition to the generation of epileptic seizures.
In adults, temporal lobe epilepsy is the most common syndrome, in which seizures arise from brain structures such as the hippocampus and amygdala [70]. A common pathological finding in patients with temporal lobe epilepsy is hippocampal sclerosis. This is characterized by selective neuron loss and reactive gliosis within the CA1, hilus/CA4 and also CA3 subfields, with relative preservation of the granule neuron population in the denate gyrus and the CA2 pyramidal neurons [79]. Experimental and clinical studies suggest hippocampal sclerosis may be both cause and consequence of temporal lobe epilepsy [80]. Indeed, prolonged or repeated seizures in animal models damage the hippocampus, as well as other brain regions, and can cause the emergence of recurrent spontaneous seizures [81,82].
Current treatments
Anti-epileptic drugs (AEDs) are the frontline treatment for epilepsy [83]. There are over 20 AEDs in clinical use, including phenytoin, sodium valproate and carbamazepine, and newer generation drugs such as levetiracetam and lacosamide. The introduction of newer generation AEDs has provided a wider range of treatment options that can reduce potential side effects and allow better tailoring of therapies to an individual patients’ specific syndrome. However, the proportion of pharmacoresistant epilepsy patients has changed relatively little, remaining at ~30% [83]. Patients with poorly-controlled seizures suffer additional reductions in quality of life, severe limitations on work and other activities, and are at increased risk of neurological deficits, accidents and death [69]. Temporal lobe epilepsy is particularly associated with pharmacoresistance.
The need for new AEDs is a widely-recognized goal for the improved treatment of epilepsy [84]. Existing AEDs target a relatively small number of proteins. These include inhibitory (GABAergic) transmission, reducing excitatory (glutamatergic) neurotransmission and modulation of neurotransmitter release, and targeting voltage-gated ion channels [85]. There is a need for new AEDs which function in different ways or target different modulatory proteins. Possible features of new AED targets include proteins with more subtle influence on excitatory neurotransmission to avoid the common side effects of many AEDs. Anti-neuroinflammatory [76] or neuroprotective properties [86] could also yield disease-modifying effects that would mitigate the underlying pathology.
A second potential clinical application is for the treatment of status epilepticus (non-terminating seizures). Current clinical practice is to use benzodiazepines such as lorazepam and midazolam or barbiturates such as phenobarbitone [87]. However, status epilepticus becomes pharmacoresistant over time, which is thought to be due to internalization or otherwise desensitization of the GABAA receptor [88]. Thus, drugs acting on a separate aspect of the pathophysiology of status epilepticus may be useful frontline or adjunctive treatments for status epilepticus.
ATP and P2X receptors in epilepsy
Early evidence of a possible role of ATP during seizures stems from studies showing increased extracellular ATP levels after direct electrical stimulation of the cortex in rodents [18]. A later study confirmed and extended this insight, showing increased ATP release after electrical stimulation of hippocampal slices [19]. Another early study showed that epileptiform activity in the CA3 region of rat hippocampal slices was possibly modulated by P2X receptors [89] and microinjection of ATP analogues into the piriform cortex or into the ventricle of mice induces or exacerbates seizure activity [62,90]. The DBA/2 strain of seizure-prone mice also has increased extracellular ATP levels, possibly due to decreased ATPase activity [19]. Glutamate released from astrocytes induced by ATP and ectonucleotidase activity/expression changes have also been implicated in epileptogenesis [23].
P2X7 receptor in epilepsy
The first studies to explore expression of the P2X7 receptor in epilepsy used tissue from chronically epileptic rats. P2X7 receptor staining was found to be increased in the hippocampus, mainly within mossy fibers and the dentate gyrus [91]. Subsequent studies in rodents found enhanced immunoreactivity of the P2X7 receptor after status epilepticus, and responsiveness to ATP, in microglia in relation to transformation to the activated state [92,93]. Dona et al also found increased P2X7 receptor immunoreactivity on microglia during the acute and chronic phase of epilepsy in rats [94]. Additionally, they also noted P2X7 receptor staining on glutamatergic nerve terminals [94]. Interestingly, P2X7 receptor immunoreactivity has not generally been reported on astrocytes, which was confirmed recently by work using P2X7 receptor reporter mice [92].
