Abstract
Introduction: Postoperative ileus (POI) is characterized by a transient inhibition of coordinated motility of the gastrointestinal (GI) tract after abdominal surgery and leads to increased morbidity and prolonged hospitalization. Currently, intestinal manipulation of the intestine is widely used as a preclinical model of POI. The technique used to manipulate the intestine is however highly variable and difficult to standardize, leading to large variations and inconsistent findings between different investigators. Therefore, we developed a device by which a fixed and adjustable pressure can be applied during intestinal manipulation. Methods: The standardized pressure manipulation method was developed using the purpose-designed device. First, the effect of graded manipulation was examined on postoperative GI transit. Next, this new technique was compared to the conventional manipulation technique used in previous studies. GI transit was measured by evaluating the intestinal distribution of orally gavaged fluorescein isothiocyanate (FITC)-labeled dextran. Infiltration of myeloperoxidase positive cells and cytokine production (ELISA) in the muscularis externa of the intestine were assessed. Results: Increasing pressures resulted in a graded reduction of intestinal transit and was associated with intestinal inflammation as demonstrated by influx of leukocytes and increased levels of IL-6, IL-1β and MCP-1 compared to control mice. With an applied pressure of 9 grams a similar delay in intestinal transit could be obtained with a smaller standard deviation, leading to a reduced intra-individual variation. Conclusions: This method provides a reproducible model with small variation to study the pathophysiology of POI and to evaluate new anti-inflammatory strategies.
Keywords: Gastrointestinal motility and physiology, intestinal transit, mice, postoperative ileus, inflammation
Introduction
Abdominal surgery commonly leads to a temporary inhibition of intestinal motility, known as postoperative ileus (POI) [1,2]. Recent evidence shows that POI is mediated by intestinal inflammation triggered by handling of the intestine [3], with activation of resident muscularis externa macrophages as a crucial step [4,5]. These macrophages release pro-inflammatory cytokines and chemokines resulting in infiltration of leukocytes, in particular monocytes and neutrophils. This inflammatory response leads to increased release of nitric oxide and prostaglandins in the muscularis and impaired intestinal smooth muscle contractility, thereby leading to a delay in GI transit. The importance of this inflammatory response in POI is underscored by the beneficial effect of pharmacological interventions reducing the intestinal inflammation [6].
Manual compression of the small intestine by means of two cotton applicators [7] is currently widely used to induce POI [8-11]. However, the amount of manual compression of the intestine is difficult to standardize and therefore may vary between experiments, animals studied and even investigators. In addition, accidental damage to the intestine, blood vessels and mesentery is very difficult to control, leading to a large inter- and intra-individual variation. This large variation has a major impact on the number of animals required to achieve statistical power and implicates a great ethical burden to animal research. Therefore, there is a large need for standardization and increased reproducibility of intestinal manipulation applied in models of POI. Here, we developed a novel method fulfilling these needs allowing us to better study the mechanisms involved in POI and to evaluate new compounds as potential treatment options for POI.
Materials and methods
Animals
Laboratory animals were kept under environmentally controlled conditions (light on from 8:00 AM to 8:00 PM with water and rodent non-purified diet ad libitum; 20°C-22°C, 55% humidity). Animals were acclimatized to the new laboratory environment. There was at least one week conventional acclimatization at the laboratory. Ten to twelve weeks old C57NL/BL6 mice were purchased from Charles River Laboratories (Maastricht, The Netherlands). Mice were maintained at the animal facility of the Academic Medical Centre in Amsterdam and were used at 11-14 weeks of age; weight 20-25 grams. Studies were performed according to the guidelines of the Dutch Central Committee for Animal Experiments. All experiments were approved by the Animal Care and Use Committee of the University of Amsterdam (Amsterdam, The Netherlands) and the Animal Experiments Committee of the Medical Faculty of the Catholic University of Leuven (Leuven, Belgium).
Experimental groups
Eleven to fourteen weeks old mice underwent control surgery of only laparotomy (L), L followed by standardized pressure intestinal manipulation or L followed by conventional intestinal manipulation.
