Abstract
Hepatoblastoma is the most common malignant liver tumor between the age of 6 months and 3 years, but it is extremely rare in adults. Only Forty-five cases of adult hepatoblastoma were reported up to now in the world. Hepatoblastoma exhibits a wide range of epithelial and mesenchymal lines differentiation, but neuroendocrine differentiation of this tumor has rare been reported in the literature. Here, we reported a case of primary giant hepatoblastoma (about 11.4*7.9*15.3cm) with neuroendocrine differentiation of a 32-year-old woman, which was misdiagnosed as neuroendocrine tumor.
Keywords: Hepatoblastoma, neuroendocrine tumour (NET), immunohistochemistry, squamous corpuscles
Introduction
Hepatoblastoma is a malignant neoplasm and is most commonly encountered in infants. Although a rare occurrence, hepatoblastomas can be also present in adults. Its pathogenesis and mechanism have not been identified, but it appears to be a genetic predisposition which has been reported to have a close association with developmental syndromes, such as the Beckwith-Wiedemann syndrome, hemihypertrophy and familial adenomatous polyposis (FAP) [1,2]. Hepatoblastomas are usually large and single in size from 5 to 22 cm in diameter, including 57% cases occurring in the right lobe and 15% in the left lobe. Hepatoblastomas have been histologically classified into 2 broad categories, epithelial and mixed type. Typically, Hepatoblastomas have been demonstrated a wide range of differentiation from epithelial to mesenchymal lines, including squamous, mucinous, melanocytic, cartilaginous, osseous, skeletal muscle, and neural elements. This feature supports the interpretation that these tumors arise from a multipotential blastema capable of differentiation to both epithelium and mesenchyma, but neuroendocrine differentiation in this tumor is extremely rare. In this paper, we reported an adult case of hepatoblastoma with neuroendocrine differentiation, which was misdiagnosed as a neuroendocrine tumor.
Case description
A 32-year-old Chinese woman presented with upper abdominal distension for two weeks. Physical examination: The abdomen was slightly protuberant and a hard mass was palpable, with smooth surface and clear boundary, no tenderness in the liver region, hepato-jugular reflux(+). The patient had no history of hepatitis and lack of icteric skin or sclera. Serum levels of AFP, bilirubin, transaminase and tumor markers including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125) were all within normal limits. Ultrasound showed a huge hyperechoic mass with heterogeneous echo-texture in the left lobe of liver, approximately 11.4*7.9*15.3cm, No duct dilatation either in the liver or in the extrahepatic bile ducts was found. The pancreas was normal in size and shape, no pancreatic duct dilatation was seen. Computed tomography (CT) scans showed a large solid mass of low density in the left lobe of liver with complete envelop and clear boundary. Magnetic resonance imaging (MRI) showed a giant mass with slightly long T1 and long T2 signal in the left hepatic lobe. T1WI showed a soft tissue mass about 11.8*7.4cm in diameter, with a slightly weak signal and a well-demarcated border (Figure 1A). T2WI showed a strong signal image, with an asteriform stronger signal in and a clear capsule of low signal around the tumor (Figure 1B). The lesion was irregular enhancement in T1 contrast-enhanced arterial phase scanning, mostly with borderline enhancement (Figure 1C). The lesion was persistent enhancement in T1 contrast-enhanced venous phase scanning, which was a little lower than the obviously enhanced liver parenchyma. The enhanced signal was gradually present from surrounding to interior of the mass, with a significant envelope enhancement (Figure 1D). Signal intensities of the capsular of delayed phase was strengthen.
Figure 1.
