Abstract
The docosahexaenoic (DHA), a ω-3 fatty acid, could play a beneficial inhibition of the incidence and progress of a series of human diseases including cancer. It has been report that DHA is involved in cell apoptosis. Recent studies show that the signal transduction pathway links with bcl-2, bax, caspase-3 and MMP-9 molecules. Therefore, we tested the relationship between DHA and cell apoptosis in human hepatocellular carcinoma cells (Bel-7402 cells). We show here that DHA induces Bel-7402 cells apoptosis after pre-treating cells with DHA. DHA down-regulates the protein expression of Bcl-2 and Bim mRNA level, and up-regulates caspase-3 activity and Bax expression level. We also found that DHA inhibits Bel-7402 cells migration. Basic on our studies, DHA may play a role in tumor invasion and survival.
Keywords: Docosahexaenoic (DHA), Bcl-2, caspase-3, MMP-9
Introduction
Omega 3 fatty acids (also called ω−3 fatty acids or n−3 fatty acids) are fats commonly found in marine and plant oils. The docosahexaenoic (DHA), a ω-3 fatty acid, was found primarily in cold-water fish and fish oils, could play a beneficial inhibition of the incidence and progress of a series of human diseases including cancer [1].
A study of 3,081 women suffering from breast cancer was done to research the effects of polyunsaturated fats on breast cancer. It has demonstrated that the consumption of high amounts of long chain omega-3 polyunsaturated fats from food produced a 25% reduced risk of additional breast cancer events.
It has shown that the risk of breast cancer in women at western countries is 4 to 5 folds higher than at Japan [2,3]. Much more seafood in daily diet may be explained why the lower risk of breast cancer in Japanese women. The seafood contains higher ω-3 andω-6 PUFA than other foods. It has been reported that DHA inhibits tumor metastases in vivo and invasion of carcinoma cells in vitro in human breast [4-6], prostate [7], urinary bladder [8], hepatocellular [9-11], colon [12,13], lung [14,15] and melanoma [16] carcinomas. Hepatocellular carcinoma is one of the most common cancers, especially in Asian countries, and is also associated with a high mortality rate which the risk is increasing worldwide, due to tumor metastasis. Extensive infection with hepatitis B virus (HBV) and hepatitis C (HCV) may account for the high prevalence of hepatocellular carcinoma in Asia and Africa [17].
It has been reported that the effect of ω-3 polyunsaturated fatty acids (PUFA), such as DHA and eicosapentaenoic (EPA), on reducing the proliferation and inducing apoptosis of cancer cells in vitro and in vivo [11-16]. It has also shown that the ω-3 PUFA can induce apoptosis and inhibit invasion of hepatoma cells, DHA in particularly. However, the cellular and molecular mechanism remains unclear. Nevertheless, some evidence suggest that DHA might be a pro-apoptotic factor can act on lipid peroxidation the same as the protein implicated in the mechanism of reactive oxygen species (ROS) which lead tumor cells death [18,19]. Recently, Lee et al. [20] have found that DHA inhibit cyclin A/Cdk2 interfered with S-phase progression to reduce the proliferation of MHCC97L.
The metastasis of hepatocellular carcinoma is a major threat the lives of patients [21]. In addition, previous studies have showed thatω-6 PUFA stimulate tumor growth and metastasis, whereasω-3 PUFA suppresses them. Matrix metalloprotease (MMP), a group of zinc-dependent endopeptidases, may play an essential role in tumor metastasis, angiogenesis and growth factor release from the ECM [22].
Here, we report that DHA has reduced Bel-7402 cell density, and it is time and dose dependence, and causing by cell apoptosis. The signal transduction pathway linked with bcl-2, bax, caspase-3 and MMP-9.
Materials and methods
Cell culture and treatment
Immortalized human hepatocellular carcinoma cell (Bel-7402) were obtained from Liver Cancer Institute of Zhongshan Hospital, Shanghai and maintained in DMEM medium containing 10% fetal bovine serum (FBS, HyClone, USA) and 1% penicillin/streptomycin solution (HyClone, USA) at 37°C in 5% CO2 atmosphere. Cells were used between passages 5 and 20. The Bel-7402 cells were seeded on standard 6-well culture plates at a density of 1.5x104 cells/well to approximately 70% confluence 24, 48 or 72 hours treatment, cells were maintained in medium without or with DHA in 25, 50, 100 or 200μM (DHA was obtained according to Van Harken described [23] or purchased from Sigma-Aldrich.), then cells were harvested.