Studies in our laboratory confirmed upregulation of the P2X7 receptor in the ipsilateral hippocampus after status epilepticus induced by the unilateral microinjection of kainic acid into the mouse amygdala [62]. Indeed, we surveyed the hippocampal expression of most of the P2X receptor family in this model and only P2X7 receptor levels increased [62]. Using the P2X7 receptor reporter mouse, we found increased transcription of the gene mainly in granule neurons in the dentate gyrus, as well as in some CA1 pyramidal neurons. The EGFP signal driven by P2X7 receptor promoter activation did not increase in cells positive for the microglial marker Iba-1 or the astrocyte marker GFAP [62]. Together, these studies reveal P2X7 receptor expression is profoundly altered after status epilepticus and in chronic epilepsy although model, time period and region studied and other technical explanations may underlie the apparent differences reported for P2X7 receptor localization in these models. Table 1 summarizes the available evidence of P2X7 receptor expression in models of seizures/epilepsy and the results of in vivo studies with P2X7 receptor ligands.
Table 1.
P2X7 receptor expression and function in epilepsy
| Model and time-point studied | Brain region | P2X7R localization | Drug effects on seizures | Drug effects on damage | Reference |
|---|---|---|---|---|---|
| Pilocarpine in rats (chronic) | Hippocampus | Mossy fibers | - | - | [91] |
| Seizure-prone gerbil | Hippocampus | Mossy fibers | - | - | [101] |
| Systemic kainate in rats (acute, 24 h) | Hippocampus, piriform cortex, amygdala, ventral striatum | Microglia | - | - | [92] |
| Systemic kainate in mice (acute, up to 48 h) | Hippocampus | Microglia | - | Increased microglia membrane currents blocked by P2X7R antagonists | [93] |
| Pilocarpine in rats (acute and chronic) | Hippocampus | Microglia and glutamatergic nerve terminals | - | - | [94] |
| Pilocarpine in rats (acute and chronic) | Hippocampus | Microglia | - | Antagonists reduced astroglial death | [95] |
| Pilocarpine in rats (acute and chronic) | Hippocampus and frontoparietal cortex | - | - | Antagonists prevented astroglial loss in some brain regions, but agonists exacerbated astroglial death in others (e.g. dentate gyrus) | [96] |
| Pilocarpine in mice (acute) | Hippocampus | - | Antagonists increased seizure time during SE | - | [97] |
| Pilocarpine in rats (acute) | Hippocampus | - | - | Antagonists increased CA3 damage, agonists increased TNF-α levels and decreased CA3 damage | [102] |
| Pilocarpine in rats (acute) | Hippocampus and piriform cortex | - | - | Antagonists inhibited microglial activation, agonists activated microglial activation | [103] |
| Intra-amygdala kainate in mice (acute) | Hippocampus | Granule neurons, CA1 neurons | Agonists increased seizure time and antagonists decreased seizure time | Antagonists protected against cell death and interleukuin-1β induction | [62] |
| Pilocarpine in rats (chronic) | Medial entorhinal cortex | - | Pro-epileptic effect of ATP in naïve rats through P2X7R. No pro-epileptic effect of ATP in chronic epileptic rats | - | [104] |
Key; P2X7R, P2X7 receptor. SE, status epilepticus.
Effects of P2X7 ligands on seizures
Research on the in vivo effects of P2X7 receptor ligands in seizure models has produced exciting discoveries in the past two years. In studies by the authors, central (intracerebroventricular) injection of mice with P2X7 receptor agonists increased the severity of seizures during status epilepticus triggered by intra-amygdala kainic acid [62]. This suggests activation of the P2X7 receptor may exacerbate seizures. P2X7 receptor antagonists strongly reduced production of interleukin-1β and seizure-damage to the hippocampus (Figure 2). Seizure severity was also strongly reduced by either pre- or early post-treatment of mice with P2X7 receptor antagonists (Figure 3). Finally, P2X7 receptor antagonists were even effective at reducing status epilepticus once seizures became partially refractory to a traditional anticonvulsant (lorazepam). In this experiment, a combination of lorazepam and the P2X7 receptor antagonist A-438079, but not either drug on its own, fully abrogated seizures (Figure 3). This indicates a potential application of P2X7 receptor antagonists in the treatment of refractory status epilepticus. Thus, P2X7 receptor antagonists may be useful new adjunctive agents for seizure suppression.