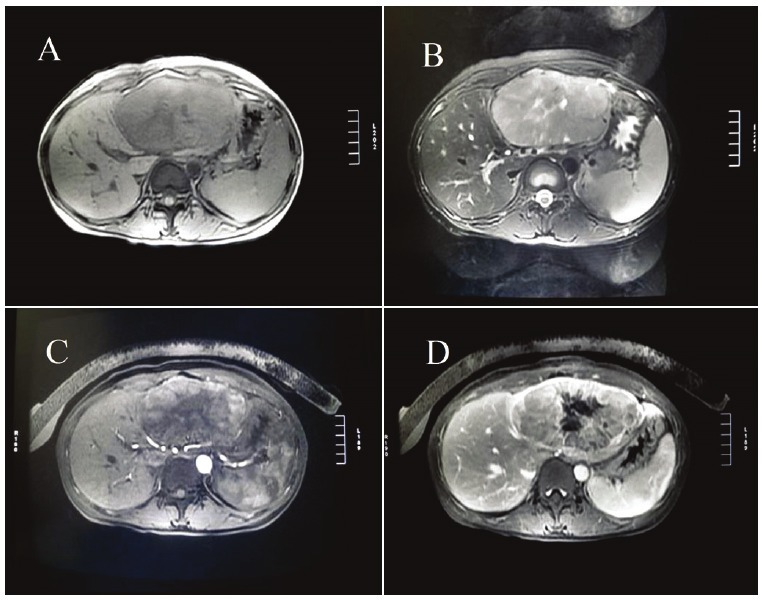
A: T1WI showed a soft tissue mass about 11.8*7.4cm in diameter, with a slightly weak signal and a welldemarcated border. B: T2WI showed a strong signal image, with an asteriform stronger signal in and a clear capsule of low signal around the tumor. C: The lesion was irregular enhancement in T1 contrast-enhanced arterial phase scanning, mostly with borderline enhancement. D: The lesion was persistent enhancement in T1 contrast-enhanced venous phase scanning, which was a little lower than the obviously enhanced liver parenchyma.
Immunohistochemical studies
The resected tumor was fixed in 10% formalin and embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Immunohistochemical staining was performed using the streptavidin-peroxidase system (Ultrasensitive, MaiXin Inc, Fuzhou, China) according to the manufacturer’s instruction. The following antibodies (MaiXin Inc, China, prediluted) were used: Cytokeratin(pan), Vimentin, synaptophysin, chromogranin, a-fetoprotein (AFP), Polyclonal carcinoembryonic antigen (CEA), Placental Alkaline Phosphatase (PLAP), CD99, CD21, CD34, CD10, β-catenin and the Ki-67. Positive and negative controls were evaluated appropriately for each procedure.
Pathological findings
The patient was diagnosed with malignant liver tumor and carried out left hepatectomy. No ascites and enlarged retroperitoneal lymph nodes were found during the operation. The tumor appeared heterogeneous, off-white and solid, which was surrounded by a slim envelope. No tumor necrosis was found and cirrhosis was absence in adjacent non-tumor liver tissues.
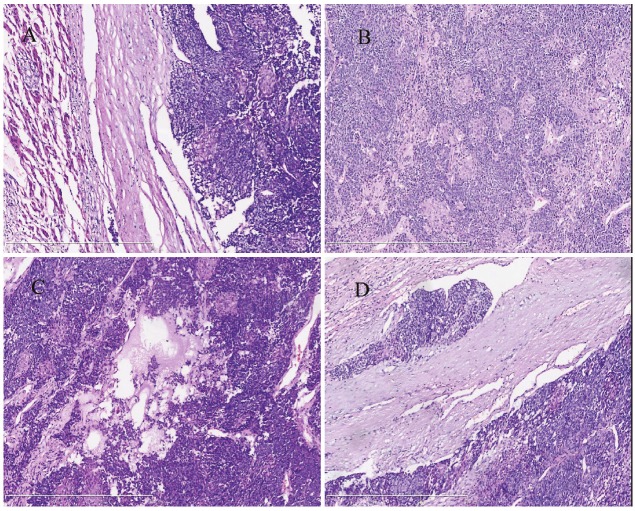
Hematoxylin and eosin sections showed that the tumor was clear bordered and characterized by cells with hyperchromatic nuclei and without prominent nucleoli and scant cytoplasm, growing in a solid pattern (Figure 2A). Occasional rosettes were seen (Figure 2B), the mitotic activity was about 5 mitoses/10HPF, mucoid degeneration (Figure 2C) and vascular invasion (Figure 2D) were seen in the tumor, but necrosis was absent. Immunohistochemical stainings showed that the tumor cells were positive for Cytokeratin(pan) (Figure 3A), and neuroendocrine markers [Including Syn (Figure 3B) and CgA (Figure 3C)], but negative for AFP (Figure 3D), Polyclonal CEA, PLAP, CD99, CD21, Vimentin, CD34, and the Ki-67 labeling index was about 35%-40%. The tumor showed high proliferative activity and presented some similar characteristics of neuroendocrine tumor, such as formed the rosettes and diffused expression of neuroendocrine markers. Therefore, the patient was diagnosed as neuroendocrine tumor (G3) and was referred to the Department of Oncology to evaluate the possibility of chemotherapy or reirradiation. Because larger neuroendocrine tumors (G3) were prone to hemorrhage and necrosis, and usually presented with high mitotic activity, the oncologist advised a revision of the pathological diagnosis.
