Abstract
The author reviewed 910 cases of consecutive esophageal biopsies in the last 15 year in the pathology laboratory of our hospital. There were 693 normal mucosa and benign lesions (76.2%) and 217 malignant lesions (23.8%). No significant changes were recognized in the esophagus in 50 biopsies (5.5%). In benign lesions, the number and frequency (percentages) were as follows: 263 chronic esophagitis (28.9%), 98 heterotopic gastric mucosa (10.8%), 3 heterotopic colonic mucosa (0.3%), 71 glycogenic acanthosis (7.8%), 68 candidiasis (7.5%), 35 benign ulcer (3.8%), 41 squamous papilloma (4.5%), 4 granular cell tumor (0.4%), 1 tubular adenoma (0.1%), 2 cytomegalovirus esophagitis (0.2%), 3 leiomyoma (0.3%), 17 basal cell hyperplasia (1.9%), and 37 Barrett’s epithelium (4%). In malignant lesions, the number and frequency (percentages) were as follows: 53 mild dysplasia (5.8%), 29 moderate dysplasia (3.2%), 31 severe dysplasia (3.4%), 13 carcinoma in situ (1.4%), 68 squamous cell carcinoma (7.5%), 7 primary adenocarcinoma (0.8%), 1 primary signet ring cell carcinoma (0.1%), 4 primary small cell carcinoma (0.4%), 2 primary amelanotic malignant melanoma (0.2%), 1 primary undifferentiated sarcoma (0.1%), 7 gastric cancer invasion (0.8%), and 1 primary adenoid cystic carcinoma (0.1%). In this article, the clinicopathologic features of these esophageal lesions were described.
Keywords: Esophagus, benign lesions, malignant lesions, clinicopathologies, immunohistochemistry
Introduction
Many kinds of pathologic lesions occur in the esophagus. They include esophageal atresia, heterotopic gastric mucosa, heterotopic pancreatic tissue, diverticula, esophageal cyst, achalasia, Lye stricture, reflex esophagitis, Barrett’s esophagus, dysplasia and carcinoma in Barrett’s esophagus, Herpes simplex esophagitis, cytomegalovirus esophagitis, eosinophilic esophagitis, Crohn’s disease, candidiasis, squamous cell carcinoma, carcinoma in situ, intraepithelial neoplasm (dysplasia), sarcomatoid carcinoma, verrucous carcinoma, adenocarcinoma, adenosquamous carcinoma, mucoepidermoid carcinoma, basaloid carcinoma, small cell carcinoma, leiomyoma, leiomyosarcoma, gastrointestinal stromal tumor, carcinoid tumor, lymphoepithelioma-like carcinoma, glycogenic acanthosis, amyloidosis, squamous papilloma, hyperplastic polyp, granular cell tumor, malignant melanoma, malignant lymphoma, plasmacytoma, malignant mesenchymal tumors, and metastatic carcinoma [1,2]. Recent advances in endoscopy have made it possible that these esophageal lesions are biopsied and diagnosed correctly. In the present study, the authors reviewed 910 archival cases of the esophageal biopsies.
Materials and methods
The authors retrospectively reviewed consecutive 910 cases of esophageal biopsy specimens in the last 15 years in the pathology laboratory in our hospital. In the person with multiple biopsies, the number of biopsy was counted as one. The ages of the patients ranged from 12 years to 95 years with a mean of 52.6 years. Male to female ratio was 546:364. Clinical and endoscopic records were also reviewed.
Histochemical stainings including PAS, Alcian blue, Grocott, Grimelius were employed in appropriate biopsies. In appropriated cases, an immunohistochemical study was performed, using Dako Envision method (Dako Corp., Glostrup, Denmark), as previously described [3-9]. The antibodies employed were anti-cytokeratin (AE1/3, Dako), anti-cytokeratin (polyclonal wide, Dako), anti-p53 protein (DO-7, Dako) anti-Ki-67 antigen (MIB-1, Dako), CD3 (M7193, Dako), CD10 (M0727, Dako), CD15 (M0733, Dako), CD30 (M0751, Dako), CD45 (M0855, DAKO), CD45RO (M0834, Dako), CD79α (M7050, Dako), CD56 (MOC-1, Dako), carcinoembryonic antigen (polyclonal, Dako), chromogranin (DAK-A3, Dako), synaptophysin (polyclonal, Dako), neuron-specific enolase (BBS/NC/VI-H14, Dako), CD56 (MOC-1, Dako), glucagon (polyclonal, Dako), KIT (polyclonal Dako), PDGFRA (polyclonal, Santa Cruz, CA, USA), CD34 (QBEND10, Dako), vimentin (Vim 3B4, Dako), desmin (D33, Dako), α-smooth muscle actin (1A4, Dako), S100 protein (polyclonal, Dako), myoglobin (polyclonal), cytomegalovirus (polyclonal, Dako), and melanosome (HMB45, Dako).