Surgical procedures
Mice were anesthetized by an intraperitoneal (i.p.) injection of a mixture of Ketamine (Ketalar 100 mg/kg) and Xylazine (Rompun 10 mg/kg). Surgery was performed under sterile conditions. Mice underwent control surgery of only laparotomy, L followed by gentle intestinal pressure manipulation or L followed by conventional intestinal manipulation. During and after the procedure, mice were positioned on a heating map (32°C) until they recovered from anesthesia. The surgery was performed as follows: the abdomen was shaved using a shaving machine and sterilized with 70% ethanol. A 1-cm midline abdominal incision was made and the peritoneal cavity was entered via another incision along the linea alba using curved forceps and sterile small scissors. The opened abdominal cavity was covered with moist (0.9% saline solution) sterile gauze [12].
Standardized pressure manipulation
The standardized pressure manipulation was performed by mounting the intestine on a plexiglas platform and manipulating the small intestine three times back and forth using a purpose-designed device. The device enables the application of a constant pressure to the intestine by a cotton applicator attached to its end (Figure 1D & Appendix).
Figure 1.
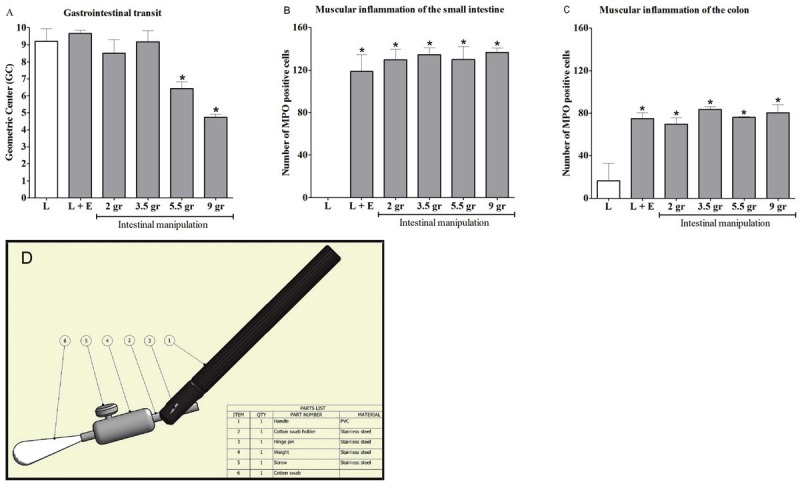
Different degrees of manipulation of the small intestine induced a dose-dependent delay in gastrointestinal (GI) transit. A: Twenty-four hours after intestinal manipulation (IM), GI transit was determined by the calculation of the Geometric Center (GC). The GC was significantly decreased after manipulation with a pressure of 5.5 and 9 grams. B & C: Twenty-four hours after IM, muscular inflammation was determined by counting the number of MPO positive cells in the muscularis of the small intestine (B) and colon (C). The number of MPO positive cells was significantly increased after manipulation, but no significant differences between the groups with different degrees of standardized pressure manipulation were found. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test; * P < 0.05 compared to laparotomy (L). Bars indicate mean ± SEM. Figure 1A & B: L: n = 4; L + externalization of small intestine and cecum (L+E): n = 5-6; IM: n = 5-8 mice per group. Figure 1C: L: n = 4; L+E: n = 5; IM: n = 2-4 mice per group. Panel D | Construction drawing of the device to apply standardized manipulation of the small intestine.