Figure 2.
A: The tumor was clear boundary (hematoxylin-eosin, original magnification ×100). B: The tumor cells presented some similar characteristics of neuroendocrine tumor, formed focal rosette and a large number of Squamous corpuscles. (hematoxylin-eosin, original magnification ×100), C: Show the mucoid degeneration in tumor interstitial (hematoxylin-eosin, original magnification ×100), D: Show the vascular invasion (hematoxylin-eosin, original magnification ×100).
Figure 3.
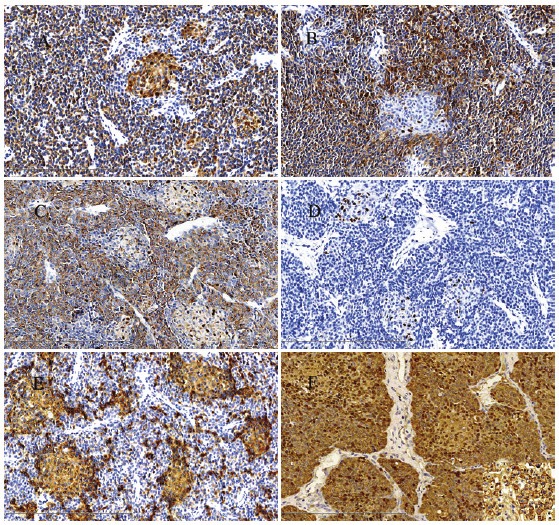
The tumor cells were positive for Cytokeratin(pan) A, Syn B and CgA C (Original magnification ×200). D: The tumor cells were negative for AFP, but Scattered cells in the squamous corpuscles showed a nuclear staining (Original magnification ×200). E: CD10 immunohistochemistry highlighting the squamoid corpuscles. (Original magnification ×200). F: β-catenin staining exhibited a mixed nuclear and cytoplasmic pattern of the tumor cells. (Original magnification ×200).
Revision was performed in the Department of Pathology in the First Affiliated Hospital of China Medical University. The hematoxylin and eosin-stained sections showed that the tumor was made up of two epithelial cell types. One epithelial component comprised small uniform embryonal cells with increased nuclear/cytoplasmic ratio, grouped in nodules and formed focal rosette. Some solid areas were found in the center of the nodules, which were composed of large, spindled squamoid cells that formed small morular arrangements, occasionally with keratinization, known as squamous corpuscles (Figure 2B). Scattered cells in the squamous corpuscles showed a nuclear staining with all the immunohistochemical markers. However, all the morular cells showed cytoplasmic positivity for CD10 (Figure 3E), which further highlighted the squamous corpuscles. β-catenin staining exhibited a mixed nuclear and cytoplasmic pattern of the tumor cells (Figure 3F), and was only present in the membrane of normal liver cells (Figure 3F, the lower right corner). Based on the histological findings and immunohistochemical stainings, we concluded that this is a rare case of adult hepatoblastoma with neuroendocrine differentiation, which has been only described twice in infants in the literature [3,4].
Discussion
Serum levels of alpha-fetoprotein (AFP) are often elevated in most infants with hepatoblastoma and act as an important marker to diagnose and predict hepatoblastoma. However, immunohistochemical stain for AFP was negative and serum level of AFP was 3.49 ng/ml (normal 0-7 ng/ml) in this case. Some studies have found areas of small cell and undifferentiated variant of hepatoblastoma in patients with low or negative AFP, which predict worse prognosis than those with elevated AFP levels [5]. This tumor was poorly differentiated in this case, which was probably the reason for the negative expression of AFP.
Activation of β-catenin signaling is a highly characteristic feature in hepatoblastoma pathogenesis, especially in the embryonal variety [6]. In this case, Immunohistochemical staining showed that β-catenin was characteristically nuclear/cytoplasmic accumulation in the tumor cells, which was only present in the membrane of normal liver cells.