Cytotoxicity assay
The Bel-7402 cells were cultured at 5×103 cells/well in 96-well micro-titer plates (Costar, USA) for cytotoxicity assay. Cytotoxicity was determined by the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] assay as previously described [24]. These cells were pretreated with DHA at 0, 25, 50, 100 and 200μM for 24, 48 or 72h, then added 20μL MTT solution (5mg/ml MTT) in each well and continue cultured for another 4h. The medium was removed, and add 150μl/well Dimethyl Sulfoxide (Amresco, USA) and incubate 10min with shaking gently to stop the reaction. The optical density (OD) was read at 495 nm by using Microplate reader (Thermo Fisher Scientific, Finland).
Untreated and DHA-treated cells were isolated by Trypsin-EDTA Solution (Beyotime, China). After homogenizing, cell viability was estimated by using the Trypan blue dye and count were performed by using a hemocytometer. Each independent experiment was reduplicated in triplicate.
Flow cytometry
Annexin-V staining (in the presence or absence of DHA) and cell scanning were conducted by using the Annexin V-FITC detection kit (Beyotime, China). The Bel-7402 cells were treated with different concentration of DHA. To detect apoptosis, cells were stained with Annexin-V. 1×105 cells were re-suspended in 195μL binding buffer in the presence of 5 μl Annexin-V FITC and 10μL PI and incubated at room temperature for 15 min avoiding the light. Mean fluorescent intensity (MFI) of triplicate samples for each cohort was used to ascertain the levels of Annexin-V. The data was analyzed by using Cell Quest software.
RT-PCR and gene expression analysis
Total RNA was extracted from Bel-7402 cells by using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA was generated by using reverse transcription 500ng of total RNA in a reaction volume of 10μL random hexamers as a priming agent with PrimeScript RT Master Mix (TAKARA, CHN). One microliters of cDNA was used as a template in 20μL PCR reaction. Quantitative real-time PCR was performed by using Roche LightCycler 7300 Real-Time PCR System with SYBR Premix Ex Taq (TAKARA, CHN). The number of PCR cycles used was determined to be within the linear range of the reactions. Sequences of primers of detecting bim expression are 5’-GGGTCTGTTGCCAGCCTGCATT-3’ and 5’-TGCTGCAGCTGGACTCTGCT-3’. Reactions were performed in at least triplicate and analyzed via relative quantitation with data as average fold change compared to no treatment.
Western blot
The Bel-7402 cells were treated with DHA in different concentration. The cells were washed with PBS twice and lyse with 500μL of lysis buffer (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instruction. The protein concentration of the lysate was determined by the BCA assay. Samples (containing protein 30μg each sample) were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the protein was transferred into polyvinylidene difluoride (PVDF) membranes (Immobilon-P. Millipore Corp., Bedford, MA). The membranes were blocked with 5% skimmed milk in PBST (phosphate-buffered saline with 0.1% Tween 20) for 1.5 h and incubated with rabbit anti-bcl-2 (Santa Crus, USA) or anti-bax antibody (1:1000) (Santa Crus, USA) at 4°C overnight. After three washing with PBST buffer, the membranes were incubated with goat anti-rabbit IgG (Santa Crus, USA) conjugated with peroxidase for 2hours at room temperature. After three washing with PBST buffer, the protein bands were detected by using enhanced chemiluminescence according to the manufacturer’s instruction. β-Actin was served as a control to confirm equal loading. Densitometry index analysis of the bands was made using gel imagery system.
Caspase-3 activity
Caspase-3 activity was measured by using the Caspase-3 Activity Assay Kit (BESTBIO, Beijing, China) according as previously described [25]. The Bel-7402 cells were treated with DHA in different concentration. The cells were collected by centrifugation at 10,000× g, 4°C, and 10min, and scraped off with a rubber policeman. Caspases-3 activity was detected by using the specific fluorogenic substrates Ac-DEVD-AMC to measure absorbance at 405 nm using a microplate reader (Thermo Fisher Scientific, Finland).
Cell invasion and migration
Invasion assays were performed in BioCoat Matrigel Invasion Chambers (BD Biosciences, USA) as previously described [26]. Matrigel (BD Science, France), a basement membrane matrix, diluted with DMEM, introduced in the upper chamber and dried 4 hours at 37°C. The Bel-7402 cells, in fatty acid free BSA-DHA-DMEM, were added to the upper well of invasion chambers in triplicate, allowed to adhere for 2 hours and migrate toward 10% fetal bovine serum (Hyclone, Logan, UT) for 48 hours at 37°C. Cells on the upper surface of the filter were removed with a cotton swab and on the underside of the membrane were fixed, stained with the crystal violet and counted by using a light microscope-attached.