The cecum and the small intestine were carefully externalized onto the gauze using two saline-moistened cotton swabs. The stomach and the colon remained in the abdominal cavity and contact with or stretch of these parts of the gut was strictly avoided. The small intestine was wrapped up in the moistened gauze and pulled through a hole in the center of a plexiglas platform. After removal of the gauze, the small intestine was spread out onto the platform encircling the central hole of the platform: first the cecum was put at three o’clock (relative to the hole), then the second distal half of the small intestine was spread out in a circle around the hole. Starting at the cecum, the most distal part of the small intestine was manipulated first in a retrograde direction (to the proximal part) and after reaching the end of the circle (i.e. halfway ileum-jejunum), the small intestine was manipulated in the same way in an aboral direction (back to the cecum). The first round consisted of placing the tip of the large cotton swab every time on the intestine to flatten the surface of the intestine and its connecting mesenteric vasculature on the plexiglas platform. The cotton swab was attached to a device, which enabled the application of a constant pressure to the surface of the small intestine (Figure 1D). The tip of the cotton swab was moved to an adjacent intestinal surface area continuing the flattening of the intestine in a retrograde direction (in steps of ± 20 mm2) till the end of the spread out intestine was reached. The second and third round consisted of placing the tip of the cotton swab just at the mesenteric attachment of the small intestine and gently sliding it towards the anti-mesenteric side. The intestine was moistened with saline every round. The duodenum (i.e. the most proximal 2 cm of small intestine) was neither spread out nor manipulated. During manipulation, rubbing of the mesentery and especially the blood vessels entering the bowel wall from the mesenteric side was strictly avoided. After finishing the manipulation, the small intestine was carefully repositioned in the abdomen with two moist cotton swabs. The abdomen was closed by two continuous sutures (Mersilene 6-0 silk). All animals recovered rapidly after the surgical procedure.
Conventional manipulation
The cecum and the small intestine were carefully externalized onto the gauze using two saline-moistened cotton swabs. The stomach and the colon remained in the abdominal cavity and contact with or stretch of these parts of the gut was strictly avoided. The conventional intestinal manipulation was performed by compression of the small bowel using moist cotton applicators such that the luminal contents was moved aborally as previously described [13]. After finishing the manipulation, first cecum and subsequently small intestine were carefully placed back into the abdomen with two moist cotton swabs. The abdomen was closed by two continuous sutures. All animals recovered rapidly after the surgical procedure.
After 24 hours animals were anesthetized and sacrificed by cervical dislocation, the complete GI tract was removed, flushed in ice-cold oxygenated KREBS solution, divided into several segments and stored for further analysis. Further analysis included gastrointestinal transit measurements, quantification of infiltration of leukocytes in the intestinal muscularis, and determination of cytokine levels in the intestinal muscularis.
Gastrointestinal transit measurements
GI transit was measured by evaluating the intestinal distribution of orally gavaged fluorescein isothiocyanate (FITC)-labeled dextran. [14]. Three hours before sacrifice, food pellets were removed from the cage. One and a half hour before sacrifice, 10 μL FITC-dextran (70,000 Da; Invitrogen, Paisley, UK) dissolved in 0.9% saline (6.25 mg/mL) was administered to the mouse via oral gavage and water was removed from the cage. Ninety minutes after administration, the animal was sacrificed, the abdomen was reopened and the complete gastrointestinal tract from stomach to distal colon was collected. The contents of the stomach, small bowel (divided into 10 segments of equal length), the cecum, and colon (3 segments of equal length) were collected and assayed in duplicate for the presence of fluorescent label (Synergy HT, BioTek Instruments Inc., VT, USA; excitation wavelength: 485 nm, emission wavelength: 528 nm) for quantification of the fluorescent signal in each bowel segment. The distribution of signal along the gastrointestinal tract was determined by calculating the geometric center (GC): Σ (percent of total fluorescent signal in each segment X the segment number) / 100 for quantitative statistical comparison among experimental groups [15]. Individual transit distribution histograms were plotted, and transits were statistically analyzed using the calculated GC.