Figure 2.
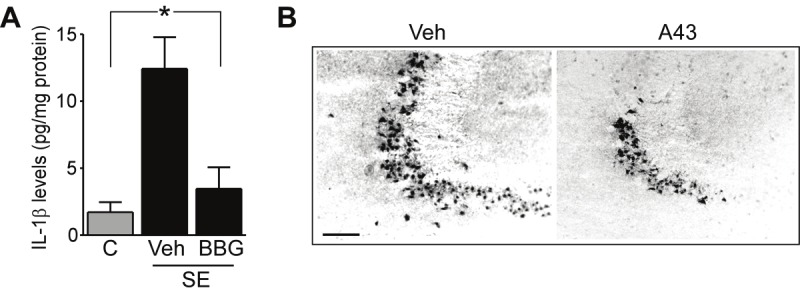
P2X7 receptor antagonists reduce release of interleukin-1β and seizure-damage after status epilepticus in mice. A. Graph showing interleukin-1β (IL-1β) levels measured by ELISA in hippocampal extracts 24 h after status epilepticus (SE). The induction of IL-1β was strongly reduced in seizure mice injected with P2X7 receptor antagonist BBG (1 pmol) 15 min after triggering SE. B. Photomicrographs from the CA3 subfield of the hippocampus 24 h after SE in mice, stained for damaged neurons using Fluoro-Jade B (black dots are damaged neurons). Injection 15 min after SE of the P2X7 receptor antagonist A-438079 (A43) strongly reduced damage. Bar, 150 μM. Data in A, B are adapted and reproduced with permission from Engel et al. FASEB J. [62].
Figure 3.
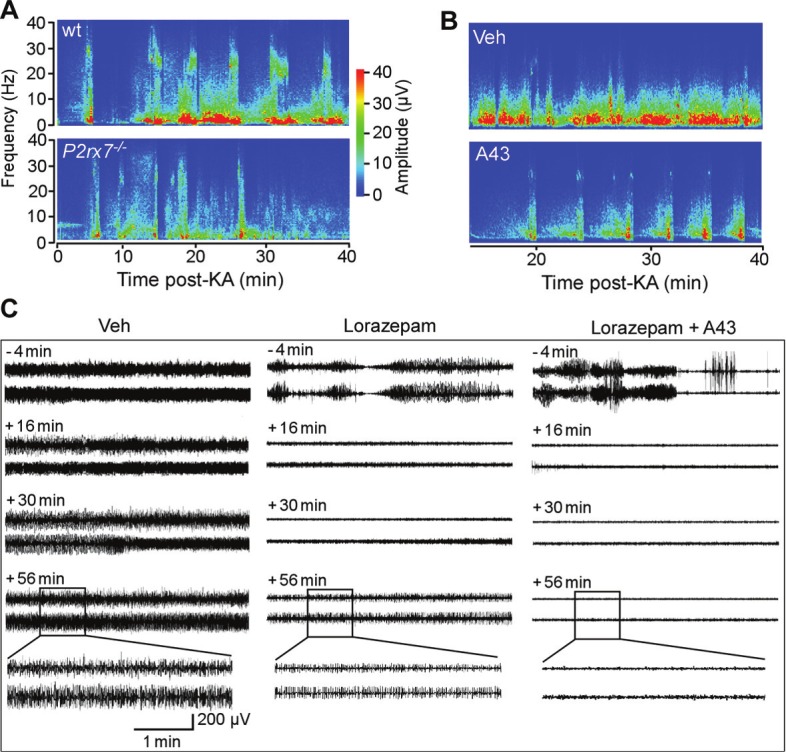
P2X7 receptor and status epilepticus. A. Representative EEG spectrograms showing frequency and amplitude data during kainate-induced status epilepticus for a wild-type (WT) mouse and a mouse lacking the P2X7 receptor (P2rx7-/-). B. Representative EEG spectrograms during recordings after triggering status epilepticus for vehicle (veh) and P2X7 receptor antagonist (A-438079 (A-43), 1.75 nmol) injected mice. Note, reduced seizure severity in P2rx7-/- and A43-treated mice. C. Representative EEG trace recordings from mice after triggering status epilepticus. Time markings on left of each panel refer to point when either Veh, lorazepam or A43 were injected 60 min post-status epilepticus (when seizures are normally continuous). Lorazepam partly reduces seizures but does not abolish them whereas the combination of lorazepam and A43 results in full seizure termination. Data are adapted and reproduced with permission from Engel et al. FASEB J. [62].