Grow Bel-7402 cells in 6-well plate at a density of 1×105 cells per well overnight. The wound was scraped by using a 2.5μl micropipette tip. After washing cells twice with PBS, the cells were growth another 24 hours in absence or presence DHA in different concentration and 1mg/ml fatty acid-free BSA-DMEM. Microphotographs were taken at 0, 12 and 24 hours.
Immunofluorescence labeling MMP-9
The Bel-7402 cells were grown to confluence 60%-70% on sterile cover slips (18×18 mm) overnight. The cells were treated with DHA in different concentration another 48 hours. After treatment, glass slides with cells were washed three times with phosphate-buffered saline (PBS) before fixing in 4% paraformaldehyde/PBS for 30 minutes at room temperature. The cells were infiltrated with 0.5% Triton X-100 in PBS for 20 min. Next, cells were rinsed in PBS three times and blocked with peroxidase inhibitor (MAIXIN, CHN) for 10 minutes. Then cells were incubated with rabbit anti-human MMP-9 antibody (Beijing Zhongshan Bio., China) overnight at 4°C, followed by incubation in a secondary antibody for one hour. The slides were mounted using anti-fade mounting medium and viewed with Leica DM4000 fluorescent microscope (Germany).
Results
DHA decreases the Bel-7402 cell density
The Bel-7402 cells were treated with DHA for 72 hours in different concentration. It had been found that the cell density was decreased in DHA treatment group, and it dependents on concentration (Figure 1A). We found that DHA decreased cell density also dependents on time, and it decreased from 24 hours. Next, we examined the treated cell viability by using Trypan blue staining. The positive control (without treatment) resulted in health cells staining. However, each of treated with DHA group shown Trypan blue positive staining, and dose dependence (Figure 1B). The cells treated with 200μM DHA have 40% positive Trypan blue staining.
Figure 1.
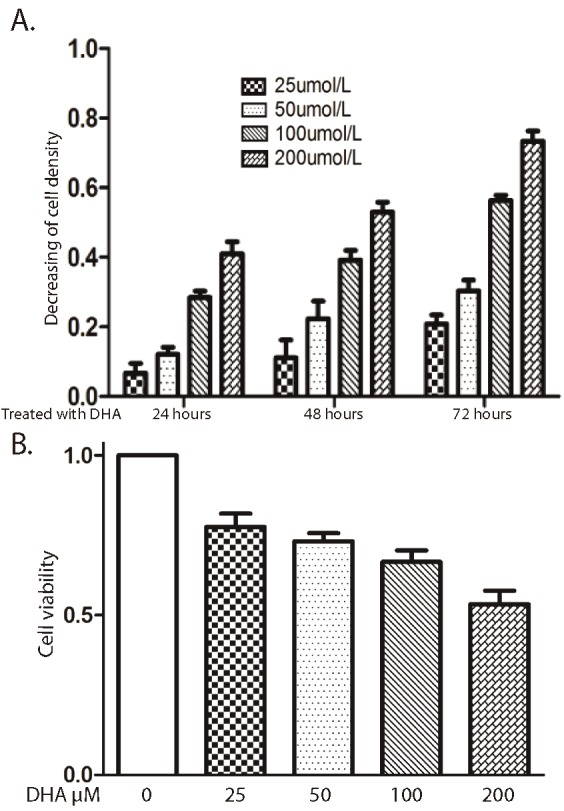
DHA inhibits Bel-7402 cells growth. A. Cells were treated with or without DHA in different concentrations at 24, 48, and 72h. The cell proliferation was detected by measuring cell density. B. Cells were exposed to different concentrations of DHA at 48h. Cells were stained by trepan blue solution. Data represent triplicate experiments ± SD.
DHA induces the Bel-7402 cells apoptosis
Based on finding, we investigated the potential link between DHA and decreased cell density in mammalian cells using the human hepatocellular carcinoma cell line Bel-7402. We found that more than 10-fold increase in apoptosis after treating Bel-7402 cells with DHA for 72 hours, as assessed by fluorescent Annexin V staining (Figure 2). It can be more than 30-fold increased when DHA concentration reached 200 μM, apoptotic cells are 35%. Prior work has shown that the Bim, Bcl-2, Bax and caspases- 3 play role in signaling transduction pathway leading to cell survival. To focus the signaling mechanism, the Bel-7402 cells were grown in absence or presence DHA in different concentration. The mRNA level of Bim gene was decreased when DHA concentration was increased (Figure 3A). The protein expression of Bcl-2 and Bax were detected by western blot (Figure 3B). Our data showed when DHA concentration was increased, the protein expression of Bcl-2 was decreased. The Bax expression level was increased when DHA concentration wa3s increased and caspases-3 activity was increased (Figure 3C).