Whole mount preparation and histochemistry
To quantify the degree of inflammation in whole mounts of the intestinal muscularis, ileal segments (approximately 12 cm proximal from the cecum) were quickly excised, and the mesenteric attachment was removed. Ileal segments were cut open along the mesentery border, fecal content was washed out in ice-cold modified Krebs solution, and segments were fixed with 100% ethanol for 10 minutes, transferred to ice-cold modified Krebs solution and pinned flat in a glass-dish. Mucosa and submucosa were removed, and the remaining full-thickness sheets of muscularis externa were stained for polymorphonuclear neutrophils with Hanker Yates reagent (Sigma-Aldrich, Zwijndrecht, The Netherlands) for 10 minutes. To quantify the extent of intestinal muscle inflammation, the number of myeloperoxidase (MPO) positive cells in 10 randomly chosen representative high-power fields were counted at a 200-fold magnification and the average was calculated. Tissue sections were coded so that the observer was unaware of the surgical treatment of the specimens.
Cytokine measurements
For cytokine measurements, 3 cm long jejunal muscularis segments were added to 500 μL lysis buffer containing 300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% Triton X-100, pepstatin A, leupeptin, and aprotinin (all 20 ng/mL; pH 7.4), homogenized, and incubated at 4°C for 30 minutes. Homogenates were centrifuged at 1500 x g at 4°C for 15 minutes and supernatants were stored at -20°C until assays were performed. IL-6, IL-1β, MCP-1 and TNF-α in supernatants were analyzed by mouse ELISA (R&D Systems, Abingdon, England) according to manufacturer’s instructions.
Statistical analysis
The results are expressed as mean ± SEM. Statistical analysis of cytokine levels was performed using the Mann Whitney U test. All other data were statistically analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test using Graph Pad Prism version 5.01. A probability level of P less than 0.05 was considered significant. Variances in GC between the groups were compared using the Levene’s test with rank transformed values. The latter was because of the non-normal distribution of the GC measurements.
Results and discussion
We first examined the effect of graded manipulation on postoperative GI transit 24 hours after surgery. Mice were subjected to laparotomy (L) only, L followed by externalization of the small intestine and cecum without manipulation (L+E), or L followed by different degrees (2, 3.5, 5.5 or 9 grams) of standardized pressure manipulation of the small intestine. In control (L) mice, the fluorescent dye was rapidly transported to the distal ileum (mean GC ± SEM: 9.2 ± 0.7). As shown in Figure 1A, increased intestinal manipulation resulted in a pressuredependent decrease in intestinal transit starting from a pressure > 3.5 grams and a pressure-dependent increase in the production of inflammatory cytokines (IL-6, IL-1β and MCP-1) 24 hours after surgery (Figure 2A-C). Interestingly, only externalization of the small intestine and cecum outside the abdominal cavity without manipulation already induced a significant influx of leukocytes into the intestinal muscularis to a similar level as the manipulated groups (Figure 1B-C). The activation and recruitment of these polymorphonuclear cells known to be the primary constituents of the acute inflammatory response, can be influenced by numerous different chemo-attractants, including bacterial products, complement, and cytokines [16] which may come into play after the exposure of the intestine to environmental air. Arriving at their designated site, they act as the first recruited wave of defense against invading pathogens [5]. These data show that handling of the intestine leads to a delay in transit with influx of leukocytes in the muscularis, while the pressure-dependent decrease in transit is more likely explained by a local pro-inflammatory cytokine mediated inflammatory response that is independent of the number of leukocytes infiltrating the muscularis.
Figure 2.