Results
There were 693 normal mucosa or benign lesions (76.2%) and 217 malignant lesions (23.8%). In the former 693 cases, 50 biopsies (5.5%) showed mature normal squamous epithelium free of significant pathologic changes. Chronic esophagitis consisting of lymphocytic and eosinophilic infiltration was recognized in 263 cases (28.9%). Cytomegalovirus esophagitis was noted in 2 cases (0.2%); one patient was SLE and another was 85-year-old woman without other diseases. Histologically, inclusion bodies were seen in the endothelium, and they were immunohistochemically positive for cytomegalovirus. Esophagitis was endoscopically recognized as reflux esophagitis and erosion in the lower esophagus near the stomach.
Heterotopic gastric mucosa was recognized in 98 cases (10.8%) (Figure 1). Two types of heterotopic gastric mucosa were recognized; one with foveolar epithelium and fundic glands (63 cases) and one consisting of only foveolar epithelium (35 cases). It was present in the cervical esophagus in 13 cases, in proximal esophagus in 19 cases, in the middle esophagus in 28 cases, and in the distal esophagus in 38 cases. The foveolar epithelium showed neutral mucins. Heterotopic colonic mucosa (Figure 2) was present in 3 cases (0.3%), 1 in the middle esophagus and 2 in the distal esophagus. It was free of gastric elements and showed acidic mucins. These heterotopic tissues were endoscopically recognized in flat or slightly elevated discoloration, and iodine reaction was negative. Barrett’s esophagus was identified in 37 cases (4%). One of the Barrett’s epithelium showed atypical glands.
Figure 1.
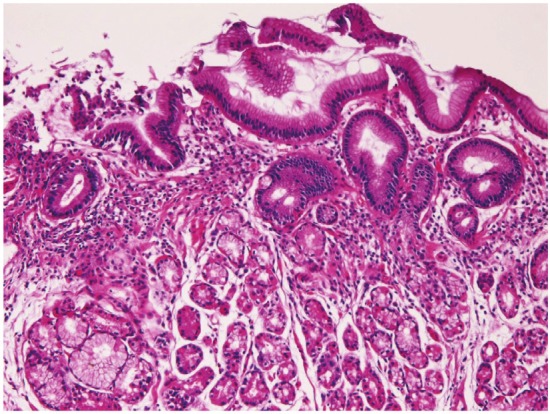
Heterotopic gastric mcuosa in the esophagus. Fundic glands are seen. HE, x100.
Figure 2.
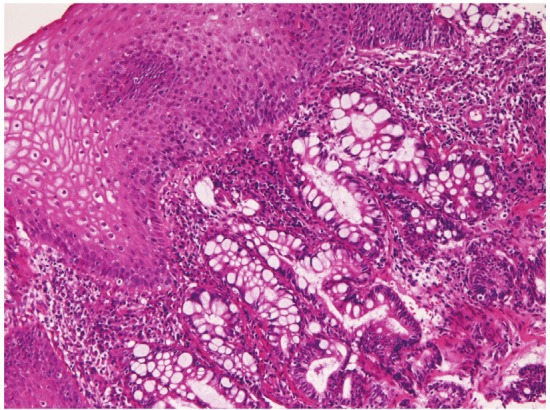
Heterotopic colonic mucosa in the esophagus.Goblet cells are seen. HE, x200.
Glycogenic acanthosis was recognized in 71 cases (7.8%). Glycogen was demonstrated by PAS stain. It was present in the cervical esophagus in 4 cases, in the proximal esophagus in 10 cases, in the middle esophagus in 11 cases, and in the distal esophagus in 46 cases. It was endoscopically recognized as slightly elevated iodine-positive small areas. Candidiasis was noted in 68 cases (7.5%). The patients with candidiasis were of middle or old age. The candida fungi were confirmed by PAS and Grocott stains. It was located in the cervical esophagus in 12 cases, in the proximal esophagus in 17 cases, in the middle esophagus in 19 cases, and in the distal esophagus in 20 cases. Benign ulcer was present in 35 cases (3.8%). It was located in the middle esophagus in 2 cases, and in the distal esophagus in 33 cases. Basal cell hyperplasia was identified in 17 cases (1.9%); 5 in the proximal esophagus, 4 in the middle esophagus, and 8 in the distal esophagus. It was endoscopically recognized as flat or elevated lesions.