The presence of squamous corpuscles is one of the most characteristic feature of the pancreatoblastoma [7], and hepatoblastoma has several similarities to pancreatoblastoma. Increasing evidence suggests that these tumors are derived from pluripotent stem cells [8], so it’s not surprised to see a large number of typical squamous corpuscles in hepatoblastoma. The patient was revealed no abnormalities in the pancreas, which fully excluded the possibility of metastatic pancreatoblastoma. So the presence of squamoid corpuscles with a morular appearance could be considered as a characteristic feature of this hepatoblastoma. Of note, the squamoid corpuscles may contain tumor cells with optically clear nuclei (OCN) rich in biotin [9], as a result that the nuclei in squamous corpuscles sometimes were non-specifically immunolabeled with a number of immunohistochemical markers. In this case, the nuclei were false-positive for all the antibodies using the avidin-biotin peroxidase complex method, except for the CD10 antibody. It has been confirmed that CD10 is a characteristic marker of tumors with biotin-rich, optically clear nuclei that forming morules and occurring in different organs [10,11]. This unique staining pattern could be helpful for making a diagnosis of hepatoblastoma.
This case was misdiagnosed with neuroendocrine tumor because of some similar histological characteristics and the positive immunoreaction toward neuroendocrine markers and negative immunoreactivity of AFP. However, the presence of embryonic epithelial cells and squamous corpuscles distinguished the hepatoblastoma from neuroendocrine tumor. This case emphasizes the important points of recognizing the rare and unusual variant of hepatoblastoma, and tells pathologists not to have overreliance on immunohistochemistry to avoid misdiagnosis of this tumor.
Conflict of interest statement and source of funding
The author(s) indicated no significant relationships with, or financial interest in any commercial companies pertaining to this article.
References
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization classification of tumours. Fourth Edition. IARC press; 2010. WHO Classification of Tumours of the Digestive System; pp. 228–236. [Google Scholar]
- 2.Juan Rosai. Rosai and Ackerman’s Surgical Pathology. 10 edition. Mosby press; 2011. p. 953. [Google Scholar]
- 3.Rabah R. Teratoid Hepatoblastoma With Abundant Neuroendocrine and Squamous Differentiation With Extensive Parenchymal Metastasis. Arch Pathol Lab Med. 2012;136:911–914. doi: 10.5858/arpa.2012-0212-CR. [DOI] [PubMed] [Google Scholar]
- 4.Ruck P, Harms D, Kaiserling E. Neuroendocrine differentiation in hepatoblastoma. An immunohistochemical investigation. Am J Surg Pathol. 1990;14:847–855. doi: 10.1097/00000478-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, Gore L, Feusner JH. Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer. 2009;52:328–334. doi: 10.1002/pbc.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Y, Fabre M, Branchereau S, Gauthier F, Perilongo G, Buendia MA. Activation of betacatenin in epithelial and mesenchymal hepatoblastomas. Oncogene. 2000;19:498–504. doi: 10.1038/sj.onc.1203356. [DOI] [PubMed] [Google Scholar]
- 7.Bien E, Godzinski J, Dall’igna P, Defachelles AS, Stachowicz-Stencel T, Orbach D, Bisogno G, Cecchetto G, Warmann S, Ellerkamp V, Brennan B, Balcerska A, Rapala M, Brecht I, Schneider D, Ferrari A. Pancreatoblastoma: a report from the European cooperative study group for paediatric rare tumours (EXPeRT) Eur J Cancer. 2011;47:2347–2352. doi: 10.1016/j.ejca.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Sell S, Leffert HL. Liver cancer stem cells. J. Clin. Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y, Ijiri R, Yamanaka S, Kato K, Nishihira H, Nishi T, Misugi K. Pancreatoblastoma: optically clear nuclei in squamoid corpuscles are rich in biotin. Mod Pathol. 1998;11:945–949. [PubMed] [Google Scholar]
- 10.Cameselle-Teijeiro J, Alberte-Lista L, Chiarelli S, Buriticá C, Gonçalves L, González-Cámpora R, Nogales FF. CD10 is a characteristic marker of tumours forming morules with biotin-rich, optically clear nuclei that occur in different organs. Histopathology. 2008;52:389–392. doi: 10.1111/j.1365-2559.2007.02911.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiarelli S, Buriticá C, Litta P, Ciani S, Guarch R, Nogales FF. An Immunohistochemical Study of Morules in Endometrioid Lesions of the Female Genital Tract: CD10 Is a Characteristic Marker of Morular Metaplasia. Clin Cancer Res. 2006;12:4251–4256. doi: 10.1158/1078-0432.CCR-06-0398. [DOI] [PubMed] [Google Scholar]