Some studies have reported different effects of P2X7 receptor ligands on seizures (see Table 1). Kim et al. reported reduced astroglial degeneration during chronic epilepsy in the dentate gyrus in animals that received a P2X7 receptor blocker [95]. Also, Kim et al showed that P2X7 antagonists exacerbated astroglial cell death in the CA1 hippocampal subfield [96]. Conversely, P2X7 receptor agonists protected against astroglial cell death in the CA1 hippocampal subfield but promoted astroglial cell death in the dentate gyrus and in the frontoparietal cortex [96]. In further studies, this group found that mice lacking P2X7 receptors were more vulnerable to status epilepticus induced by pilocarpine (although not kainate), and P2X7 receptor antagonists exacerbated seizures in the pilocarpine model [97]. However, this study also reported that pannexin channels mediated anticonvulsant effects, which strongly contrasts findings by others on the pro-convulsive properties of these channels [98,99]. Regardless, in vivo studies now suggest P2X7 receptor ligands have excitability-modulating effects that might make them suitable for the treatment or prevention of seizures.
Final comments and summary
There remains a need for alternative drug targets for seizure control in pharmacoresistant epilepsy and refractory status epilepticus. The P2X7 receptor may be one such target. Potent seizure-suppressing effects have been reported for drugs which block the P2X7 receptor and, in combination with traditional benzodiazepine anticonvulsants, seem to stop seizures in refractory status epilepticus [62]. These drugs may also have direct effects on neuronal and glial survival which could be beneficial in mitigating the pathophysiology associated with temporal lobe epilepsy (Figures 2 and 3).
There are a number of questions, however, which must be addressed in driving forward this area of epilepsy research. First, given the apparently paradoxical performance of P2X7 receptor antagonists in the pilocarpine and kainate models [62,97] groups must now test P2X7 receptor antagonists in other seizure models (e.g. electroshock, kindling). Such experiments would ideally be supplemented by testing the drug for specificity, perhaps using the available P2X7 receptor knockout animals. Administration route is another area that should be explored further. In the studies by our group, P2X7 receptor ligands were delivered intracerebroventricularly but work to optimize blood-brain barrier permeability of P2X7 receptor ligands is critical. Another potential application for P2X7 receptor antagonists would be for the treatment of neonatal seizures, where current pharmacotherapy is often ineffective [100].
Another aspect of the P2X7 receptor research is whether altered seizure-induced cell death after treatment with P2X7 receptor ligands in some studies (see Table 1) is a result of altered seizure time alone or due to direct effects on pathways downstream of P2X7 receptors. Finally, do P2X7 receptor antagonists prevent seizures in already-epileptic mice and in models of drug-resistant epilepsy? These drugs are likely to perform differently in models where P2X7 receptor expression is particularly increased in microglia versus neurons. Expression of the P2X7 receptor has not been well characterized in human epilepsy and has not been examined at all in non-temporal lobe epilepsies. Do P2X7 receptor ligands work in human epileptic tissue?
In summary, the present review supports the P2X7 receptor as a promising new target of interest for seizure control and also as a contributing factor to the pathophysiologic mechanisms underlying epilepsy.
Acknowledgements
The authors would like to thank the Health Research Board (Ireland) for support.