Squamous papilloma (Figure 3) was noted in 41 cases (4.5%). It was located in the cervical esophagus in 6 cases, in the proximal esophagus in 12 cases, in the middle esophagus in 11 cases, and in the distal esophagus in 12 cases. It was endoscopically recognized as small polypoid tumor. Granular cell tumor (Figure 4) was present in 4 cases (0.4%); in the proximal esophagus in 3 cases, and in the middle esophagus in 1 case. The granular cell tumor was immunohistochemically positive for vimentin and S100 protein. It was endoscopically recognized by elevated small lesions. Tubular adenoma was noted in 1 case (0.1%) in the distal esophagus. It was endoscopically an elevated lesion. Leiomyoma was seen in 3 cases (0.3%); in the cervical esophagus in 1 case and in the proximal esophagus in 2 cases. It was immunohistochemically positive for vimentin, smooth muscle actin, and desmin. It was endoscopically recognized as a submucosal tumor.
Figure 3.
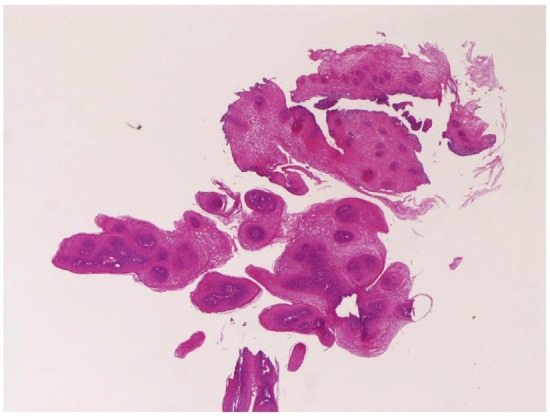
Sqaumous papilloma of the esophagus. HE, x20.
Figure 4.
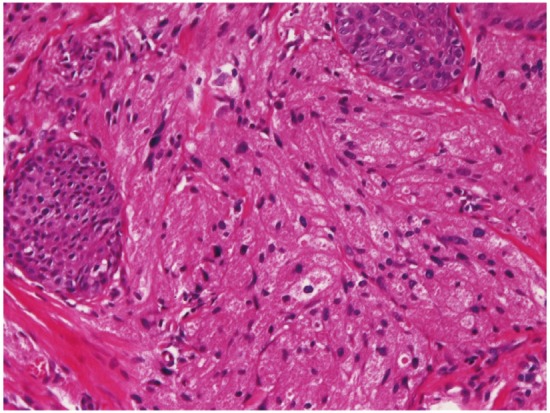
Granular cell tumor of the esophagus. HE,x200.
In preneoplastic and malignant lesions, mild dysplasia (Figure 5) was identified in 53 cases (5.8%); in the proximal esophagus in 14 cases, in the middle esophagus 18 cases, in the distal esophagus in 21 cases. Moderate dysplasia (Figure 6) was recognized in 29 cases (3.2%); in the cervical esophagus in 2 cases, in the proximal esophagus in 8 cases, in the middle esophagus in 9 cases and in the distal esophagus in 10 cases. Severe dysplasia (Figure 7) was seen in 31 (3.4%); in the cervical esophagus in 2 cases, in the proximal esophagus in 8 cases, in the middle esophagus in 10 cases, and in the distal esophagus in 11 cases. Carcinoma in situ (Figure 8) was present in 13 cases (1.4%); in the middle esophagus in 5 cases and in the distal esophagus in 8 cases. Immunohistochemically, p53 protein expression was 20/53 in mild dysplasia, 23/29 in moderate dysplasia, 30/31 in severe dysplasia, and 13/13 in carcinoma in situ. Mean Ki-67 labeling was 8% in mild dysplasia, 13 % in moderate dysplasia, 23 % in severe dysplasia, and 36 % in carcinoma in situ. These intraepithelial neoplasms were endoscopically recognized as flat, elevated, or depressed iodine-negative areas.
Figure 5.
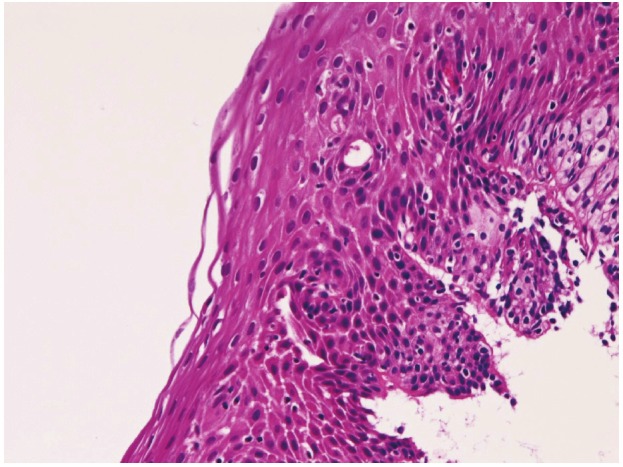
Mild dysplasia of the esophagus, HE, x100.