Figure 2.
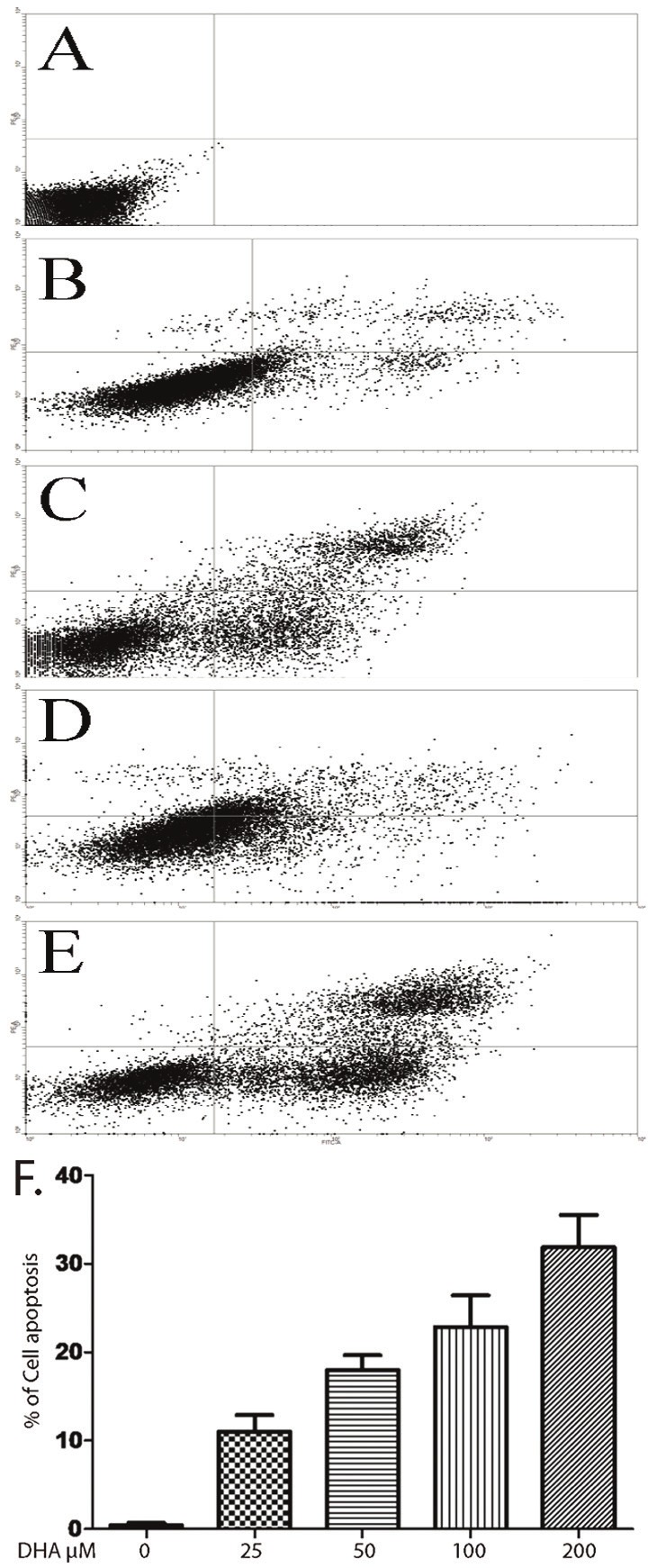
The Bel-7402 cells were treated with or without DHA in 25, 50, 100 or 200 μM for 48h. A-E has shown Annexin V-FITC/propidium staining by flow cytometry without or with DHA 25, 50, 100 or 200 μM. F. quantification of apoptosis cells, data represent triplicate experiments ± SD.
Figure 3.