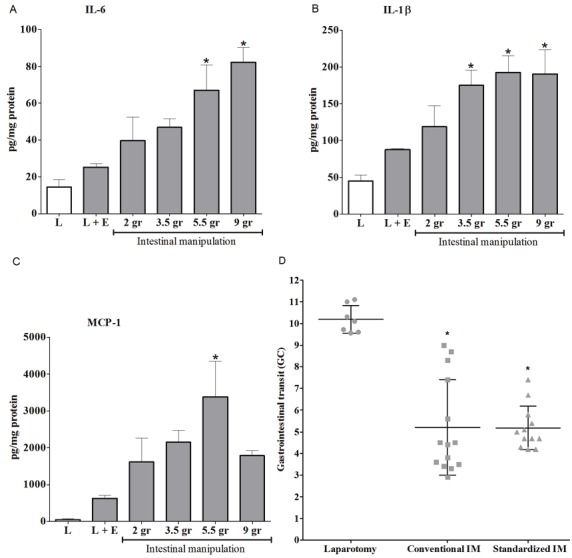
Different degrees of manipulation of the small intestine induced a pressure-dependent production of proinflammatory cytokines. A-C: Twenty-four hours after intestinal manipulation (IM), cytokine production in the muscle layer of the small intestine was determined by ELISA. IL-6 (Panel A), IL-1β (Panel B) and MCP-1 (Panel C) levels were significantly increased after manipulation with a pressure of 5.5 grams. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test; * P < 0.05 compared to laparotomy (L). Bars indicate mean ± SEM. L: n = 3, L + externalization of small intestine and cecum (L+E): n = 5, IM: n = 3-6 per group. Panel D | Both conventional intestinal manipulation (IM) and standardized pressure IM of the small intestine induced a delay in gastrointestinal (GI) transit. Twenty-four hours after IM, GI transit was determined by the calculation of the Geometric Center (GC). The GC was significantly decreased by both methods, but standardized pressure manipulation resulted in a smaller variation. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test; * P < 0.05 compared to laparotomy. Bars indicate mean ± SD. Laparotomy: n = 7; conventional IM: n = 14; standardized IM: n = 12.
We next compared this new technique to the conventional manipulation technique used in previous studies [5,8,10,17-19]. To this end, mice were subjected to L only, L followed by standardized pressure (9 grams) manipulation or L followed by conventional manipulation. In the conventional technique, manipulation of the small intestine is performed by compressing the small bowel with the tips of two cotton applicators such that the lumenal contents are moved aborally. Both the conventional- and standardized manipulation technique induced a significant delay in GI transit, but the intraindividual variability of GC was smaller for the standardized method compared to the conventional manipulation technique (standardized GC = 5.2 ± 1.00, conventional GC = 5.2 ± 2.19, n= 12 and 14 respectively; mean ± SD) (Figure 2D). The variances in GC in the group treated with the standardized method was significantly (p<0.013) smaller than those in the group treated with the conventional method. This difference in standard deviation has a major impact on the number of animals required to achieve the desired statistical power. Taken together, these data indicate that conventional and standardized IM resulted in a similar delay in GI transit, while the standardized pressure method was more reproducible with a smaller intraindividual variability.
In summary, we have developed a new technique to manipulate the intestine in a more controlled manner that results in a pressure-dependent decrease in intestinal transit with small intra-individual variability. This model recapitulates important clinical phenomena (e.g. an inflammatory response in the muscle layer of the small intestine) of the POI seen in surgical patients, suggesting that this novel method provides a methodologically convenient and useful model for investigation of the underlying mechanisms of POI. Additionally, this innovative model offers the capability to study the potential of new anti-inflammatory strategies in a reliable and adequately controlled manner.
Acknowledgements
We would like to thank Gerrit Burger and Arie Steenbeek of the intrumental developmental office of the Academic Medical Centre for their support and intellectual input during the construction of the device.
G.E.E. Boeckxstaens is supported by a grant (Odysseus program, G.0905.07) of the Flemish “Fonds Wetenschappelijk Onderzoek” (FWO) and a governmental NWO-VICI grant.
Appendix
Materials
Equipment for the preparation of anesthetic (coagulation tube).
Equipment for the induction of anesthesia (25 gauge i.p. needles).
Heating map covered with blanket.
Shaving machine (Wella).
Scissors, surgical forceps, straight forceps, curved forceps.
Sterile cotton gauze (NW Drain compress 10x10 cm split compress 4 layers, Medeco b.v. REF 175051).
Plexiglas platform (self made).
Small cotton swabs (Stoelting 50975).
Large cotton swabs (MEDICA EUROPE BV, Oss, the Netherlands): cut off the wooden shaft, but leave 5 mm of the wooden shaft extending from the cotton applicator.