Figure 6.
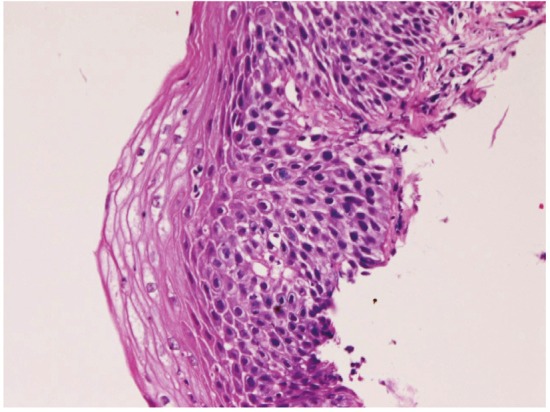
Moderate dysplasia of the esophagus. HE,x100.
Figure 7.
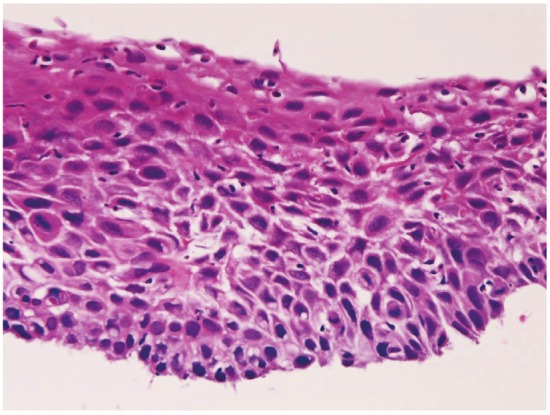
Severe dysplasia of the esophagus. HE,x100.
Figure 8.
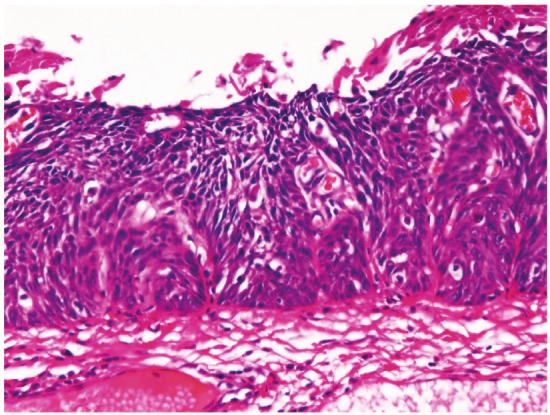
Carcinoma in situ of the esophagus. HE,x100.
Squamous cell carcinoma (Figure 9) was recognized 68 cases (7.5%); in the cervical esophagus in 1 cases, in the distal esophagus in 9 cases, in the middle esophagus in 20 cases, and in the distal esophagus in 38 cases. Of the 68 cases, 23 were well, 25 were moderately, and 20 were poorly differentiated squamous cell carcinomas. Of the 68 cases, 56 cases showed dysplasia or carcinoma in situ in the vicinity of squamous cell carcinoma. Expression of p53 protein was recognized in 66/68 cases, and mean Ki-67 labeling was 54%. Endoscopically, it was identified as a polypoid, elevated or ulcerated tumor. Primary ordinary adenocarcinoma (Figure 10) was demonstrated 7 cases (0.8%); in the proximal esophagus in 1 case, in the middle esophagus in 4 cases, and in the distal esophagus in the 2 cases. Of the 7 cases, 3 were well, 2 were moderately and 2 were poorly differentiated adenocarcinomas. Immunohistochemically, carcinoembryonic antigen and p53 protein were positive in all cases. Mean Ki-67 labeling was 67%. Primary signet ring cell carcinoma (Figure 11) was noted in 1 case (0.1%) in the middle esophagus. It contained acidic and neutral mucins, and immunohistochemically positive for cytokeratins and p53 protein. The Ki-67 labeling was 29%. These primary adenocarcinoma cases were endoscopically recognized in elevated or ulcerated tumors.
Figure 9.
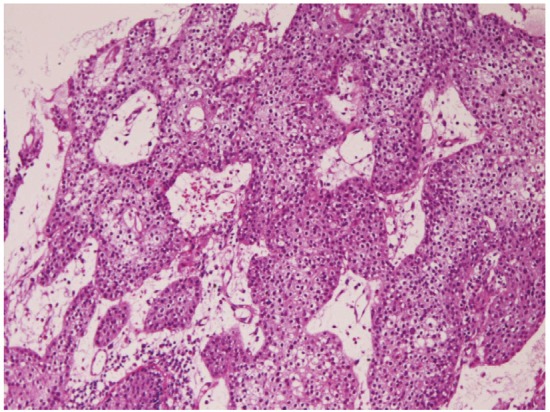
Squamous cell carcinoma of the esophagus. HE, x100.