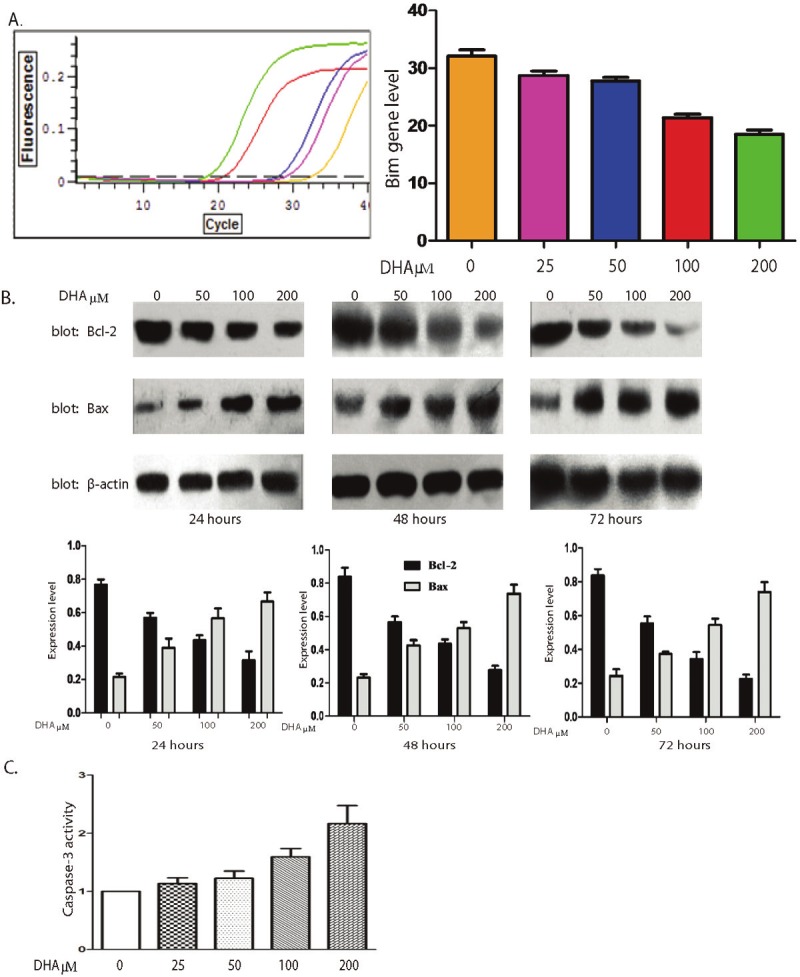
DHA activate the mitochondrial apoptotic pathway on Bel-7402 cells. A. After treatment with 0-200μM DHA for 48h, the real-time PCR shown that the expressions level of Bim gene was increased (left panel). The quantification of the expression level is shown in right panel, data represent triplicate experiments ± SD. B. The Bel-7402 cells were treated with DHA in different concentrations (0-200μM) for 48h. Protein levels of Bcl-2 and Bax were monitored by Western blot. The protein level of β-actin was used as loading control (upper panel). The quantification of the expression level is shown in lower panel, data represent triplicate experiments ± SD. C. The Bel-7402 cells were treated with DHA in different concentrations (0-200μM) for 48h. The activity of Caspase-3 was detected by measuring absorbance at 405 nm. Result was quantified, data represent triplicate experiments ± SD.
DHA inhibits the Bel-7402 cells migration
To further understand the function of DHA, we examined the functional consequence of DHA using a 24 hour scratch wound assay. We found that Bel-7402 cells migrate to close the wound in response to culture medium with serum. There are some of growth factors, such as EGF and HGF, in the serum, and the cells migrate in responding stimulation of those growth factors. It should be tested which growth factor causes the wound closed. However, in the Bel-7402 cells migration is severely blunted by treatment with DHA (Figure 4A), and depending concentration. In another approach to show that DHA inhibits Bel-7402 cells migration, the cells were growth in Matrigel and pretreated with DHA. It was further confirmed that DHA blocks cells migration, and depending concentration (Figure 4B and 4C). These results suggest that DHA inhibits cell migration by blocking cell signal transduction pathway. It has been reported that MMP-9 is required in the human hepatocellular carcinoma cell migration. Last, we have detected MMP-9 expression by immunofluorescences (Figure 5). The MMP-9 expression has decreased after treating Bel-7402 cell with DHA, and also depending concentration.
Figure 4.