Large cotton swab attached to a device (self made) (Figure 1D) with different weights: to apply a standardized pressure of 9 grams, mount the appropriate weight and check that the balance indicates 9 grams when the tip of the cotton applicator (attached to the device with the right weight) is resting on the balance.
Needle holder.
Suture material (6-0 soft silk, Mersilene).
Procedure for intestinal pressure manipulation
● Timing 30 minutes per animal.
1. Induce anesthesia by an intraperitoneal injection of a mixture of ketamine (Ketalar 100 mg/kg), xylazine (Rompun 10 mg/kg) and dH2O.
2. Check the level of anesthesia by pitching the tail or toe and position the mouse on a heating map until the mouse recovers from anesthesia.
3. Shave the abdomen using a shaving machine, sterilize the abdomen with 70% ethanol and label the tail of the mouse.
4. Using a sterile small scissor, make a vertical 1 cm mid-line abdominal incision downwards distally from the xiphisternum. Enter the peritoneal cavity via a second incision in the peritoneum along the linea alba using curved forceps and sterile small scissors.
5. Place sterile moist cotton gauze around the incision and carefully externalize the cecum and the small intestine with two saline-moistened cotton swabs onto the sterile cotton gauze. Leave the stomach and the colon in the abdominal cavity and strictly avoid contact with or stretch of these parts of the gut.
6. Wrap up the small intestine in the moistened gauze and pull the gauze containing the small intestine through a hole in the center of a plexiglas platform.
7. Spread out the gauze and the small intestine onto the platform. First spread out the cecum on the right side and then spread out the second distal half of the small intestine in a circle around the hole (refer to troubleshooting below).
8. Starting at the cecum, first manipulate the most distal part of the small intestine using the cotton swab attached to a device (Figure 1D) in a retrograde direction (to the proximal part) and after reaching the end of the circle, manipulate the small intestine in the same way in an aboral direction (back to the cecum). The manipulation takes 15 minutes (approximately 6,5 minutes for the distal part, 2 minutes to switch from the distal part to the proximal part and 6,5 minutes for the proximal part). The first round consists of placing the tip of the cotton swab on an adjacent proximal area (in steps of ± 20 mm2 intestinal surface area), only to smooth the surface of the intestine and its connecting mesenteric vasculature on the plexiglas platform. This cotton swab is attached to a device, which enables the application of a constant pressure to the surface of the small intestine with the tip of the cotton swab.
9. The second round consists of placing the tip of the cotton swab on the small intestine and rubbing the small intestine from the mesenteric towards the anti-mesenteric side. When the cotton swab does not touch the small intestine anymore, move the cotton swab upwards and manipulate the next adjacent area.
10. The third and last round is exactly the same as the second round. Moisten the small intestine with saline before every round to prevent dehydration.
11. After manipulating the distal half of the small intestine, replace the distal half by the proximal half of the small intestine by moving the distal half to the right with two saline-moistened cotton swabs and spread out the proximal half around the hole. Manipulate this part of the small intestine three times there and back in exactly the same manner as the distal part of the small intestine. Do not manipulate the last most proximal 2 cm of the small intestine. Strictly avoid rubbing of the mesentery (especially the blood vessels entering the bowel wall from the mesenteric site) during the manipulation (refer to troubleshooting below).
12. Carefully wrap up the small intestine in the moistened gauze and push the gauze, containing the small intestine, back through the hole in the platform on the abdomen of the mouse. Open the gauze and carefully place the cecum followed by the small intestine back into the abdomen with two moist cotton swabs.
13. Complete surgical closure of the abdomen by two continuous sutures using the needle holder, curved forceps and suture material.
14. Allow the animal to recover from the surgery positioned on a heating map (32°C). Complications are rare but might include torsion of the intestine, local intestinal hematoma and postoperative infection of the laparotomy wound. The risk of bleeding and complications can be minimized by strictly avoiding rubbing the mesentery, especially the blood vessels entering the bowel wall from the mesenteric site.
Timing
The procedure of anesthesia, laparotomy, intestinal manipulation and wound closure (steps 1-13) takes approximately 30 minutes per animal.