Figure 10.
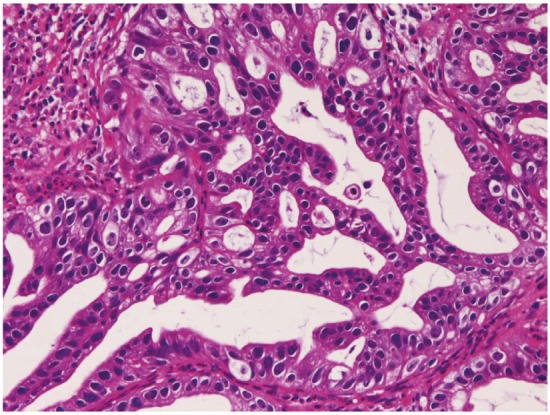
Ordinary adenocarcinoma of the esophagus.HE, x200.
Figure 11.
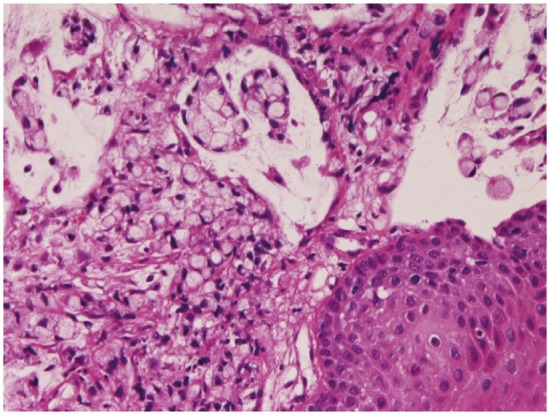
Signet ring cell carcinoma of the esophagus.HE, x200.
Primary small cell carcinoma (Figure 12) was identified in 4 cases (0.4%); in the proximal esophagus in 1 case, in the middle esophagus in 2 cases, and in the distal esophagus in 1 case. Immunohistochemically, small cell carcinoma was positive for cytokeratin and also positive for at least one of neuroendocrine markers (neuron-specific enolase, chromogranin, synaptophysin, and CD56). All cases were immunoreactive for KIT and PDGFRA. Small cell carcinoma was negative for lymphoma markers such as CD3, CD20 and CD45. It was endoscopically demonstrated as a polypoid tumor. Malignant melanoma (Figure 13A) was present in 2 cases (0.2%), in the middle esophagus in both cases. The melanomas were amelanotic, and immunohistochemically positive for melanosome (Figure 13B) and S100 protein. Both cases were endoscopically recognized as polypoid lesions. Primary undifferentiated sarcoma was seen in 1 case (0.1%) in the cervical esophagus. The sarcoma was immunohistochemically positive for vimentin but negative for cytokeratins and other mesenchymal markers. Histologically, no specific patterns were recognized; therefore the exact diagnosis was impossible. Invasion from gastric adenocarcinoma was seen in 7 cases (0.8%) in the distal esophagus. Adenoid cystic carcinoma (Figure 14) was identified as elevated lesion in the middle esophagus in 1 case (0.1%). Immunohistochemically, tumor cells were positive for cytokeratins, p53 protein and smooth muscle actin. The Ki-67 labeling was 26%.
Figure 12.
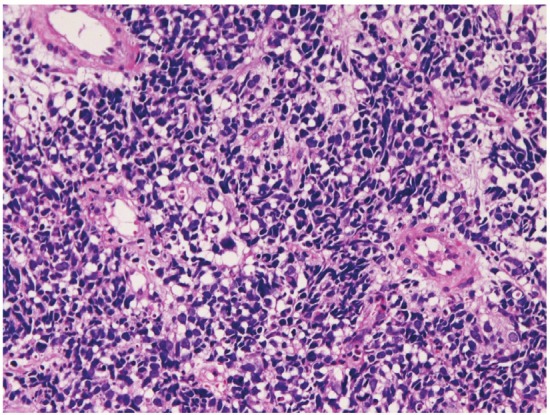
Small cell carcinoma of the esophagus. HE, x200.
Figure 13.
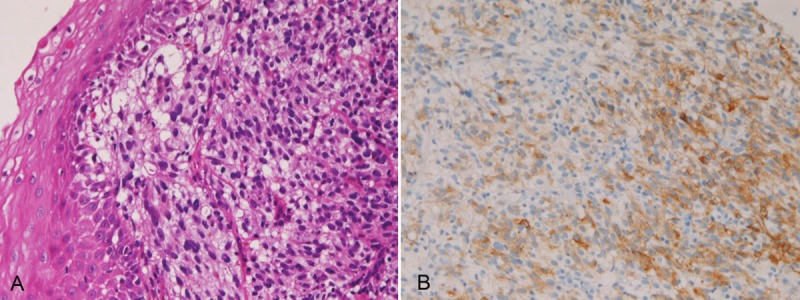
A: Amelanotic melanoma of the esophagus. HE, x200. B: Melanosome (HMB45) is positive in melanomaof A. x200.