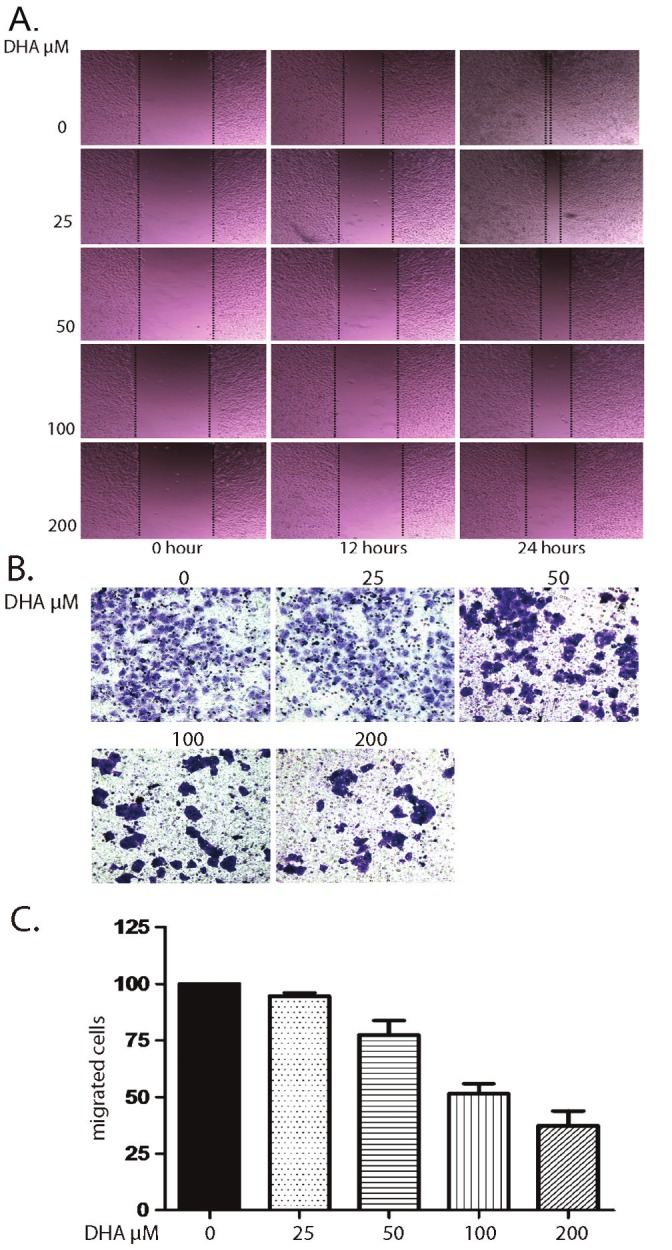
DHA inhibits invasion and migration of Bel-7402 cells. A. The Bel-7402 cells were growth in 6 well plate, then treated with DHA in different concentrations with 1mg/ml fatty acid-free BSA-DMEM another 24 hours. The cell migration was detected by measuring wound closure. B. The Bel-7402 cells were growth in Matrigel and pretreated with DHA in different concentration for 48h. The migrated cells were stained by the crystal violet. C. Quantification of migrated cells, data represent triplicate experiments ± SD.
Figure 5.
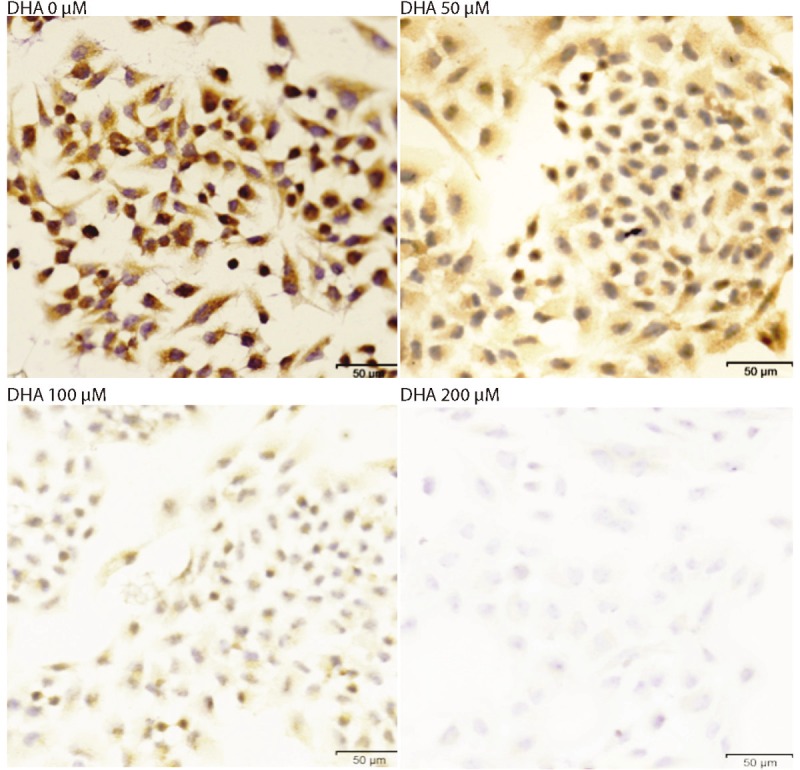
The Bel-7402 cells were treated with DHA in different concentration for 48 hours. The MMP-9 was detected by immunofluorescences. The representative photographs were taken at 400X.