Step 8, 9 & 10: Pressure manipulation of the distal part of the small intestine takes approximately 6.5 minutes.
Step 11: Changing from the distal part to the proximal part takes approximately 2 minutes.
Step 11: Pressure manipulation of the proximal part of the small intestine takes approximately 6.5 minutes.
Troubleshooting
Step 7: Twisting of the intestine must be strictly avoided to prevent a mechanical obstruction.
Step 11: Damage to the intestinal blood vessels and mesentery must be strictly avoided.
Abbreviations
- GI
gastrointestinal
- GC
geometric center
- IM
intestinal manipulation
- I.P.
intraperitoneal
- L
laparotomy
- MPO
myeloperoxidase
- PBS
phosphate buffered saline
- POI
postoperative ileus
Disclosure statement
All authors concur with the submission. The authors state that there is no conflicting financial interest.
References
- 1.Manabe N, Camilleri M, Rao A, Wong BS, Burton D, Busciglio I, Zinsmeister AR, Haruma K. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970–975. doi: 10.1152/ajpgi.00043.2010. [DOI] [PubMed] [Google Scholar]
- 2.Tokita Y, Yuzurihara M, Sakaguchi M, Satoh K, Kase Y. The Pharmacological Effects of Daikenchuto, a Traditional Herbal Medicine, on Delayed Gastrointestinal Transit in Rat Postoperative Ileus. J Pharmacol Sci. 2007;104:303–310. doi: 10.1254/jphs.fp0070831. [DOI] [PubMed] [Google Scholar]
- 3.Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterol. 1999;117:378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge WJ, van den Wijngaard RM, The FO, ter Beek ML, Bennink RJ, Tytgat GN, Buijs RM, Reitsma PH, van Deventer SJ, Boeckxstaens GE. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterol. 2003;125:1137–1147. doi: 10.1016/s0016-5085(03)01197-1. [DOI] [PubMed] [Google Scholar]
- 5.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228:652–663. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 7.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228:652–663. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347–356. doi: 10.1136/gut.2008.155481. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, Muller MH, Karpitschka M, Mittler S, Kasparek MS, Renz B, Sibaev A, Glatzle J, Li Y, Kreis ME. Role of the vagus nerve on the development of postoperative ileus. Langenbecks Arch Surg. 2010;395:407–411. doi: 10.1007/s00423-010-0594-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, Hori M, Ozaki H. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–647. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterol. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Kalff JC, Schwarz NT, Walgenbach KJ, Schraut WH, Bauer AJ. Leukocytes of the intestinal muscularis: their phenotype and isolation. J Leukoc Biol. 1998;63:683–691. doi: 10.1002/jlb.63.6.683. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt J, Stoffels B, Moore BA, Chanthaphavong RS, Mazie AR, Buchholz BM, Bauer AJ. Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterol. 2008;135:926–936. 936.e1–2. doi: 10.1053/j.gastro.2008.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz NT, Kalff JC, Türler A, Speidel N, Grandis JR, Billiar TR, Bauer AJ. Selective jejunal manipulation causes postoperative panenteric inflammation and dysmotility. Gastroenterol. 2004;126:159–169. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 16.Kozol RA. Neutrophil recruitment to the gastrointestinal tract. J Surg Res. 1992;53:310–315. doi: 10.1016/0022-4804(92)90053-3. [DOI] [PubMed] [Google Scholar]
- 17.Wehner S, Straesser S, Vilz TO, Pantelis D, Sielecki T, de la Cruz VF, Hirner A, Kalff JC. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterol. 2009;136:619–629. doi: 10.1053/j.gastro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 18.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 19.Mueller MH, Karpitschka M, Gao Z, Mittler S, Kasparek MS, Renz B, Sibaev A, Glatzle J, Li Y, Kreis ME. Vagal Innervation and Early Postoperative Ileus in Mice. J Gastrointest Surg. 2011;15:891–900. doi: 10.1007/s11605-011-1481-2. [DOI] [PubMed] [Google Scholar]