References
- 1.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 2.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 6.Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 7.Vizi ES, Liang SD, Sperlagh B, Kittel A, Juranyi Z. Studies on the release and extracellular metabolism of endogenous ATP in rat superior cervical ganglion: support for neurotransmitter role of ATP. Neuroscience. 1997;79:893–903. doi: 10.1016/s0306-4522(96)00658-6. [DOI] [PubMed] [Google Scholar]
- 8.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 9.Gereau RW th, Conn PJ. Potentiation of cAMP responses by metabotropic glutamate receptors depresses excitatory synaptic transmission by a kinase-independent mechanism. Neuron. 1994;12:1121–1129. doi: 10.1016/0896-6273(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 10.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naumann N, Siratska O, Gahr M, Rosen-Wolff A. P-glycoprotein expression increases ATP release in respiratory cystic fibrosis cells. J Cyst Fibros. 2005;4:157–168. doi: 10.1016/j.jcf.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 14.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 15.Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 17.Franke H, Grummich B, Hartig W, Grosche J, Regenthal R, Edwards RH, Illes P, Krugel U. Changes in purinergic signaling after cerebral injury -involvement of glutamatergic mechanisms? Int J Dev Neurosci. 2006;24:123–132. doi: 10.1016/j.ijdevneu.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Wu PH, Phillis JW. Distribution and release of adenosine triphosphate in rat brain. Neurochem Res. 1978;3:563–571. doi: 10.1007/BF00963759. [DOI] [PubMed] [Google Scholar]
- 19.Wieraszko A, Seyfried TN. Increased amount of extracellular ATP in stimulated hippocampal slices of seizure prone mice. Neurosci Lett. 1989;106:287–293. doi: 10.1016/0304-3940(89)90178-x. [DOI] [PubMed] [Google Scholar]
- 20.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–128. discussion 128-130, 233-117, 275-181. [PubMed] [Google Scholar]
- 22.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 23.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 24.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 26.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 27.Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- 28.Paukert M, Hidayat S, Grunder S. The P2X(7) receptor from Xenopus laevis: formation of a large pore in Xenopus oocytes. FEBS Lett. 2002;513:253–258. doi: 10.1016/s0014-5793(02)02324-4. [DOI] [PubMed] [Google Scholar]
- 29.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 30.Torres GE, Egan TM, Voigt MM. Heterooligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 31.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 32.Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, Adam D, Bulfone-Paus S. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 34.Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, Laye S. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. J Neurochem. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 36.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 37.Ortega F, Perez-Sen R, Delicado EG, Miras-Portugal MT. P2X7 nucleotide receptor is coupled to GSK-3 inhibition and neuroprotection in cerebellar granule neurons. Neurotox Res. 2009;15:193–204. doi: 10.1007/s12640-009-9020-6. [DOI] [PubMed] [Google Scholar]
- 38.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 42.Arbeloa J, Perez-Samartin A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz-Hernandez JI, Gomez-Villafuertes R, Leon-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, Diaz-Hernandez M. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3beta and secretases. Neurobiol Aging. 2012;33:1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 45.Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23:1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 46.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 47.Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN, Collo G, Di Virgilio F. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis. 2005;192:149–155. doi: 10.1086/430622. [DOI] [PubMed] [Google Scholar]
- 49.Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D’Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7:2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- 50.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 51.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 53.Papp L, Balazsa T, Kofalvi A, Erdelyi F, Szabo G, Vizi ES, Sperlagh B. P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J Pharmacol Exp Ther. 2004;310:973–980. doi: 10.1124/jpet.104.066712. [DOI] [PubMed] [Google Scholar]