Discussion
The current study demonstrates that DHA inhibits human hepatocellular carcinoma cell (Bel- 7402) growth. The cell density has decreased after DHA treatment. Further, our data has shown that cell apoptosis is a major reason of decreasing cell density, and caspase-3, Bcl-2, Bax and Bim are involved.
It has known that intracellular signaling pathways affected by DHA, DHA and its metabolites are involved in the regulation of many cell signaling pathways, contributing to the activation or inhibition of various components. DHA influences not only transduction of signals from the extracellular space, it also functions as a mediator and modulator in the intercellular and intracellular signaling network [19]. DHA has been also shown to actively modulate protein kinase C (PKC)-related signaling.
Studies using human colon adenocarcinoma cell lines in vitro showed that DHA inhibited cell proliferation and induced apoptosis by decreasing signal transduction through the PI3K/Akt cell survival pathway. DHA reduced phosphorylation of phosphatase and tensin homolog protein (PTEN), which functions as a negative regulator of the PI3K/Akt pathway. Our immune-staining studies show that pre-treat Bel-7402 cells with DHA, the Annex V staining positive. Our data suggest that DHA blocks signal transduction pathway for cell survival, and induces Bel-7402 cells apoptosis. We also found that DHA inhibits the migration of Bel- 7402 cells.
Furthermore, we have examined the Bim and Bax expression level of protein. Bim is a proapoptotic proteins released from the binding protein, combined with the tubulin. After treatment with DHA, the Bim gene expression has increased in Bel-7402 cells. Bax is a pro-apoptotic Bcl-2 protein containing BH1, BH2 and BH3 domains, and it initiates apoptotic signaling. The expression of Bax is up-regulated by the tumor suppressor protein p53, and Bax has been shown to be involved in p53-mediated apoptosis. We found that DHA has also increased Bax expression in the Bel-7402 cells.
Caspase-3 is a Caspase protein that interacts with caspase-8 and caspase-9, and encoded by the CASP3 gene. Caspase-3 has been activated in the apoptotic cell both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways. Bax is involved in the intrinsic pathway. The activated Caspase-3 induces Caspase-8 activation, then actives Bax. Bax activates Cyt c, and induces apoptosis. We found that the same signal pathway is in the Bel-7402 cells. The Caspase-3 activity has been increased by treating cells with DHA. The Bax expression has also increased after treating cells with DHA.
It has been accepted that mitochondria are a major subcellular site where n-3 fatty acids are rapidly incorporated. DHA interacts with mitochondrial membranes and alters their permeability by opening the permeability transition pores and decreasing the mitochondrial membrane potential (MMP). DHA has caused structural changes and increased mitochondrial membrane unsaturation.
The changes in mitochondria can influence the activity of pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family. It has been demonstrated that DHA contributes to down-regulation of Bcl-2 expression, a well-known anti-apoptotic molecule, and block lipid peroxidation and thus apoptosis induction. Our data has shown that DHA suppress Bcl-2 expression, and also decreases MMP-9 expression.
Based on published description and our studies, DHA inhibits human hepatocellular carcinoma cell migration, and induces apoptosis. The signal transduction is through Caspase-3 pathway. The further study on this signal pathway would give us more detail information, and understand the function of DHA in tumor invasion and survival.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30772097 and No. 30972892), Anhui Provincial Natural Science Foundation (No. 070413073), the Science and Technological Fund of Anhui Province for Outstanding Youth (No. 08040106818), Anhui Provincial Natural Science Foundation of Institution of Higher Education (No. KJ2010B386), and the Anhui Provincial ‘115’ Industrial Innovation Programmer.