Figure 14.
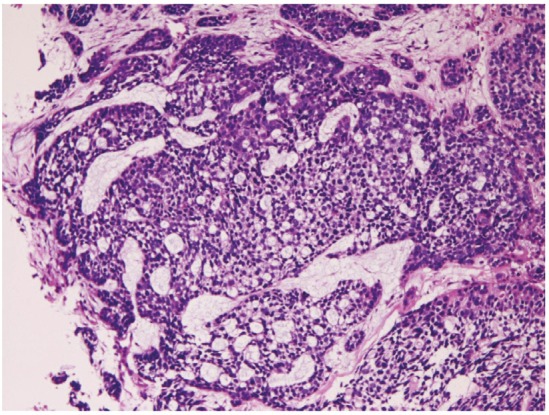
Adenoid cystic carcinoma of the esophagus.HE, x100.
Discussion
In the present study, 50 biopsies (5.5%) showed mature normal squamous epithelium free of significant pathologic changes, suggesting that normal mucosa is present in endoscopic lesions, or endoscopy does not always take the lesions. In the present study, chronic esophagitis in 263 cases (28.9%). Esophagitis was endoscopically recognized as reflux esophagitis and erosion in the lower esophagus near the stomach, suggesting that esophagitis predominantly occurs in the distal esophagus and it is due to reflux of gastric juice. In the present study, cytomegalovirus esophagitis was noted in 2 cases (0.2%). Cytomegalovirus esophagitis is very rare and is known to occur in collagen diseases and in immunocompromised persons [10]. One of the present patients was SLE, and another was 85-year-old woman.
In the present study, heterotopic gastric mucosa was recognized in 98 cases (10.8%). Heterotopic gastric mucosa has been observed in the esophagus and its frequency has been reported in 3.8-10 % in the esophagus, and has been though to be a congenital developmental anomaly [11-13]. The preferential site is thought to be upper esophagus and it is also called inlet patch [11,12] The present study indicates that heterotopic gastric mucosa may occur in the middle and distal esophagus. In the present study, two types of heterotopic gastric mucosa were recognized. The one with foveolar epithelium and fundic glands may be congenital anomaly, and the one consisting of only foveolar epithelium may be congenital anomaly or acquired lesion (gastric foveolar metaplasia). In the present study, heterotopic colonic mucosa was observed in 3 cases (0.3%). It was devoid of gastric elements, suggesting that it was not intestinal metaplasia of heterotopic gastric mucosa, but a true heterotopic colonic mucosa. A review of the literature does not reveal such a heterotopic colonic mucosa in the esophagus. Therefore, this is a new finding. Numerous studies have been performed in Barrett’s esophagus. Adenocarcinoma and dysplastic glands are frequently observed in Barrett’s esophagus [1,2,14,15]. In the present series, Barrett’s esophagus was present in 37 cases (4%), and only one case showed atypical glands. No adenocarcinoma in Barrett’s esophagus was seen in this series, suggesting that malignant transformation is rare in Barrett’s esophagus in Japanese population.
Glycogenic acanthosis is a common condition, its frequency is 3.5%, and may be associated with reflux esophagitis [16]. In the present study, glycogenic acanthosis was recognized in 71 cases (7.8%). Esophageal candidiasis occurs in persons with deficiency of cell-mediated immunity or old persons [17]. In the present study, candidiasis was noted in 68 cases (7.5%). All persons were of middle or old age. In the present study, benign ulcer was present in 35 cases (3.8%). It was located preferentially in the distal esophagus, suggesting that it is associated with gastroesophageal reflux. Basal cell hyperplasia has been considered associated with high risk of squamous cell carcinoma [1,2]. In the present study, basal cell hyperplasia was identified in 17 cases (1.9%).
Squamous papilloma of the esophagus is a rare condition. It may be associated with human papilloma virus [18]. However, contradictory studies have been reported [19]. In the present series, squamous papilloma was noted in 41 cases (4.5%). Granular cell tumor is very rare in the esophagus; only a few case reports are present [20]. In the present series, granular cell tumor was present in 4 cases (0.4%). Adenoma of the esophagus is extremely rare [21]. In the present series, only one tubular adenoma (0.1%) was recognized. Leiomyoma of the esophagus is rare but the most common mesenchymal tumor in the esophagus [22]. In the present study, leiomyoma was observed in 3 cases (0.3%).