- 54.Franke H, Grosche J, Schadlich H, Krugel U, Allgaier C, Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001;108:421–429. doi: 10.1016/s0306-4522(01)00416-x. [DOI] [PubMed] [Google Scholar]
- 55.Hervas C, Perez-Sen R, Miras-Portugal MT. Presence of diverse functional P2X receptors in rat cerebellar synaptic terminals. Biochem Pharmacol. 2005;70:770–785. doi: 10.1016/j.bcp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 56.Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkinson L, Milligan CJ, Buckley NJ, Deuchars J. An ATP-gated ion channel at the cell nucleus. Nature. 2002;420:42. doi: 10.1038/420042a. [DOI] [PubMed] [Google Scholar]
- 58.Diaz-Hernandez M, del Puerto A, Diaz-Hernandez JI, Diez-Zaera M, Lucas JJ, Garrido JJ, Miras-Portugal MT. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci. 2008;121:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- 59.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 60.Matute C. P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol. 2008;38:123–128. doi: 10.1007/s12035-008-8028-x. [DOI] [PubMed] [Google Scholar]
- 61.Miras-Portugal MT, Diaz-Hernandez M, Giraldez L, Hervas C, Gomez-Villafuertes R, Sen RP, Gualix J, Pintor J. P2X7 receptors in rat brain: presence in synaptic terminals and granule cells. Neurochem Res. 2003;28:1597–1605. doi: 10.1023/a:1025690913206. [DOI] [PubMed] [Google Scholar]
- 62.Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, Miras-Portugal MT, Henshall DC, Diaz-Hernandez M. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012;26:1616–1628. doi: 10.1096/fj.11-196089. [DOI] [PubMed] [Google Scholar]
- 63.Atkinson L, Batten TF, Moores TS, Varoqui H, Erickson JD, Deuchars J. Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience. 2004;123:761–768. doi: 10.1016/j.neuroscience.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 64.Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, Gorecki DC, Murrell-Lagnado RD, Soto F. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem. 2009;284:25813–25822. doi: 10.1074/jbc.M109.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Nogueiro J, Marin-Garcia P, Miras-Portugal MT. Characterization of a functional P2X(7)-like receptor in cerebellar granule neurons from P2X(7) knockout mice. FEBS Lett. 2005;579:3783–3788. doi: 10.1016/j.febslet.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 66.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 67.Armstrong JN, Brust TB, Lewis RG, MacVicar BA. Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci. 2002;22:5938–5945. doi: 10.1523/JNEUROSCI.22-14-05938.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 69.Pugliatti M, Beghi E, Forsgren L, Ekman M, Sobocki P. Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia. 2007;48:2224–2233. doi: 10.1111/j.1528-1167.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- 70.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 71.Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, Grinton B, Szoeke C, Murphy K, Kinirons P, O’Rourke D, Ge D, Depondt C, Claeys KG, Pandolfo M, Gumbs C, Walley N, McNamara J, Mulley JC, Linney KN, Sheffield LJ, Radtke RA, Tate SK, Chissoe SL, Gibson RA, Hosford D, Stanton A, Graves TD, Hanna MG, Eriksson K, Kantanen AM, Kalviainen R, O’Brien T J, Sander JW, Duncan JS, Scheffer IE, Berkovic SF, Wood NW, Doherty CP, Delanty N, Sisodiya SM, Goldstein DB. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol. 2007;6:970–980. doi: 10.1016/S1474-4422(07)70247-8. [DOI] [PubMed] [Google Scholar]
- 72.Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, Muhle H, de Kovel C, Baker C, von Spiczak S, Kron KL, Steinich I, Kleefuss-Lie AA, Leu C, Gaus V, Schmitz B, Klein KM, Reif PS, Rosenow F, Weber Y, Lerche H, Zimprich F, Urak L, Fuchs K, Feucht M, Genton P, Thomas P, Visscher F, de Haan GJ, Moller RS, Hjalgrim H, Luciano D, Wittig M, Nothnagel M, Elger CE, Nurnberg P, Romano C, Malafosse A, Koeleman BP, Lindhout D, Stephani U, Schreiber S, Eichler EE, Sander T. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 74.Henshall DC, Simon RP. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab. 2005;25:1557–1572. doi: 10.1038/sj.jcbfm.9600149. [DOI] [PubMed] [Google Scholar]