References
- 1.Salem N Jr, Kim HY, Yergey JA. Health effects of polyunsaturated fatty acids in sea foods. Academic Press; 1986. Docosahexaenoic acids: membrane function and metabolism; pp. 263–317. [Google Scholar]
- 2.Holmes MD, Willett WC. Does diet affect breast cancer risk? Breast Cancer Res. 2004;6:170–178. doi: 10.1186/bcr909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinlen L. Diet and breast cancer. Br Med Bull. 1991;47:462–469. doi: 10.1093/oxfordjournals.bmb.a072484. [DOI] [PubMed] [Google Scholar]
- 4.Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chénais B. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int J Oncol. 2010;36:737–742. doi: 10.3892/ijo_00000549. [DOI] [PubMed] [Google Scholar]
- 6.Chiu LC, Wong EY, Ooi VE. Docosahexaenoic acid from a cultured microalga inhibits cell growth and induces apoptosis by upregulating Bax/Bcl-2 ratio in human breast carcinoma MCF-7 cells. Ann N Y Acad Sci. 2004;1030:361–368. doi: 10.1196/annals.1329.045. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 8.D’Eliseo D, Manzi L, Merendino N, Velotti F. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J Nutr Biochem. 2012;23:452–7. doi: 10.1016/j.jnutbio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Hagi A, Nakayama M, Miura Y, Yagasaki K. Effects of a fish oil-based emulsion on rat hepatoma cell invasion in culture. Nutrition. 2007;23:871–877. doi: 10.1016/j.nut.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M, Bae MA. Docosahexaenoic acid induces apoptosis in CYP2E1-containing HepG2 cells by activating the c-Jun N-terminal protein kinase related mitochondrial damage. J Nutr Biochem. 2007;18:348–354. doi: 10.1016/j.jnutbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Fasano E, Serini S, Piccioni E, Toesca A, Monego G, Cittadini AR, Ranelletti FO, Calviello G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822:1762–72. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Giros A, Grzybowski M, Sohn VR, Pons E, Fernandez-Morales J, Xicola RM, Sethi P, Grzybowski J, Goel A, Boland CR, Gassull MA, Llor X. Regulation of colorectal cancer cell apoptosis by the n-3 polyunsaturated fatty acids docosahexaenoic and eicosapentaenoic. Cancer Prev Res. 2009;2:732–742. doi: 10.1158/1940-6207.CAPR-08-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikawa KD, Herrick JS, Tateo RE, Mouradian M, Tay JS, Pardini RS. Induced oxidative stress and cell death in the A549 lung adenocarcinoma cell line by Ionizing radiation is enhanced by supplementation with docosahexaenoic acid. Nutr Cancer. 2010;62:1017–1024. doi: 10.1080/01635581.2010.492084. [DOI] [PubMed] [Google Scholar]
- 15.Xia SH, Wang J, Kang JX. Decreased n-6/n-3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes. Carcinogenesis. 2005;26:779–784. doi: 10.1093/carcin/bgi019. [DOI] [PubMed] [Google Scholar]
- 16.Denkins Y, Kempf D, Ferniz M, Nileshwar S, Marchetti D. Role of omega-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J Lipid Res. 2005;46:1278–84. doi: 10.1194/jlr.M400474-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Seitz HK, Stickel F. Risk factors and mechanisms of hepato-carcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 18.Vibet S, Goupille C, Bougnoux P, Steghens JP, Goré J, Mahéo K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathioneperoxidase (GPx1) response. Free Radic Biol Med. 2008;44:1483–1491. doi: 10.1016/j.freeradbiomed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui RA, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee CY, Sit WH, Fan ST, Man K, Jor IW, Wong LL, Wan ML, Tan-Un KC, Wan JM. The cell cycle effects of docosahexaenoic acid on human metastatic hepatocellular carcinoma proliferation. Int J Oncol. 2010;36:991–998. doi: 10.3892/ijo_00000579. [DOI] [PubMed] [Google Scholar]
- 21.Liotta LA, Wewer U, Rao NC, Schiffmann E, Stracke M, Guirguis R, Thorgeirsson U, Muschel R, Sobel M. Biochemical mechanism of tumor invasion and metastasis. Prog Clin Biol Res. 1988;256:3–16. [PubMed] [Google Scholar]
- 22.Nart D, Yaman B, Yilmaz F, Zeytunlu M, Karasu Z, Kiliç M. Expression of matrix metalloproteinase- 9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010;16:621–630. doi: 10.1002/lt.22028. [DOI] [PubMed] [Google Scholar]
- 23.Van Harken DR, Dixon CW, Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969;244:2278–85. [PubMed] [Google Scholar]
- 24.Kanno S, Shouji A, Asou K, Ishikawa M. Effects of naringin on hydrogenperoxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci. 2003;92:166–170. doi: 10.1254/jphs.92.166. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Lechon MJ, Ponsoda X, O’Connor E, Donato T, Castell JV, Jover R. Diclofenac induces apoptosis in hepatocytes by alteration of mitochondrial function and generation of ROS. Biochem Pharmacol. 2003;66:2155–2167. doi: 10.1016/j.bcp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 26.D’Eliseo D, Pisu P, Romano C, Tubaro A, De Nunzio C, Morrone S, Santoni A, Stoppacciaro A, Velotti F. Granzyme B is expressed in urothelial carcinoma and promotes cancer cell invasion. Int J Cancer. 2010;127:1283–94. doi: 10.1002/ijc.25135. [DOI] [PubMed] [Google Scholar]