In preneoplastic and malignant lesions, it is well established that mild dysplasia and moderate dysplasia were classified as low-grade intraepithelial neoplasm, and severe dysplasia and carcinoma in situ as high-grade intraepithelial neoplasm [1,2,23]. The intraepithelial neoplasm may evolve into squamous cell carcinoma, and patients with this lesion have 30-60 fold increase for the development of squamous cell carcinoma [2]. In the present study, mild dysplasia was identified in 53 cases (5.8%), moderate dysplasia in 29 cases (3.2%), severe dysplasia in 31 cases (3.4%), and carcinoma in situ in 13 cases (1.4%). The frequent presence of dysplasia in the vicinity of squamous cell carcinoma in the present study highly suggests that these dysplastic lesions lead to the development of squamous cell carcinoma. The choice of therapy is endoscopic mucosal resection.
As is well known, squamous cell carcinoma is the most common aggressive tumor in the esophagus. In the present study, squamous cell carcinoma was recognized 68 cases (7.5%); it occurred preferentially middle and distal esophagus. The frequent p53 expression and high Ki-67 labeling suggest that p53 gene is mutated and proliferative fraction is very high. Primary ordinary adenocarcinoma is a very rare entity. It was demonstrated 7 cases (0.8%) in the present study. Primary signet ring cell carcinoma is an extremely rare tumor in the esophagus [24]. In the present study, it was recognized in only 1 case (0.1%).
Primary small cell carcinoma is a very rare neoplasm in the esophagus [25-29]. In the present series, it was identified in 4 cases (0.4%). Immunohistochemically, it was characterized by positive neuroendocrine markers and positive KIT and PDGFRA. The latter two were new findings. Primary malignant melanoma is also an extremely rare neoplasm in the esophagus [29,31]. In the present series, it was recognized in 2 cases (0.2%). Both cases were amelanotic melanomas. In the present series, undifferentiated sarcoma was seen in 1 case (0.1%). The cell type of the sarcoma was indeterminate despite of various immunohistochemical procedures. Adenoid cystic carcinoma is very rare in the esophagus [1,2,32]. It is believed to originate from esophageal glands [1,2]. In the present series, it was identified in only 1 case (0.1%). In the present series, invasion from gastric adenocarcinoma was seen in 7 cases (0.8%). It must be differentiated from primary adenocarcinoma of the esophagus.
In summary, the author described clinical and pathological features of esophageal lesions in 910 cases.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Rosai J. Rosai and Ackermann’s Pathology. Ninth edition. Mosby; Esophagus; pp. 615–647. [Google Scholar]
- 2.Hamilton SR, Aaltonen LA, editors. WHO Classification of tumors. Pathology and genetics, Tumor of the digestive system. Lyon: IARC press; 2000. Chapter 1, tumors of oesophagus; pp. 10–30. [Google Scholar]
- 3.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 5.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 7.Terada T. Gall bladder adenocarcinoma arising in Rokitansky-Schoff sinuses. Pathol Int. 2008;58:806–809. doi: 10.1111/j.1440-1827.2008.02316.x. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Intraductal tubular carcinoma, intestinal type, of the pancreas. Pathol Int. 2009;59:53–58. doi: 10.1111/j.1440-1827.2008.02325.x. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Large endocervical polyp with cartilaginous and osseous metaplasia: a hitherto unreported entity. Int J Gynecol Pathol. 2009;28:98–100. doi: 10.1097/PGP.0b013e31817eb796. [DOI] [PubMed] [Google Scholar]
- 10.Sekigawa I, Nawata M, Seta N, Yamada M, Iida N, Hashimoto H. Cytomegalovirus infection in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2002;20:559–564. [PubMed] [Google Scholar]
- 11.Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut. 1991;32:968–972. doi: 10.1136/gut.32.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabbari M, Goresky CK, Lough J, Yaffe C, Daly D, Cote C. The inlet patch: heterotopic gastric mucosa in the upper esophagus. Gastroenterology. 1985;89:352–356. doi: 10.1016/0016-5085(85)90336-1. [DOI] [PubMed] [Google Scholar]