- 75.Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 78.Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–572. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meldrum BS. First Alfred Meyer Memorial Lecture. Epileptic brain damage: a consequence and a cause of seizures. Neuropathol Appl Neurobiol. 1997;23:185–201. discussion 201-182. [PubMed] [Google Scholar]
- 81.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 82.Raol YH, Brooks-Kayal AR. Experimental models of seizures and epilepsies. Prog Mol Biol Transl Sci. 2012;105:57–82. doi: 10.1016/B978-0-12-394596-9.00003-2. [DOI] [PubMed] [Google Scholar]
- 83.Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8:669–677. doi: 10.1038/nrneurol.2012.181. [DOI] [PubMed] [Google Scholar]
- 84.Baulac M, Pitkanen A. Research Priorities in Epilepsy for the Next Decade-A Representative View of the European Scientific Community. Epilepsia. 2008 [Epub ahead of print] [Google Scholar]
- 85.MacDonald RL, Rogawski MA. Cellular effects of antiepileptic drugs. In: Engel J Jr, Pedley TA., editors. Epilepsy: A comprehensive textbook. Philadelphia: Lippincott, Williams & Wilkins; 2008. pp. 1433–1445. [Google Scholar]
- 86.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(Suppl 9):63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 88.Wasterlain CG, Liu H, Naylor DE, Thompson KW, Suchomelova L, Niquet J, Mazarati AM, Baldwin RA. Molecular basis of self-sustaining seizures and pharmacoresistance during status epilepticus: The receptor trafficking hypothesis revisited. Epilepsia. 2009;50(Suppl 12):16–18. doi: 10.1111/j.1528-1167.2009.02375.x. [DOI] [PubMed] [Google Scholar]
- 89.Ross FM, Brodie MJ, Stone TW. Modulation by adenine nucleotides of epileptiform activity in the CA3 region of rat hippocampal slices. Br J Pharmacol. 1998;123:71–80. doi: 10.1038/sj.bjp.0701586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knutsen LJS, Murray TF. Adenosine and ATP in epilepsy. In: Kenneth AJ, Michael FJ, editors. Purinergic Approaches in Experimental Therapeutics. New York: Wiley-Liss; 1997. pp. 423–447. [Google Scholar]
- 91.Vianna EP, Ferreira AT, Naffah-Mazzacoratti MG, Sanabria ER, Funke M, Cavalheiro EA, Fernandes MJ. Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: fluorimetric, immunohistochemical, and Western blot studies. Epilepsia. 2002;43(Suppl 5):227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- 92.Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 93.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28:9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dona F, Ulrich H, Persike DS, Conceicao IM, Blini JP, Cavalheiro EA, Fernandes MJ. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83:157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Kim JE, Kwak SE, Jo SM, Kang TC. Blockade of P2X receptor prevents astroglial death in the dentate gyrus following pilocarpine-induced status epilepticus. Neurol Res. 2009;31:982–988. doi: 10.1179/174313209X389811. [DOI] [PubMed] [Google Scholar]
- 96.Kim JE, Ryu HJ, Yeo SI, Kang TC. P2X7 receptor differentially modulates astroglial apoptosis and clasmatodendrosis in the rat brain following status epilepticus. Hippocampus. 2011;21:1318–1333. doi: 10.1002/hipo.20850. [DOI] [PubMed] [Google Scholar]
- 97.Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121:2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 99.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 101.Kang TC, Park SK, Hwang IK, An SJ, Won MH. GABA(B) receptor-mediated regulation of P2X7 receptor expression in the gerbil hippocampus. Brain Res Mol Brain Res. 2004;121:12–18. doi: 10.1016/j.molbrainres.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 102.Kim JE, Ryu HJ, Kang TC. P2X7 receptor activation ameliorates CA3 neuronal damage via a tumor necrosis factor-α-mediated pathway in the rat hippocampus following status epilepticus. J Neuroinflammation. 2011;8:62. doi: 10.1186/1742-2094-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi HK, Ryu HJ, Kim JE, Jo SM, Choi HC, Song HK, Kang TC. The roles of P2X7 receptor in regional-specific microglial responses in the rat brain following status epilepticus. Neurol Sci. 2012;33:515–525. doi: 10.1007/s10072-011-0740-z. [DOI] [PubMed] [Google Scholar]
- 104.Klaft ZJ, Schulz SB, Maslarova A, Gabriel S, Heinemann U, Gerevich Z. Extracellular ATP differentially affects epileptiform activity via purinergic P2X7 and adenosine A(1) receptors in naive and chronic epileptic rats. Epilepsia. 2012;53:1978–1986. doi: 10.1111/j.1528-1167.2012.03724.x. [DOI] [PubMed] [Google Scholar]