- 13.Terada T. Heterotopic gastric mucosa of the gastrointestinal tract: A histopathologic study of 158 cases. Pathol Res Pract. 2011;207:148–150. doi: 10.1016/j.prp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Paraf F, Flejou JF, Pignon JP, Fekete F, Potet F. Surgical pathology of adenocarcinoma arising in Barrett’s esophagus: analysis of 67 cases. Am J Surg Pathol. 1995;19:183–191. doi: 10.1097/00000478-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Lee RG. Dysplasia in Barrett’s esophagus: a clinicopathologic study in six patients. Am J Surg Pathol. 1985;9:845–852. doi: 10.1097/00000478-198512000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Vadva MD, Triadafilopoulos G. Glycogenic acanthosis of the esophagus and gastroesophageal reflux. J Clin Gastroenterol. 1993;17:79–83. doi: 10.1097/00004836-199307000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Kalfa VC, Roberts RL, Stiehm ER. The syndrome of chronic mucocutaneous candidiasis with selective antibody deficiency. Ann Allergy Asthma Immunol. 2003;90:259–264. doi: 10.1016/S1081-1206(10)62152-7. [DOI] [PubMed] [Google Scholar]
- 18.Odze R, Antoniolli D, Shocket D, Noble-Topham S, Goldman H, Upton M. Esophageal squamous papillomas: a clinicopathologic study of 38 lesions and analysis for human papilloma virus by the polymerase chain reaction. Am J Surg Pathol. 1993;17:803–812. [PubMed] [Google Scholar]
- 19.Carr NJ, Bratthauer GL, Lichy JH, Taubenberger JK, Monihan JM, Sobin LH. Squamous cell papillomas of the esophagus: a study of 23 lesions for human papilloma virus by in situ hybridization and the polymerase chain reaction. Hum Pathol. 1994;25:536–540. doi: 10.1016/0046-8177(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 20.Narra SL, Tombazzi C, Datta V, Ismail MK. Granular cell tumor of the esophagus: report of five cases and review of the literature. Am J Med Sci. 2008;335:338–341. doi: 10.1097/MAJ.0b013e3181568197. [DOI] [PubMed] [Google Scholar]
- 21.Bajbouj M, von Weyhern C, Becker V, Seidi S, Ott R, Schatke W, Fend F, Schmid RM, Meining A. True adenoma of the cardia: a case of 3 patients. Digestion. 2008;77:65–67. doi: 10.1159/000121413. [DOI] [PubMed] [Google Scholar]
- 22.Mutrie CJ, Donahue DM, Wain JC, Wright CD, Gaissert HA, Grillo HC, Mathisen DJ, Allan JS. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg. 2005;79:1122–1125. doi: 10.1016/j.athoracsur.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Maezato K, Nishimaki T, Oshiro M, Yamashiro T, Sasaki H, Sashida Y. Signet-ring cell carcinoma of the esophagus associated with Barrett’s esophagus: report of a case. Surg Today. 2007;37:1096–1101. doi: 10.1007/s00595-007-3533-5. [DOI] [PubMed] [Google Scholar]
- 24.Terada T. Esophageal cancers: a clinicopathologic and immunohistochemical study of 223 cases. Gastroenterol Res. 2009;2:148–151. doi: 10.4021/gr2009.05.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terada T. Neuroendocrine carcinoma of the esophagus: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Med Oncol. 2011;28:509–512. doi: 10.1007/s12032-010-9499-y. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Primary esophageal small cell carcinoma with brain metastasis and with CD56, KIT, and PDGFRA expressons. Pathol Oncol Res. 2012;19:1091–1093. doi: 10.1007/s12253-011-9374-y. [DOI] [PubMed] [Google Scholar]
- 27.Terada T. KIT and PDGFRA in esophageal pure small cell carcinoma. Int J Clin Exp Pathol. 2011;4:718–721. [PMC free article] [PubMed] [Google Scholar]
- 28.Terada T. Esophageal carcinoma with triplicate differentiation into squamous cell carcinoma, small cell carcinoma, and adenocarcinoma: a case report. Gastroenterol Res. 2009;2:118–121. doi: 10.4021/gr2009.04.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly J, Liang J, Wang J, Wang L, He J, Xiao Z, Yin W. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3:1460–1465. doi: 10.1097/JTO.0b013e31818e1247. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. Amelanotic malignant melanoma of the esophagus: report of two cases with immunohistocheimcal and molecular genetic study of KIT and PDGFRA. World J Gastroenterol. 2009;15:2679–2683. doi: 10.3748/wjg.15.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez AA, Wu TT, Prieto VG, Rashid A, Hamilton SR, Wang H. Comparison of primary and metastatic malignant melanoma of the esophagus: clonicopathologic review of 10 cases. Arch Pathol Lab Med. 2008;132:1623–1629. doi: 10.5858/2008-132-1623-COPAMM. [DOI] [PubMed] [Google Scholar]
- 32.Cerar A, Jutersek A, Vidmar S. Adenoid cystic carcinoma of the esophagus: a clinicopathologic study of three cases. Cancer. 1991;15:2159–2164. doi: 10.1002/1097-0142(19910415)67:8<2159::aid-cncr2820670826>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]


